Abstract
AIM: To investigate the serum level and expression of insulin growth factor II (IGF-II) in liver tissues of rats with early experimental hepatocellular carcinomas (HCC) and its significance in early diagnosis.
METHODS: Early experimental hepatocellular carcinomas were induced by diethylnitrosamine (DENA) in 180 male SD rats. Another 20 male SD rats served as control. The IGF-II serum level was measured by ELISA. Immunohistochemistry and electron microscopic immunohistochemistry were used to observe the expression of IGF-II in normal and tumor liver tissues and its ultrastructural location in malignant hepatocytes. The expressions of IGF-II in human hepatoma cell lines HepG2, SMMC7721 and human embryonic liver cell line L-02 were measured by immunocytochemistry. IGF-II mRNA level was studied by in situ hybridization.
RESULTS: IGF-II was expressed in the cytoplasm of both sinusoidal cells in paracancerous cirrhotic liver tissue and malignant hepatocytes in early experimental HCC tissues. Gold particles were seen on the rough endoplasmic reticulum and the mitochondrion in malignant hepatocytes. IGF-II was expressed in the human hepatoma cell lines. The mRNA level of IGF-II was higher in rat liver tumor tissue than in normal rat liver tissue. The serum IGF-II level of the early experimental HCC group was 34.67 ± 10.53 ng·mL-1 and that of the control group was 11.75 ± 5.84 ng·mL-1. The rank sum test was used for statistical analysis. There was a significant difference between the two groups (P < 0.01).
CONCLUSION: During the induction of early experimental HCC by DENA, IGF-II may promote hepatocytic proliferation via a paracrine mechanism in the pre-cancerous stage. When hepatocytes are transformed into malignant cells, they may secrete IGF-II and promote malignant cell proliferation by an autocrine mechanism. IGF-II may be a possible biological marker in the early diagnosis of HCC.
INTRODUCTION
IGF-II is a 67-amino-acid polypeptide growth factor which is mainly produced by liver cells and is crucial in normal fetal growth[1-2]. The main researches about the relationship between IGF-II and HCC have been focused on: (1) the mechanism of IGF-II in the development of HCC[3-6], (2) to decrease the expression of IGF-II searching the treatment mothods for HCC[7-9], and (3) its prognostic significance[10]. Although IGF-II is believed to be implicated in normal and neoplastic liver growth, the mechanism of IGF-II in early hepatocellular carcinomas development and its significance in the early diagnosis of such cancer are unclear. So we used ELISA, immunohistochemistry, electron microscopic immunocytochemistry and in situ hybridization to search for possible answers to the questions.
MATERIALS AND METHODS
Animal model and tissue preparation
180 male SD rats were given DENA (diethylnitrosamine), once every week for 17 wk in a dose of 70 mg/kg, to induce early experimental HCC. In the control group, 20 male SD rats were maintained on a standard laboratory diet and tap water. From the 4th week, 2 random experimental rats were sacrificed at the end of every week for pathological study of their livers. The remainder were killed at the 17th week for study. Sera were collected and stored at -70 °C. Liver tissues were fixed in 40 mL·L-1 paraformaldehyde or 40 mL·L-1 paraformaldehyde containing 5 mL·L-1glutaraldehyde for light and electron microscopic studies.
Cell lines culture
Human hepatoma cell lines HepG2 and SMMC7721, and human embryonic liver cell line L-02 (Wuhan University Cell Center) in PRMI 1640 (GIBCO), with 100 mL·L-1 fetal calf serum (GIBCO), and 37 °C, 50 mL·L-1 CO2.
