Abstract
AIM: To investigate the characteristics of the vascularity of hepatic metastasis.
METHODS: Six New Zealand rabbits, weighing averagely 2.7 ± 0.4 kg, were selected and operated to establish hepatic VX2 tumor carrier model. Hepatic VX2 tumors were then imaged with conventional B mode US, second harmonic imaging (SHI), color Doppler flow imaging (CDFI), power Doppler imaging (PDI) and harmonic PDI by a transducer S8 connected to HP-5500 ultrasound system. A kind of self made echo contrast agent was intravenously injected at a dose of 0.01 mL/kg through ear vein, and then the venous passage was cleaned with sterilized saline.
RESULTS: Totally, 6 hypoechoic lesions and 3 hyperechoic lesions were found in the six carrier rabbits with a mean size about 2.1 ± 0.4 cm under conventional B mode ultrasound, they were oval or round in shape with a clear outline or a hypoechoic halo at the margin of the lesions. Contrast agent could not change the echogenicity of the lesions under conventional B mode and SHI, however, it could greatly increase the flow sensitivity of the lesions under PDI and harmonic PDI. Nutrient artery of these metastatic lesions might also be well depicted under contrast enhanced PDI and harmonic PDI.
CONCLUSION: Our result suggested that contrast enhanced PDI, especially harmonic PDI, was a promised method in the detection of vascularity of hepatic tumor nodules.
INTRODUCTION
Power Doppler ultrasonography (US) has been shown more sensitive than color Doppler US in the depiction of vascular flow in focal hepatic lesions, such as hepatocellular carcinoma (HCC), focal nodular hyperplasia (FNH) and metastasis (Mets). In addition, a wide variety of US contrast agents have been developed by using different gases and coating materials, especially those called the third generation contrast agents manufactured through perflurocarbon gases. These US contrast agents are promised to improve the quality of vascularity in Doppler studies, and its potential role in hepatic US has already been widely investigated. Researches have revealed that power Doppler US performed with the application of the contrast agent depicts more intratumoral vascularity in HCC than non-contrast power Doppler US does. Meanwhile, less knowledge is known about the vascularity of hepatic metastasis by harmonic power Doppler US. In this study, we intended to depict the characterization of the vascularity of hepatic Metastasis.
MATERIALS AND METHODS
Preparation of animal models
Six New Zealand rabbits weighing 2.6-3.2 kg, averagely 2.7 ± 0.4 kg were anaesthetized by Sumianxin (a product of the Changchun Argo-Pastoral University) at 0.2 mL/kg through intramuscular injection. Hairs in the abdominal region were moulted by 8% sodium sulfide, then the region was cleaned by saline water. Median incision right beneath the metasternum was made to expose the right lobe of liver. A tunnel about 3cm deep at the lobe was established with an ophthalmic nipper. Viable VX2 tumor tissue masses about 2-3 mm3 were implanted into the tunnel, locally stanched and then the each layer of abdominal wall was sutured accordingly. 2 or 3 wk later, these rabbits were ready for the experiment. VX2 tumor is a kind of dermatological squamous cancer induced by Shope virus, viable VX2 tumor can be transplanted and generated through New Zealand rabbits, and therefore is used as simulate metastatic hepatic tumor models.
Preparation of echo contrast agent
Self made echo contrast agent was made from 5% (g/L) human albumin and 40% (g/L) Dextran in a ratio of 1:3 (v/v), the mixture was then underwent electromechanical sonication (Sonication machine JY92-2D was manufactured by Ninbo Xinzhi Research Institute) for 90 s under mechanical energy of 280 W. During the sonication process, perfluropropane gas was mixed into the mixture. Microbubbles manufactured in this way were counted by a Coulter counter, which concentration was about 1.6 × 109 bubbles/L with an average size 4.3 ± 2.1 μm.
Equipments
A transducer S8 connected to HP-5500 ultrasound system was used, which fundamental wave frequency was 3 MHz and the second harmonic imaging was transmitted at frequency 3 MHz receiving at frequency 6 MHz. Power Doppler imaging was tuned to PRF = 2.2. During the whole process of experiment, the image depth, color gain and TGC should be kept constant.
