Abstract
AIM: To investigate the inhibition effects on the production of collagen type I, III secreted by activated rat hepatic stellate cells (rHSCs) by antisense tissue inhibitors of metalloproteinase 1 (TIMP-1) recombinant plasmid through elevating interstitial collagenase activity.
METHODS: rHSCs were extracted from normal rat liver by pronase and collagenase digestion and purified by centrifugal elutriation, and were cultured on plastic dishes until they were activated to a myofibroblastic phenotype after 7-10 d. RT-Nest-PCR and gene recombinant techniques were used to construct the rat antisense TIMP-1 recombinant plasmids which can express in eucaryotic cells. The recombinant plasmid and the pcDNA3 empty plasmid were transfected in rHSCs by Effectene (QIAGEN) separately. Cells were selected after growing in DMEM containing 400 μg/mL G418 for 2-3 wk. Expression of exogenous gene was assessed by Northern blot, and expression of TIMP-1 in rHSCs was determined by Northern blot and Western blot. We tested the interstitial collagenase activity with FITC-labled type I collagen as substrate. Ultimately, we quantified the type I, III collagen by Western blot.
RESULTS: The exogenous antisense TIMP-1 recombinant plasmid could be expressed in rHSCs well, which could block the expression of TIMP-1 greatly, the ratio of TIMP-1/GAPDH was 0.67, 2.41, and 2.97 separately at mRNA level (P < 0.05); the ratio of TIMP-1/β-actin was 0.31, 0.98 and 1.32 separately at protein level (P < 0.05); It might elevate active and latent interstitial collagenase activity, the collagenase activity was 0.3049, 0.1411 and 0.1196 respectively. (P < 0.05), which led to promotion the degradation of type I, III collagen, the ratio of collagen I/β-actin was 0.63, 1.78 and 1.92 separately (P < 0.05); and the ratio of collagen III/β-actin was 0.59, 1.81 and 1.98 separately (P < 0.05).
CONCLUSION: These data shows that the antisense TIMP-1 recombinant plasmid has the inhibitory effects on the production of type I, III collagens secreted by activated rHSCs in vitro. It could be a novel method to reverse hepatic fibrosis in the future.
INTRODUCTION
The incidence of liver fibrosis and cirrhosis is still high in China[1-4], and fibrosis is the common pathological basis of chronic hepatic disease[5]. Many factors inducing liver injury and inflammation may lead to hepatic fibrosis[6,7]. At present, the common sense is that hepatic fibrosis could be reversed effectively when the right therapeutic strategy was applied[8].
Recently, studies on the role of tissue inhibitors of metalloproteinases (TIMPs) in the process of hepatic fibrosis have attracted more attentions[9-14]. Roeb et al[15] reported that there were no expression of TIMP-3, 4 mRNA in normal and fibrotic liver tissues; the expression of TIMP-1, 2 mRNA could be detected in normal liver tissue at lower level[15,16]. The expression of TIMP-1 increased greatly in liver tissues of experimental rat hepatic fibrosis models induced by CCl4 or bile compared to TIMP-2[15].
Hepatic stellate cells (HSCs) represent up to 15% of the resident cells of the liver and play a pivotal role in hepatic fibrosis[17]. In response to liver injury of any etiology, the normally quiescent HSC undergoes a progressive process of trans-differentiation into a proliferating myofibroblast-like activated HSC[17]. Through increased secretion of extracellular matrix proteins and the tissue inhibitor of metalloproteinases (TIMP)-1, activated HSCs are responsible for deposition and accumulation of the majority of the excess extracellular matrix in the fibrotic liver[18]. Furthermore, activated HSCs can contribute to the fibrogenic process through their ability to secrete and respond to a wide range of cytokines and growth factors[19].
It had been considered that the antisense technique was used to inhibit the target genes and proteins expressed in experimental rat hepatic fibrosis. The main aim of our study is to try to block the gene and protein expression of TIMP-1 in rHSCs in vitro and investigate the effects of antisense-TIMP-1 recombinant plasmid on the production of collagen type I, III of activated rHSCs, so as to find out the possible mechanism of reversing hepatic fibrosis.