ELISA detection for the serum level of IGF-II
100 μL of goat anti-IGF-II polyclonal antibody (Santa Cruz Co.) (25 ug/mL in 0.1 mol/L NaHCO3) was put into each well of a 96-well ELISA plate, and incubated for 36 h at 4 °C. Nonspecific protein binding was blocked by 100 ul, 10 mL·L-1 bovine serum albumin. Then 100 μL of sera of rat and recombinant human IGF-II (Pepro Tech Co.) were separately added to the wells, 1 h at 37 °C. The 100 μL of Rabbit anti-IGF-II polyclonal antibody (Santa Cruz Co.) (1:200 dilution) was added to the wells, 1 h at 37 °C. HRP-conjugated goat anti-rabbit immunoglobulin (Southern Biotech Co.) (1:2000 dilution), 100 ul, 1 h at 37 °C. Binding was developed with ortho-phenylene diamine. Absorbance was read at 490 nm. During the performance, washing the plate was according to the ELISA routine method.
Immunohistochemical detection for IGF-II expression in early experimental HCC tissues
Immunohistochemical detection was made using rabbit anti-IGF-II polyclonal antibody (Santa Cruz Co.) by SABC technique. The experiment was performed following the manufacturer's recommendation of the SABC kit (Beijing Zhongshan Bioech Co.).
Immunocytochemical detection for IGF-II expression in human cell lines
Human hepatoma cell lines HepG2 and SMMC7721 and human embryonic cell line L-02 were cultivated to let them climb on coverslips, which then fixed in acetone at -20 °C for 10 min. The antibody, SABC kit and experimental method were the same as those for the immunohistochemical detection.
Electron microscopic immunocytochemistry for IGF-II ultrastructural location in malignant hepatocytes
Early experimental HCC tissues were cut into 500 nm ultrathin sections, and the sections were etched in 10% hydrogen peroxide solution for 10 min at room temperature. After the grids were drained, the sections were placed into 40 mL·mL-1 BSA solution, and then were treated overnight at 4 °C with rabbit anti-IGF-II polyclonal antibody (1:50 dilution). The sections were incubated with colloidal gold (5 nM) conjugated goat anti-rabbit IgG (Wuhan Best Co.) (1:40 dilution), 37 °C,10 min. The sections were examined with a transmission electron microscopy after counter staining. The solution for diluting antibody replacing the rabbit anti-IGF-II polyclonal antibody for those sections served as the negative control.
In situ hybridization detection for IGF-II mRNA level in early experimental HCC tissues
IGF-II in situ hybridization kit was purchased from the Wuhan Best Co. The experiment was conducted according to the manual of the kit.
Statistical analysis
SAS software and Rank Sum Test were used for statistical analysis.
RESULTS
Microscopic observation and histopathological examination
In the DENA group, the liver surfaces were covered with scattered small white nodules in the 4th week. In the 17th week, the diameter of the largest nodule was 8 mm. These rats were diagnosed as having early experimental hepatocellular carcinomas. The rat livers in the control group showed no pathological changes.
IGF-II serum level of early experimental HCC
The mean ± standard deviation of IGF-II serum level was 34.67 ± 10.53 ng·mL-1 in the DENA group and 11.75 ± 5.84 ng·mL-1 in the control group. There was significant difference between the two groups (P < 0.01).
Expression of IGF-II in early experimental HCC tissues
Strong immunostaining of IGF-II was seen in the cytoplasm of sinusoidal cell in paracancerous cirrhotic tissues (Figure 1) and the malignant hepatocytes in HCC tissues. Only a few hepatocytes in paracancerous tissues had weakly positive expression. In the control group, however, the liver tissues showed no positive results.
Figure 1.
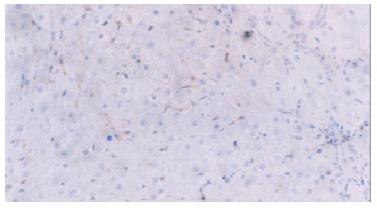
IGF-II expression in the cytoplasm of sinusoidal cells in the paracancerous cirrhotic tissues. Immunohistochemical staining, × 200.