Methods
Hepatic VX2 tumors were imaged with conventional B mode US, second harmonic imaging (SHI), color Doppler flow imaging (CDFI), power Doppler imaging (PDI) and harmonic PDI. Echo agent was intravenously injected at a dose of 0.01 mL/kg through ear vein, and then the venous passage was cleaned with sterilized saline. All images were recorded realtimely by magnetic optics (MO), and they were analyzed further by at least two independent experienced sonographer.
RESULTS
Features of VX2 tumor under conventional and harmonic B mode US
Totally 6 hypoechoic lesions and 3 hyperechoic lesions were found in the six carrier rabbits with a mean size about 2.1 ± 0.4 cm under conventional B mode ultrasound, no even echoic lesions were found. They were oval or round in shape with a clear outline or a hypoechoic halo at the margin of the lesions. These images didn't seem to be improved by SHI. Contrast images under conventional B mode US also showed no improvement at all, meanwhile, SHI revealed a short duration of enhancement of the hepatic arteries and tumor lesions at the early phase, and, an enhancement of the liver parenchyma and an decreased echo at the later phase. A pronounced arterial enhancement was also found at one side of the lesion, which may be considered as the nutrient artery of the tumor. (Figure 1, Figure 2, Figure 3).
Figure 1.

Image of VX2 tumor lesion under conventional B mode US. Arrow indicates the VX2 tumor lesion at the anterior part of the right lobe. It is oval and hypoechoic with a small hyperechoic scar at the center of the lesion.
Figure 2.
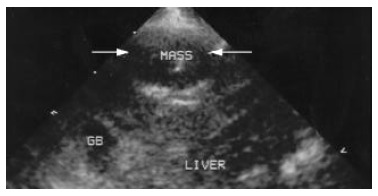
Image of VX2 tumor lesion under conventional second harmonic imaging. Arrow indicates the same VX2 tumor lesion at the anterior part of the right lobe. The echogenicity of the lesion and the around liver parenchyma seem unchanged at all.
Figure 3.
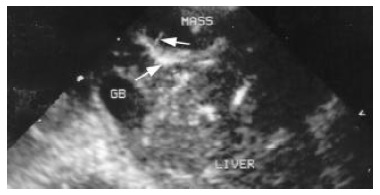
Contrast enhanced second harmonic image. It shows the enhancement of the liver arteries in the parenchyma, arrows indicate the afferent artery and its branches.
Changes of intralesional blood flow under CDFI, PDI and harmonic PDI
Color Doppler flow imaging could only reveal a spot-like or short bar-like flow signals around the lesion, and rarely reveal intralesional flow signals (Figure 4). Contrast agent may increase CDFI signals, but not so ideally as we expected. PDI could reveal a clear bar-like flow signal at one side of the lesion, and relatively more signals around and inside the lesions as compared with CDFI (Figure 5). Contrast agent can increase PDI signals quite remarkably from the instant of injection of gas bubbles, and a short duration of blooming artifacts were clearly seen, then the intralesional blood flow signals and even its branches were well revealed (Figure 6). These phenomena may better demonstrated what had been revealed by contrast SHI described above. An interesting fact was shown when tumor lesion was imaged under contrast enhanced harmonic PDI. Under this situation, intralesional and perilesional blood flow was much clearer and blooming artifacts became less (Figure 7).
Figure 4.
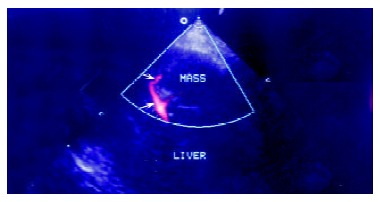
Conventional power Doppler image of the lesion. Arrows indicate the clear afferent blood flow signals of the lesion and its branches.
Figure 5.
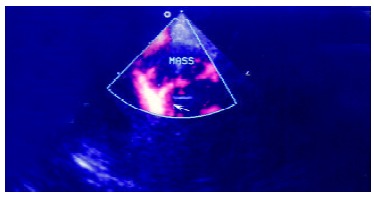
Contrast enhanced power Doppler image. It shows the enhancement of the afferent arteries in the lesion, arrow indicates especially the pronounced blooming artifacts around the artery and its branches.