MATERIALS AND METHODS
Isolation and culture of rat hepatic stellate cells
rHSCs were isolated from normal male Sprague-Dawley rats (300 ± 30 g) by sequential perfusion with Pronase and collagenase as previously described[20]. rHSCs were separated and purified from the cell suspension by single-step density gradient centrifugation with gradient buffer (Ficoll and glycan 1.053, Pharmacia). The purity of cell was assessed by light microscopic appearance and vitamin A autofluorescence at 295 nm. rHSCs were activated at day 7 after seeding on the plastic surface and became myofibroblast (MFB) phenotype. The cells were cultured in Dulbecco's modified Eagle medium (DMEM, GIBCO U.S.A) containing 4 mM L-glutamine and 10% fetal calf serum (GIBCO U.S.A). Additionally, all culture media were supplemented with penicillin (100 IU/mL) and streptomycin (100 μg/mL) respectively. They were maintained at 37°C in an atmosphere of 5% CO2.
Transfection of rat hepatic myofibroblast cells
rHSCs were transfected 8 d after seeding with pcDNA3/antisense-TIMP-1 recombinant plasmid. Briefly, total RNAs were extracted from rat liver with Trizol (Life Tech, U.S.A) reagent. According to the full-length cDNA sequence that encoded rat TIMP-1, we designed special sense and antisense primers and obtained target sequence with the RT-NEST- PCR technique, which were linked into T4 vector with T4 DNA ligase. After transformation, selection, and appraisal with restriction enzymes EcolRI and XhoI, the resulting insert was subcloned into the plasmid pcDNA3 (Invitrogen CA) and sequenced (PE377 Auto sequencer). One day before transfection, cells were dispersed with trypsin-EDTA solution and counted, then the cells were pipetted into 6 -well dishes at a density of 1 × 104 cells per well so they would attain 70% confluence the next day. Transfection was performed with the commercially available cationic liposome reagents Effectene (Qiagen, Germany), using pcDNA3/antisense-TIMP-1 recombinant plasmid (1.0 μL/well) and pcDNA3 empty plasmid, following essentially the instructions of the manufacturers. Transfecting cells were selected after growing in DMEM containing 400 μL/mL G418 for 2-3 wk. Following selection, 10 drug-resistant (neo+) clones of pcDNA3/antisense-TIMP-1 recombinant plasmid transfecting cells were picked randomly from different plates and studied after expansion; in the meantime, eight neo+ clones were picked from rMFB transfected with the pcDNA3 vector lacking the antisense sequences and eight non-transfected rMFB were studied as controls.
RNA isolation and Northern analysis for TIMP-1 and exogenous gene
The cells were rinsed with ice cold HBSS, the total RNAs were extracted from rMFBs with Trizol (Life Tech,Grand Island, NY) reagent. The concentration of RNA was determined by absorbance at 260 nm. For Northern analysis, 30 μg of total cellular RNAs were separated by electrophoresis in a 1% denaturing agarose gel, transferred to a Hybond-N membrane (Amersham,UK) and fixed by baking for 2 h at 80 °C. The probe for pcDNA3 according to the special T7 promoter sequence (the probe sequence 5'-CAGAGGGATATCACTCAGCATAAT-3' and TIMP-1 cDNA probes were labeled with [α-32P] dCTP (Amersham,UK) by random primer labeling method to detect exogenous gene and TIMP-1 expression. Blots were pre- hybridized for at least 3 h, and then hybridized for 20 h at 37 °C in a buffer containing 50% formamide, 6 × SSC, 5 × Denhardt's solution [0.1% Ficoll 400, 0.1% BSA, 0.1% polyvinyl- pyrrolidone], 5 mM EDTA, 0.5% SDS, and 100 μg/mL sheared denatured herring sperm DNA (Roche, Germany). The filters were washed once at 55 °C for 20 min in a solution containing 2 × SSC, 1 mM EDTA, and 0.1% SDS, then twice at 50 °C for 20 min in a solution containing 0.4 × SSC, 1 mM EDTA, and 0.1% SDS. Autoradiographs were exposed to indicated times to Kodak films at -70 °C for 7 d. As an internal standard (loading control) the blots were re-hybridized with a GAPDH specific cDNA.