Expression of IGF-II in human cell lines
Human hepatoma cell lines HepG2 and SMMC7721 and human embryonic liver cell line L-02 all expressed IGF-II. The positive immunolocalization of the three cell lines was in the cytoplasm. SMMC7721 cell line showed the most strongly positive immunostaining (Figure 2).
Figure 2.
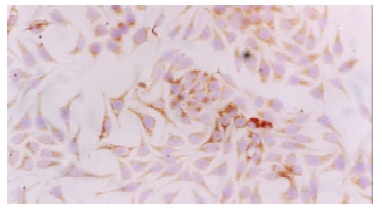
IGF-II expression in the cytoplasm of SMMC7721 cell line. Immunocytochemical staining, × 200.
Ultrastructural localization of IGF-II in malignant hepatocytes of early experimental HCC tissues
Electron microscopic immunocytochemistry confirmed the light microscopic findings. Gold particles were seen in the rough endoplasmic reticulum, mitochondrion and the paranuclear region of malignant hepatocytes. A few gold particles were found in the nuclei of malignant hepatocytes (Figure 3). The negative control sections did not show positive results.
Figure 3.
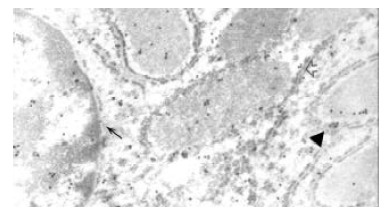
Colloidal gold particles in rough endoplasmic reticulum (↑), mitochondrion (▲), nucleus and paranuclear region (←). Electron microscopic immunocytochemical staining for IGF-II, × 16000.
IGF-II mRNA level in early experimental HCC tissues
IGF-II mRNA was distributed in the cytoplasm of hepatocytes in the tissues of early experimental HCC, and IGF-II mRNA was overexpressed in HCC tissues (Figure 4).
Figure 4.
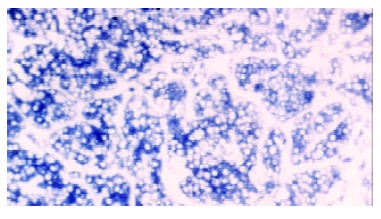
Expression of IGF-II mRNA in the cytoplasm of malignant hepatocytes. In situ hybridization, × 200.
DISCUSSION
Among the many growth factors, the insulin-like growth factor, transformation growth factor-α and hepatocyte growth factor are mostly strongly implicated in normal and neoplastic liver growth[11-17]. IGF-II exerts its effect via two specific high affinity receptors, IGF1R and M6P/IGF2R, in paracrine and autocrine manner[18-22]. IGF1R is a transmembrane glycoprotein which exists on the surface of hepatocytes with an intracellular tyrosine kinase domine. M6P/IGF2R is a single chain polypeptide associated with a G-protein signal pathway. In the present study, it has clearly been shown that: (1) the sinusoidal cells in paracancerous cirrhotic nodule tissues expressed IGF-II; (2) the malignant hepatocytes in rat early experimental HCC tissues expressed IGF-II. As we know that liver cirrhotic nodules are the pre-cancerous condition of HCC[23], so it is clearly suggested that, in the pre-cancerous condition, IGF-II mediated hepatocyte proliferation mainly via IGF1R by a paracrine mechanism. However, after the hepatocytes were transformed into malignant cells, they could secrete IGF-II themselves, suggesting that IGF-II mediated malignant hepatocytes proliferation in an autocrine manner at that time and that M6P/IGF2R might be involved. Although several reports have shown that M6P/IGF2R protein and mRNA level were reduced and that the M6P/IGF2R gene had loss of heterozygosity in HCC[24,25], Gomez-Angelats and Wada reported that no mutation was found in the M6P/IGF2R gene in the model of rat hepatocarcinogenesis and human HCC[26,27]. By electron microscopic immunocytochemistry, we found that IGF-II existed in the mitochondria of malignant hepatocytes. M6P/IGF2R is a G-protein receptor which is closely related to mitochondrion. So we favor Gomez-Angelats’ view that M6P/IGF-II was in an activated condition in the process of rat hepatocarcinogenesis.