Figure 6.
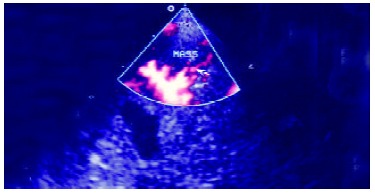
Contrast enhanced harmonic power Doppler image. It shows the enhancement of the afferent arteries in the lesion, arrow indicates the newly appeared intralesional blood signals and less blooming artefacts around the artery and its branches.
Figure 7.

Contrast enhanced harmonic power Doppler image at a later stage. It shows the enhancement of the afferent arteries in the lesion, where intralesional blood signals and rare blooming artefacts are observable around the artery and its branches.
DISCUSSION
Ultrasound is the most common used imaging modality to scan for focal liver lesions. Populations at a high risk of developing either primary or secondary hepatic tumors are usually followed up with US examination performed every 6 mo in order to detect malignant lesions at the early stage. Early detection of either primary or secondary malignancies may greatly enhance the possibilities of curative surgical operation or other ablation treatments. The major limitation of US in the detection of small tumor lesions is its sensitivity. However, the introduction of microbubble contrast agents and the development of contrast specific techniques have revealed many new prospects of US detection and characteristics of focal liver tumor lesions. Hepatic metastasis typically appears as multiple focal discrete but solid lesions, and occasionally appears diffuse infiltrate hepatic involvement. Metastasis[1-5], under conventional B mode US, has a nonspecific appearance which shows hypoechoic, hyperechoic, or mixed echogenicity. The sensitivity for total tumor detection has been reported as 57% to 92% with a specificity of 76%-96%[6-8]. However, the reported sensitivity of US is only about 41% for accurate detection in the total numbers of intrahepatic lesions[9]. Color Doppler flow imaging and power Doppler imaging are often used in the differentiation of hepatocellular carcinoma and metastatic liver lesions. Tanaka et al[1] had reported a description of vascular pattern on different hepatic malignancies using CDFI and PDI. In particular, the basket and vessels- within-the-tumor pattern were supposed to be characteristics of HCC, the spot pattern more frequently appeared in hemangioma, and the detour pattern was often seen in metastasis. PDI has already been demonstrated to be superior to conventional CDFI, particularly in depicting vessels in small tumors, and contrast enhanced PDI may even increase the sensitivity of HCC to as high as 100% in comparison with the conventional techniques[10-13]. While, metastatic liver lesions were usually considered as hypovascular. Choi et al[8,21] had reported that most of the metastatic lesions were absent of flow signals under both CDFI and PDI. Another report revealed that in a series of 64 metastatic liver lesions 67% appeared avascular, and only 32% of these cases appeared intralesional flow signals in comparison to 76% of HCC[14-18].
In our study, conventional B mode US and CDFI results are in accord with other researches. However, contrast enhanced second harmonic imaging and harmonic PDI in this study demonstrated that vascularity of metastasis might overlap with that of HCC. Although metastatic lesion may be considered as avascular under conventional ultrasonography, the introduction of new techniques such as SHI and pulse inversion harmonic imaging and the use of echo contrast agent may greatly increase the sensitivity in cheking of perilesional and intralesional vasculature. Even the nutrient artery of the metastatic lesion could be clearly seen under these new techniques. Therefore, we should reconsider the differentiating criteria of HCC and metastasis, especially when lesions were all less than 3 cm. The major problems in the use of power Doppler were that the detected velocities were too slow in the tumor, there were too many blooming artifacts associated with microbubble injection, the duration of enhancement was short and the were motion artifacts resulted from respiration[19-26]. Second harmonic imaging (SHI) US technique involves transmitting at frequency f and receiving at frequency 2f, and contrast enhanced echoes could be therefore obtained at second harmonic frequency because of the non-linear motion of the gas bubbles when destroyed by high acoustic pressures[27-29]. Thus, harmonic power Doppler US used in accord with contrast agent might provide vascular information in detail, and at the same time decreasing the artifacts associated with the contrast agent[30-36]. Our findings confirmed that contrast enhanced harmonic PDI could reveal increased color sensitivity of vessels either inside the tumor lesion or in the surrounding liver parenchyma with less blooming artifacts. It was suggested that contrast enhanced harmonic PDI was convinced to be much more superior to conventional CDFI, PDI and contrast enhanced SHI.