Western blotting for TIMP-1 and type collagen I, III
Rat TIMP-1 and collagen type I, III proteins in total cell extracts of transfected and non-transfected rMFBs were subjected to Western-blot analyses. rMFB cells (1 × 106) were pelleted by centrifugation, washed twice with ice-cold PBS, and lysed on ice in RIPA buffer [30 mM HEPES, 150 mM NaCl, 1% Triton X-100, 0.1% SDS and 1% deoxycholic acid (pH7.6)], with added proteinase inhibitors, pepstatin A (1.0 μg/mL), aprotinin (3.5 μg/mL), leupeptin (10 μg/mL) and 0.2 mM PMSF. Cell lysates were centrifuged at 3000 g for 10 min at 4 °C, and BCA protein assay was performed. Protein samples (100 μg) were heated for 5 min at 100 °C and were separated on 12% SDS-PAGE and transferred to PVDF membranes (Schleicher&Schuell Germany) in Tris-glycine buffer (pH8.5) plus 20% methanol. The membranes were blocked overnight in 5% non-fat dried milk in Tris-buffer containing 0.1% Tween-20 and then washed with Tris-buffer. The blots were incubated for 2 h at room temperature with mouse TIMP-1 monoclonal IgG (Oncogene) and rabbit anti-collagen type I, III diluted 1:500 in Tris-buffer. The blots were washed and then incubated with AP-conjugated secondary antibodies (Santcruz California U.S.A) at 1:2000 dilution. The protein bands were visualized with BCIP/NBT (Wuhan, China) system. β-actin as the internal control.
Sample preparation and Enzyme assays
After transfection and selection, rMFBs were seeded into 6-well plates with 105 cells/well and incubated for 2 wk; the medium was changed twice a week. The media were harvested. Prior to assays, 225 μL of each medium was treated with 25 mL DTT (100mM) to give a final concentration of 10 mM for 30 min at 35 °C to inactivate endogenous collagenase inhibitors. Collagenase activity was determined by Type I Collagenase Activity Assay (Chemicon, Temecula CA). Briefly, the medium had been preincubated at room temperature for 30 min with APMA to obtain 1 mM final concentration to activate latent collagenase. The reaction mixture contained 50 μL (1 μg/μL) FITC-labeled type I collagen in a final volume of 100 μL, was further incubated at 37 °C for 3 h. The reaction was terminated by adding 200 μL enzyme stop reagent/extraction (0.05 M Tris-HCl pH9.5, ethanol containing NaCl, o-phenanthroline). After centrifugation at 4000 rpm for 10 min, fluorescence in the supernatant was measured by Fluorometer (Hitachi Japan) at an excitation wavelength of 490 nm and an emission wavelength of 530nm. These experiments were performed in triplicate for each tissue sample.
One unit (U) of collagenase was defined as the amount of enzyme that hydrolyzed 1 μg of collagen substrate per min. The results shown in the figures were expressed as the mean with standard deviations of collagenase activity of samples.
Statistics
All values were expressed as mean ± SD, ANOVA was used to determined the significance of differences among the three groups. A values of P < 0.05 was considered statistically significant.
RESULTS
Expression level of exogenous gene and TIMP-1 gene in rMFB
The exogenous gene expression could be detected in transfection groups (pcDNA3/antisense-TIMP-1 recombinant group and pcDNA3 empty vector group) by Northern blot, but not in non-transfected rMFB (Figure 1); the expression of TIMP-1 was much higher in pcDNA3 empty vector group and non-transfected rMFB control group than pcDNA3/antisense-TIMP-1 recombinant group at mRNA level (Figure 2), the ratio of TIMP-1/GAPDH was 0.67, 2.41, and 2.97 separately (P < 0.05); There was no significant difference (P > 0.05) between pcDNA3 empty vector group and non-transfected rMFB control group.
Figure 1.

The expression of exogenous gene in rMFB detected by Northern blot after transfection and selection. 1: pcDNA3/antisense-TIMP-1 transfecting group; 2: pcDNA3 vector control group; 3: non-transfecting rMFB control group.
Figure 2.

The expression of TIMP-1 mRNA in rMFB detected by Northern blot after transfection and selection. 1: pcDNA3/antisense-TIMP-1 transfecting group; 2: pcDNA3 vector control group; 3: non-transfecting rMFB control group.