Human hepatoma cell lines HepG2 and SMMC7721 expressed IGF-II strongly. This fact confirms the above postulate in another aspect, i.e., IGF-II could be secreted by malignant hepatocytes themselves and stimulate their proliferation via an autocrine mechanism. Human embryonic liver cell line L-02 expressed IGF-II too, suggesting that hepatoma cells may regain some embryonic development characteristics, like α-FP secretion.
α-FP is a diagnostic marker of HCC, but its significance in the early diagnosis of HCC is unclear and the positive rate is not high[28-30]. In our research, the serum IGF-II level in the rat early experimental HCC group was significantly higher than that of the control group. IGF-II is a polypeptide hormone secreted by many organs of the fetus. After birth, however, it is mainly produced by liver cells[31,32]. We may postulate that, in rat early experimental HCC, extrahepatic organs may regain the ability to secrete IGF-II and that their intrahepatic cells express IGF-II more strongly than those in normal condition[33,35]. These might be the main reasons for the increase in serum IGF-II level. Hayakawa reported that the serum level of IGF-II was significantly lower in primary hepatocellular carcinomas than in control[36], but we haven't found any report on the serum IGF-II level in early HCC. So it is possible that serum IGF-II level increases temporarily in early HCC and then decreases gradually in the process of HCC development, IGF-II maybe a biological marker in the early diagnosis of HCC.
Footnotes
Supported by National Natural Science Foundation of China, No. 30070847
Edited By Lu HM
References
- 1.Kawamoto K, Onodera H, Kan S, Kondo S, Imamura M. Possible paracrine mechanism of insulin-like growth factor-2 in the development of liver metastases from colorectal carcinoma. Cancer. 1999;85:18–25. doi: 10.1002/(sici)1097-0142(19990101)85:1<18::aid-cncr3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann W, Waha A, Koch A, Albrecht S, Gray SG, Ekström TJ, von Schweinitz D, Pietsch T. Promoter-specific transcription of the IGF2 gene: A novel rapid, non-radioactive and highly sensitive protocol for mRNA analysis. Virchows Arch. 2001;439:803–807. doi: 10.1007/s004280100509. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson T, Frisk T, Gray SG, von Schweinitz D, Pietsch T, Larsson C, Sandstedt B, Ekström TJ. Methylation changes in the human IGF2 p3 promoter parallel IGF2 expression in the primary tumor, established cell line, and xenograft of a human hepatoblastoma. Exp Cell Res. 2001;270:88–95. doi: 10.1006/excr.2001.5336. [DOI] [PubMed] [Google Scholar]
- 4.Vernucci M, Cerrato F, Besnard N, Casola S, Pedone PV, Bruni CB, Riccio A. The H19 endodermal enhancer is required for Igf2 activation and tumor formation in experimental liver carcinogenesis. Oncogene. 2000;19:6376–6385. doi: 10.1038/sj.onc.1204024. [DOI] [PubMed] [Google Scholar]
- 5.Song BC, Chung YH, Kim JA, Lee HC, Yoon HK, Sung KB, Yang SH, Yoo K, Lee YS, Suh DJ. Association between insulin-like growth factor-2 and metastases after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma: A prospective study. Cancer. 2001;91:2386–2393. [PubMed] [Google Scholar]
- 6.Bae SK, Bae MH, Ahn MY, Son MJ, Lee YM, Bae MK, Lee OH, Park BC, Kim KW. Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res. 1999;59:5989–5994. [PubMed] [Google Scholar]