Footnotes
Supported by the National Science Foundation of China, No. 30070227
Edited By Xu XQ
References
- 1.Numata K, Tanaka K, Mitsui K, Morimoto M, Inoue S, Yonezawa H. Flow characteristics of hepatic tumors at color Doppler sonography: correlation with arteriographic findings. AJR Am J Roentgenol. 1993;160:515–521. doi: 10.2214/ajr.160.3.8381573. [DOI] [PubMed] [Google Scholar]
- 2.Choi BI, Kim TK, Han JK, Kim AY, Seong CK, Park SJ. Vascularity of hepatocellular carcinoma: Assessment with contrast-enhanced second-harmonic versus conventional power Doppler US. Radiology. 2000;214:381–386. doi: 10.1148/radiology.214.2.r00fe01381. [DOI] [PubMed] [Google Scholar]
- 3.Carter R, Hemingway D, Cooke TG, Pickard R, Poon FW, McKillop JA, McArdle CS. A prospective study of six methods for detection of hepatic colorectal metastases. Ann R Coll Surg Engl. 1996;78:27–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Machi J, Isomoto H, Yamashita Y, Kurohiji T, Shirouzu K, Kakegawa T. Intraoperative ultrasonography in screening for liver metastases from colorectal cancer: comparative accuracy with traditional procedures. Surgery. 1987;101:678–684. [PubMed] [Google Scholar]
- 5.Lundstedt C, Ekberg H, Hederström E, Stridbeck H, Torfason B, Transberg KG. Radiologic diagnosis of liver metastases in colo-rectal carcinoma. Prospective evaluation of the accuracy of angiography, ultrasonography, computed tomography and computed tomographic angiography. Acta Radiol. 1987;28:431–438. [PubMed] [Google Scholar]
- 6.Park SH, Kim TK, Lee KH, Kim AY, Choi JI, Han JK, Choi BI. Quantitative comparison of tumor vascularity of hepatocellular carcinoma after intravenous contrast agent: conventional versus harmonic power Doppler US. Abdom Imaging. 2001;26:178–183. doi: 10.1007/s002610000129. [DOI] [PubMed] [Google Scholar]
- 7.Kim AY, Choi BI, Kim TK, Han JK, Yun EJ, Lee KY, Han MC. Hepatocellular carcinoma: power Doppler US with a contrast agent--preliminary results. Radiology. 1998;209:135–140. doi: 10.1148/radiology.209.1.9769824. [DOI] [PubMed] [Google Scholar]
- 8.Choi BI, Kim TK, Han JK, Chung JW, Park JH, Han MC. Power versus conventional color Doppler sonography: comparison in the depiction of vasculature in liver tumors. Radiology. 1996;200:55–58. doi: 10.1148/radiology.200.1.8657945. [DOI] [PubMed] [Google Scholar]
- 9.Kubota K, Hisa N, Fujiwara Y, Fukumoto M, Yoshida D, Yoshida S. Evaluation of the intratumoral vasculature of hepatocellular carcinoma by power doppler sonography: Advantages and disadvantages versus conventional color doppler sonography. Abdom Imaging. 2000;25:172–178. doi: 10.1007/s002619910038. [DOI] [PubMed] [Google Scholar]
- 10.Gaiani S, Volpe L, Piscaglia F, Bolondi L. Vascularity of liver tumours and recent advances in doppler ultrasound. J Hepatol. 2001;34:474–482. doi: 10.1016/s0168-8278(01)00021-6. [DOI] [PubMed] [Google Scholar]
- 11.Leen E, McArdle CS. Ultrasound contrast agents in liver imaging. Clin Radiol. 1996;51 Suppl 1:35–39. [PubMed] [Google Scholar]
- 12.Ramnarine KV, Kyriakopoulou K, Gordon P, McDicken NW, McArdle CS, Leen E. Improved characterisation of focal liver tumours: dynamic power Doppler imaging using NC100100 echo-enhancer. Eur J Ultrasound. 2000;11:95–104. doi: 10.1016/s0929-8266(00)00074-4. [DOI] [PubMed] [Google Scholar]