Antisense-TIMP-1 recombinant plasmid treated rMFB have reduced the content of TIMP-1 protein and type I, III collagen
The content of TIMP-1 protein and collagen type I, III in recombinant transfection group was lower than those of the control groups (P < 0.05) as shown by the SDS-PAGE. (Figure 3, Figure 4). the ratio of TIMP-1/β-actin was 0.31, 0.98 and 1.32 separately at protein level (P < 0.05); the ratio of collagen I/β-actin was 0.63, 1.78 and 1.92 (P < 0.05); and the ratio of collagen III/β-actin was 0.59, 1.81 and 1.98 (P < 0.05). Moreover, there was no significant difference (P > 0.05) between pcDNA3 empty vector group and non- transfected control group.
Figure 3.
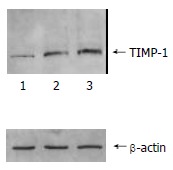
The expression of TIMP-1 protein level in rMFB detected by Western blot after transfection and selection. 1: pcDNA3/antisense-TIMP-1 transfecting group; 2: pcDNA3 vector control group; 3: non-transfecting rMFB control group.
Figure 4.

The expression of type I, III collagen in rMFB detected by Western blot after transfection and selection. 1: pcDNA3/antisense-TIMP-1 transfecting group; 2: pcDNA3 vector control group; 3: non-transfecting rMFB control group.
Collagenase activity of rMFB in response to antisense-TIMP-1 recombinant plasmid
The collagenolytic results were shown in Figure 5. Conditioned media from control groups showed low levels of collagenolytic activity;on the contrary, collagen degradation activity of the recom- binant plasmid transfection group was significantly elevated, the collagenase activity was 0.3049, 0.1411 and 0.1196 respectively. (P < 0.05). Experiments were repeated twice separately.
Figure 5.

The enzymatic activity of MMP-1 in conditioned media from rMFB detected by Type I Collagenase Activity. Assay after transfection and selection. A: pcDNA3/antisense-TIMP-1 transfecting group; B: pcDNA3 vector control group;C: non-transfecting rMFB control group.
Antisense-TIMP-1 recombinant plasmid treated rMFB have reduced the content of TIMP-1 protein and type I, III collagen
The content of TIMP-1 protein and collagen type I, III in recombinant transfection group was lower than those of the control groups (P < 0.05) as shown by the SDS-PAGE. (Figure 3, Figure 4). the ratio of TIMP-1/β-actin was 0.31, 0.98 and 1.32 separately at protein level (P < 0.05); the ratio of collagen I/β-actin was 0.63, 1.78 and 1.92 (P < 0.05); and the ratio of collagen III/β-actin was 0.59, 1.81 and 1.98 (P < 0.05). Moreover, there was no significant difference (P > 0.05) between pcDNA3 empty vector group and non- transfected control group.
Collagenase activity of rMFB in response to antisense-TIMP-1 recombinant plasmid
The collagenolytic results were shown in Figure 5. Conditioned media from control groups showed low levels of collagenolytic activity;on the contrary, collagen degradation activity of the recom- binant plasmid transfection group was significantly elevated, the collagenase activity was 0.3049, 0.1411and 0.1196 respectively. (P < 0.05). Experiments were repeated twice separately.
DISCUSSION
Liver fibrosis represents the final common pathological outcome for the majority of chronic liver insults[21]. Pathological accumulation of extracellular matrix (ECM) in cases of liver fibrosis reflects imbalance of production and degradation of matrix proteins. At present, the common sense is that it is a reversible process. Matrix metalloproteinases (MMPs) are a family of secreted zinc proteases capable of degrading collagen and other ECM components. Recent studies suggest that MMPs may participate in pathological responses involved in liver fibrosis. Especially, interstitial collagenase (MMP-1) plays a key role in degrading collagen type I, III. (the main component of ECM). MMP-1 is tightly regulated by tissue inhibitors of matrix metalloproteinases1 (TIMP-1).