- 7.Scharf JG, Dombrowski F, Ramadori G. The IGF axis and hepatocarcinogenesis. Mol Pathol. 2001;54:138–144. doi: 10.1136/mp.54.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huynh H, Chow PK, Ooi LL, Soo KC. A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation. Cell Growth Differ. 2002;13:115–122. [PubMed] [Google Scholar]
- 9.Zhang JY, Zhu W, Imai H, Kiyosawa K, Chan EK, Tan EM. De-novo humoral immune responses to cancer-associated autoantigens during transition from chronic liver disease to hepatocellular carcinoma. Clin Exp Immunol. 2001;125:3–9. doi: 10.1046/j.1365-2249.2001.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barozzi C, Ravaioli M, D'Errico A, Grazi GL, Poggioli G, Cavrini G, Mazziotti A, Grigioni WF. Relevance of biologic markers in colorectal carcinoma: A comparative study of a broad panel. Cancer. 2002;94:647–657. doi: 10.1002/cncr.10278. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N, Siegel K, Odenthal M, Becker R, Oesch F, Dienes HP, Schirmacher P, Steinberg P. The role of insulin-like growth factor II in the malignant transformation of rat liver oval cells. Hepatology. 1997;25:900–905. doi: 10.1002/hep.510250419. [DOI] [PubMed] [Google Scholar]
- 12.Chung YH, Kim JA, Song BC, Lee GC, Koh MS, Lee YS, Lee SG, Suh DJ. Expression of transforming growth factor-alpha mRNA in livers of patients with chronic viral hepatitis and hepatocellular carcinoma. Cancer. 2000;89:977–982. doi: 10.1002/1097-0142(20000901)89:5<977::aid-cncr6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Nagata K, Hirono S, Ido A, Kataoka H, Moriuchi A, Shimomura T, Hori T, Hayashi K, Koono M, Kitamura N, et al. Expression of hepatocyte growth factor activator and hepatocyte growth factor activator inhibitor type 1 in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2001;289:205–211. doi: 10.1006/bbrc.2001.5916. [DOI] [PubMed] [Google Scholar]
- 14.Tavian D, De Petro G, Benetti A, Portolani N, Giulini SM, Barlati S. u-PA and c-MET mRNA expression is co-ordinately enhanced while hepatocyte growth factor mRNA is down-regulated in human hepatocellular carcinoma. Int J Cancer. 2000;87:644–649. [PubMed] [Google Scholar]
- 15.von Schweinitz D, Faundez A, Teichmann B, Birnbaum T, Koch A, Hecker H, Glüer S, Fuchs J, Pietsch T. Hepatocyte growth-factor-scatter factor can stimulate post-operative tumor-cell proliferation in childhood hepatoblastoma. Int J Cancer. 2000;85:151–159. doi: 10.1002/(SICI)1097-0215(20000115)85:2<151::AID-IJC1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Xie Q, Liu KD, Hu MY, Zhou K. SF/HGF-c-Met autocrine and paracrine promote metastasis of hepatocellular carcinoma. World J Gastroenterol. 2001;7:816–820. doi: 10.3748/wjg.v7.i6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su Q, Liu YF, Zhang JF, Zhang SX, Li DF, Yang JJ. Expression of insulin-like growth factor II in hepatitis B, cirrhosis and hepatocellular carcinoma: its relationship with hepatitis B virus antigen expression. Hepatology. 1994;20:788–799. doi: 10.1002/hep.1840200404. [DOI] [PubMed] [Google Scholar]
- 19.Costello M, Baxter RC, Scott CD. Regulation of soluble insulin-like growth factor II/mannose 6-phosphate receptor in human serum: measurement by enzyme-linked immunosorbent assay. J Clin Endocrinol Metab. 1999;84:611–617. doi: 10.1210/jcem.84.2.5488. [DOI] [PubMed] [Google Scholar]