- 13.Solbiati L, Tonolini M, Cova L, Goldberg SN. The role of contrast-enhanced ultrasound in the detection of focal liver leasions. Eur Radiol. 2001;11 Suppl 3:E15–E26. doi: 10.1007/pl00014125. [DOI] [PubMed] [Google Scholar]
- 14.Leen E. The role of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Eur Radiol. 2001;11 Suppl 3:E27–E34. doi: 10.1007/pl00014128. [DOI] [PubMed] [Google Scholar]
- 15.Blomley M, Albrecht T, Cosgrove D, Jayaram V, Butler-Barnes J, Eckersley R. Stimulated acoustic emission in liver parenchyma with Levovist. Lancet. 1998;351:568. doi: 10.1016/S0140-6736(05)78554-8. [DOI] [PubMed] [Google Scholar]
- 16.Blomley MJ, Albrecht T, Cosgrove DO, Jayaram V, Eckersley RJ, Patel N, Taylor-Robinson S, Bauer A, Schlief R. Liver vascular transit time analyzed with dynamic hepatic venography with bolus injections of an US contrast agent: Early experience in seven patients with metastases. Radiology. 1998;209:862–866. doi: 10.1148/radiology.209.3.9844688. [DOI] [PubMed] [Google Scholar]
- 17.Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio A, Summaria V, Maresca G, Pezzoli C, Llull JB. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200–206. doi: 10.1016/s0720-048x(01)00457-0. [DOI] [PubMed] [Google Scholar]
- 18.Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Maekawa K. Hepatocellular carcinoma: depiction of tumor parenchymal flow with intermittent harmonic power Doppler US during the early arterial phase in dual-display mode. Radiology. 2001;220:349–356. doi: 10.1148/radiology.220.2.r01au07349. [DOI] [PubMed] [Google Scholar]
- 19.Nisenbaum HL, Rowling SE. Ultrasound of focal hepatic lesions. Semin Roentgenol. 1995;30:324–346. doi: 10.1016/s0037-198x(05)80021-5. [DOI] [PubMed] [Google Scholar]
- 20.Zardi EM, Uwechie V, Picardi A, Costantino S. Liver focal lesions and hepatocellular carcinoma in cirrhotic patients: from screening to diagnosis. Clin Ter. 2001;152:185–188. [PubMed] [Google Scholar]
- 21.Choi BI, Kim TK, Han JK, Kim AY, Seong CK, Park SJ. Vascularity of hepatocellular carcinoma: Assessment with contrast-enhanced second-harmonic versus conventional power Doppler US. Radiology. 2000;214:381–386. doi: 10.1148/radiology.214.2.r00fe01381. [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R, Pinto F, Armillotta N, Bartolozzi C. Assessment of tumor vascularity in hepatocellular carcinoma: comparison of power Doppler US and color Doppler US. Radiology. 1996;201:353–358. doi: 10.1148/radiology.201.2.8888222. [DOI] [PubMed] [Google Scholar]
- 23.Hosoki T, Mitomo M, Chor S, Miyahara N, Ohtani M, Morimoto K. Visualization of tumor vessels in hepatocellular carcinoma. Power Doppler compared with color Doppler and angiography. Acta Radiol. 1997;38:422–427. doi: 10.1080/02841859709172094. [DOI] [PubMed] [Google Scholar]
- 24.Koito K, Namieno T, Morita K. Differential diagnosis of small hepatocellular carcinoma and adenomatous hyperplasia with power Doppler sonography. AJR Am J Roentgenol. 1998;170:157–161. doi: 10.2214/ajr.170.1.9423624. [DOI] [PubMed] [Google Scholar]
- 25.Lagalla R, Caruso G, Finazzo M. Monitoring treatment response with color and power Doppler. Eur J Radiol. 1998;27 Suppl 2:S149–S156. doi: 10.1016/s0720-048x(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 26.Kim AY, Choi BI, Kim TK, Han JK, Yun EJ, Lee KY, Han MC. Hepatocellular carcinoma: power Doppler US with a contrast agent--preliminary results. Radiology. 1998;209:135–140. doi: 10.1148/radiology.209.1.9769824. [DOI] [PubMed] [Google Scholar]