Current evidence indicates that the central mediator of liver fibrosis is the hepatic stellate cell (HSC)[18]. During fibrotic injury, these retinoid-rich perisinusoidal cells proliferate and undergo a phenotypic transformation to myofibroblast (MFB)-like cells, a process termed activation[22]. Previous work has demonstrated that activated HSC can express TIMP-1, leading to the hypothesis that matrix degradation is inhibited during progressive fibrosis[23]. This hypothesis is supported by findings that overexpression of TIMP-1 enhances experimental fibrosis[24] and that spontaneous recovery from liver fibrosis is associated with a diminution of TIMP-1 expression and an increase in collagenase activity with consequent matrix degradation[25]. Nucleic acid complemented with DNA/RNA of genome is called antisense nucleic acid, including antisense DNA, antisense RNA, and ribozyme[26] According to these theories, we designed a special antisense sequence which may complement with the mRNA of TIMP-1, constructing the rat pcDNA3/antisense-TIMP-1 recombinant plasmid which can express in eucaryotic cells. After transfection and selection, we observed that the exogenous antisense-TIMP-1 recombinant plasmid could be expressed in rMFB well by Northern blot analysis. In order to evaluate the intervening or blocking effects on the expression of TIMP-1, Northern and Western blots were used. The results showed that antisense-TIMP-1 recombinant plasmid could block the expression of TIMP-1 greatly in rMFB compared to that of the control groups (P < 0.05); though the expression of TIMP-1 could not be blocked thoroughly, there maybe existed other factors which regulated the expression of TIMP-1.
We have chosen TIMP-1 as the target gene so as to elevate latent and active interstitial collagenase (MMP-1) activity in rMFB. In our study, we found that the enzymatic activity of MMP-1 in the pcDNA3/antisense-TIMP-1 recombinant transfecting group was 2-3 times higher than that in the control groups. However, the enzymatic activity was lower in the control groups (P > 0.05). These results were proven by FITC-labeled type I collagenase Activity Assay.
In healthy human liver, the collagen type I, III account for about 80% of the total collagen of liver, while it rises up to more than 95% in fibrotic livers. The collagen type I covers about 60%-70% of the total collagen of fibrotic liver, and type III was 20%-30%[27-29]. Collagen I, III are the main target of MMP-1. MMP-1 has similar capacity of degrading collagen I, III. Therefore, collagen I, III are regarded as the important parameters to reflect the metabolism of collagen, and thus we can judge the therapeutic effect of the anti-fibrotic strategies of the liver[30-32].
The content of collagen I, III was lower in the pcDNA3/antisense-TIMP-1 recombinant transfecting group than that in control groups (P < 0.05) determined by Western blotting. These data showed that this recombinant plasmid had stronger effects on increasing the degradating capacity of collagen I, III, decreasing the deposition of ECM, and reversing probably the hepatic fibrosis.
In conclusion, our experiment results have demonstrated that the pcDNA3/antisense-TIMP-1 recombinant has anti-hepatic fibrosis effect in vitro strongly through inhibiting the TIMP-1.
Footnotes
Supported by the National Natural Scientific Foundation of China, No. 39970339
Edited by Wu XN
References
- 1.Huang ZG, Zhai WR, Zhang YE, Zhang XR. Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J Gastroenterol. 1998;4:206–209. doi: 10.3748/wjg.v4.i3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia JB, Han DW, Xu RL, Gao F, Zhao LF, Zhao YC, Yan JP, Ma XH. Effect of endotoxin on fibronectin synthesis of rat primary cultured hepatocytes. World J Gastroenterol. 1998;4:329–331. doi: 10.3748/wjg.v4.i4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397–403. doi: 10.3748/wjg.v5.i5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng ML, Wu YY, Huang KF, Luo TY, Ding YS, Lu YY, Liu RC, Wu J. Clinical study on the treatment of liver fibrosis due to hepatitis B by IFN-alpha (1) and traditional medicine preparation. World J Gastroenterol. 1999;5:267–269. doi: 10.3748/wjg.v5.i3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 6.Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836–847. doi: 10.1016/s0168-8278(98)80269-9. [DOI] [PubMed] [Google Scholar]