- 20.Fan ZR, Yang DH, Cui J, Qin HR, Huang CC. Expression of insulin like growth factor II and its receptor in hepatocellular carcinogenesis. World J Gastroenterol. 2001;7:285–288. doi: 10.3748/wjg.v7.i2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharf JG, Ramadori G, Dombrowski F. Analysis of the IGF axis in preneoplastic hepatic foci and hepatocellular neoplasms developing after low-number pancreatic islet transplantation into the livers of streptozotocin diabetic rats. Lab Invest. 2000;80:1399–1411. doi: 10.1038/labinvest.3780147. [DOI] [PubMed] [Google Scholar]
- 22.Fan Z, Yang D, Qin H. [Significance and expression of insulin-like growth factor II and its receptor in hepatocellular carcinogenesis] Zhonghua Ganzangbing Zazhi. 2000;8:84–86. [PubMed] [Google Scholar]
- 23.Mei MH, Xu J, Shi QF, Yang JH, Chen Q, Qin LL. Clinical significance of serum intercellular adhesion molecule-1 detection in patients with hepatocellular carcinoma. World J Gastroenterol. 2000;6:408–410. doi: 10.3748/wjg.v6.i3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Souza AT, Hankins GR, Washington MK, Orton TC, Jirtle RL. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nat Genet. 1995;11:447–449. doi: 10.1038/ng1295-447. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci USA. 1997;94:10351–10355. doi: 10.1073/pnas.94.19.10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gómez-Angelats M, Teeguarden JG, Dragan YP, Pitot HC. Mutational analysis of three tumor suppressor genes in two models of rat hepatocarcinogenesis. Mol Carcinog. 1999;25:157–163. doi: 10.1002/(sici)1098-2744(199907)25:3<157::aid-mc1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Wada I, Kanada H, Nomura K, Kato Y, Machinami R, Kitagawa T. Failure to detect genetic alteration of the mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R) gene in hepatocellular carcinomas in Japan. Hepatology. 1999;29:1718–1721. doi: 10.1002/hep.510290635. [DOI] [PubMed] [Google Scholar]
- 28.Zhai SH, Liu JB, Liu YM, Zhang LL, Du ZP. Expression of HBsAg, HCV-Ag and AFP in liver cirrhosis and hepatocarcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:524–527. [Google Scholar]
- 29.Zhang L, Li SN, Wang XN. CEA and AFP expression in human hepatoma cells transfected with antisense IGF-I gene. World J Gastroenterol. 1998;4:30–32. doi: 10.3748/wjg.v4.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Zhang BH, Qian GX, Chen H, Wu MC. Detection of blood AFPmRNA in nude mice bearing human HCC using nested RT-PCR and its significance. World J Gastroenterol. 1998;4:268–270. doi: 10.3748/wjg.v4.i3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998;58:348–351. [PubMed] [Google Scholar]
- 32.Lin SB, Hsieh SH, Hsu HL, Lai MY, Kan LS, Au LC. Antisense oligodeoxynucleotides of IGF-II selectively inhibit growth of human hepatoma cells overproducing IGF-II. J Biochem. 1997;122:717–722. doi: 10.1093/oxfordjournals.jbchem.a021814. [DOI] [PubMed] [Google Scholar]
- 33.Fan ZR, Yang DH, Tan HR. The expression and mutation of IGF-II gene in hepatocarcinoma and para-cancerous tissues. Shijie Huaren Xiaohua Zazhi. 2000;8:704–705. [Google Scholar]
- 34.Liang QM, Zhou ZM, Yang DH, Xu Z. The expression of IGF-II and its receptor for proliferation of hepatocytesin liver tissue. Shijie Huaren Xiaohua Zazhi. 2000;8:545. [Google Scholar]
- 35.Ling CQ, Qian Y, Zhao JA, Jin Y. The genes of C-myc, IGF-II and the expression of CyclinD1 in experimental liver carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2001;9:1452–1453. [Google Scholar]
- 36.Hayakawa T, Kondo T, Shibata T, Kitagawa M, Ono H, Sakai Y, Kato K, Katada N, Sugimoto Y, Takeichi M. Serum insulin-like growth factor II in chronic liver disease. Dig Dis Sci. 1989;34:338–342. doi: 10.1007/BF01536252. [DOI] [PubMed] [Google Scholar]