- 27.Ohishi H, Hirai T, Yamada R, Hirohashi S, Uchida H, Hashimoto H, Jibiki T, Takeuchi Y. Three-dimensional power Doppler sonography of tumor vascularity. J Ultrasound Med. 1998;17:619–622. doi: 10.7863/jum.1998.17.10.619. [DOI] [PubMed] [Google Scholar]
- 28.Hirai T, Ohishi H, Yamada R, Yoshimura H, Hirohashi S, Uchida H, Hashimoto H, Jibiki T, Takeuchi Y. Three-dimensional power Doppler sonography of tumor vascularity. Radiat Med. 1998;16:353–357. [PubMed] [Google Scholar]
- 29.Imamura M, Shiratori Y, Shiina S, Sato S, Obi S, Okudaira T, Teratani T, Kato N, Akahane M, Ohtomo K, et al. Power Doppler sonography for hepatocellular carcinoma: factors affecting the power Doppler signals of the tumors. Liver. 1998;18:427–433. doi: 10.1111/j.1600-0676.1998.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki T, Itani T, Nakase H, Mimura J, Komori H, Sugimoto K. Power Doppler imaging of hepatic tumours: differential diagnosis between hepatocellular carcinoma and metastatic adenocarcinoma. J Gastroenterol Hepatol. 1998;13:1152–1160. doi: 10.1111/j.1440-1746.1998.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 31.Hosten N, Puls R, Lemke AJ, Steger W, Zendel W, Zwicker C, Felix R. Contrast-enhanced power Doppler sonography: improved detection of characteristic flow patterns in focal liver lesions. J Clin Ultrasound. 1999;27:107–115. doi: 10.1002/(sici)1097-0096(199903/04)27:3<107::aid-jcu2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 32.Chen RC, Chen WT, Tu HY, Cheng NY, Wang CK, Liao LY, Wang CS, Chen PH. Assessment of vascularity in hepatic tumors: comparison of power Doppler sonography and intraarterial CO (2)-enhanced sonography. AJR Am J Roentgenol. 2002;178:67–73. doi: 10.2214/ajr.178.1.1780067. [DOI] [PubMed] [Google Scholar]
- 33.Cioni D, Lencioni R, Rossi S, Garbagnati F, Donati F, Crocetti L, Bartolozzi C. Radiofrequency thermal ablation of hepatocellular carcinoma: using contrast-enhanced harmonic power doppler sonography to assess treatment outcome. AJR Am J Roentgenol. 2001;177:783–788. doi: 10.2214/ajr.177.4.1770783. [DOI] [PubMed] [Google Scholar]
- 34.Meloni MF, Goldberg SN, Livraghi T, Calliada F, Ricci P, Rossi M, Pallavicini D, Campani R. Hepatocellular carcinoma treated with radiofrequency ablation: comparison of pulse inversion contrast-enhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. AJR Am J Roentgenol. 2001;177:375–380. doi: 10.2214/ajr.177.2.1770375. [DOI] [PubMed] [Google Scholar]
- 35.Cedrone A, Pompili M, Sallustio G, Lorenzelli GP, Gasbarrini G, Rapaccini GL. Comparison between color power Doppler ultrasound with echo-enhancer and spiral computed tomography in the evaluation of hepatocellular carcinoma vascularization before and after ablation procedures. Am J Gastroenterol. 2001;96:1854–1859. doi: 10.1111/j.1572-0241.2001.03883.x. [DOI] [PubMed] [Google Scholar]
- 36.Gaiani S, Casali A, Serra C, Piscaglia F, Gramantieri L, Volpe L, Siringo S, Bolondi L. Assessment of vascular patterns of small liver mass lesions: value and limitation of the different Doppler ultrasound modalities. Am J Gastroenterol. 2000;95:3537–3546. doi: 10.1111/j.1572-0241.2000.03372.x. [DOI] [PubMed] [Google Scholar]