- 7.Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2–13. doi: 10.1111/j.1600-0676.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DA. Signal transduction during liver regeneration. J Gastroenterol Hepatol. 1998;13 Suppl:S93–S95. doi: 10.1111/jgh.1998.13.s1.93. [DOI] [PubMed] [Google Scholar]
- 9.Torres L, García-Trevijano ER, Rodríguez JA, Carretero MV, Bustos M, Fernández E, Eguinoa E, Mato JM, Avila MA. Induction of TIMP-1 expression in rat hepatic stellate cells and hepatocytes: A new role for homocysteine in liver fibrosis. Biochim Biophys Acta. 1999;1455:12–22. doi: 10.1016/s0925-4439(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 10.George DK, Ramm GA, Walker NI, Powell LW, Crawford DH. Elevated serum type IV collagen: A sensitive indicator of the presence of cirrhosis in haemochromatosis. J Hepatol. 1999;31:47–52. doi: 10.1016/s0168-8278(99)80162-7. [DOI] [PubMed] [Google Scholar]
- 11.Murawaki Y, Ikuta Y, Idobe Y, Kawasaki H. Serum matrix metalloproteinase-1 in patients with chronic viral hepatitis. J Gastroenterol Hepatol. 1999;14:138–145. doi: 10.1046/j.1440-1746.1999.01821.x. [DOI] [PubMed] [Google Scholar]
- 12.Arthur MJ, Iredale JP, Mann DA. Tissue inhibitors of metalloproteinases: role in liver fibrosis and alcoholic liver disease. Alcohol Clin Exp Res. 1999;23:940–943. doi: 10.1111/j.1530-0277.1999.tb04208.x. [DOI] [PubMed] [Google Scholar]
- 13.Sakaida I, Uchida K, Hironaka K, Okita K. Prolyl 4-hydroxylase inhibitor (HOE 077) prevents TIMP-1 gene expression in rat liver fibrosis. J Gastroenterol. 1999;34:376–377. doi: 10.1007/s005350050277. [DOI] [PubMed] [Google Scholar]
- 14.Murawaki Y, Ikuta Y, Kawasaki H. Clinical usefulness of serum tissue inhibitor of metalloproteinases (TIMP)-2 assay in patients with chronic liver disease in comparison with serum TIMP-1. Clin Chim Acta. 1999;281:109–120. doi: 10.1016/s0009-8981(98)00215-0. [DOI] [PubMed] [Google Scholar]
- 15.Roeb E, Purucker E, Breuer B, Nguyen H, Heinrich PC, Rose-John S, Matern S. TIMP expression in toxic and cholestatic liver injury in rat. J Hepatol. 1997;27:535–544. doi: 10.1016/s0168-8278(97)80359-5. [DOI] [PubMed] [Google Scholar]
- 16.Geisler S, Lichtinghagen R, Böker KH, Veh RW. Differential distribution of five members of the matrix metalloproteinase family and one inhibitor (TIMP-1) in human liver and skin. Cell Tissue Res. 1997;289:173–183. doi: 10.1007/s004410050863. [DOI] [PubMed] [Google Scholar]
- 17.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 18.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 19.Arthur MJ, Mann DA, Iredale JP. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J Gastroenterol Hepatol. 1998;13 Suppl:S33–S38. [PubMed] [Google Scholar]
- 20.Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–184. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- 21.Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond) 1997;92:103–112. doi: 10.1042/cs0920103. [DOI] [PubMed] [Google Scholar]
- 22.Bachem MG, Meyer D, Melchior R, Sell KM, Gressner AM. Activation of rat liver perisinusoidal lipocytes by transforming growth factors derived from myofibroblastlike cells. A potential mechanism of self perpetuation in liver fibrogenesis. J Clin Invest. 1992;89:19–27. doi: 10.1172/JCI115561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–831. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- 24.Yoshiji H, Kuriyama S, Miyamoto Y, Thorgeirsson UP, Gomez DE, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, et al. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000;32:1248–1254. doi: 10.1053/jhep.2000.20521. [DOI] [PubMed] [Google Scholar]
- 25.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol. 2001;7:363–369. doi: 10.3748/wjg.v7.i3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis H, Le Moine A, Quertinmont E, Peny MO, Geerts A, Goldman M, Le Moine O, Devière J. Repeated concanavalin A challenge in mice induces an interleukin 10-producing phenotype and liver fibrosis. Hepatology. 2000;31:381–390. doi: 10.1002/hep.510310218. [DOI] [PubMed] [Google Scholar]
- 28.Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 29.Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149–159. doi: 10.1002/hep.510310123. [DOI] [PubMed] [Google Scholar]
- 30.Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: A review. Semin Liver Dis. 1990;10:56–65. doi: 10.1055/s-2008-1040457. [DOI] [PubMed] [Google Scholar]
- 31.Arthur MJ. Collagenases and liver fibrosis. J Hepatol. 1995;22:43–48. [PubMed] [Google Scholar]
- 32.Arthur MJ. Degradation of matrix proteins in liver fibrosis. Pathol Res Pract. 1994;190:825–833. doi: 10.1016/S0344-0338(11)80985-4. [DOI] [PubMed] [Google Scholar]


