Abstract
AIM: To study the effects of palmatine, a known inhibitor on delayed rectifier potassium current and L-type calcium current (ICa,L) in guinea pig ventricular myocytes, on the potassium and calcium currents in isolated rat hepatocytes.
METHODS: Tight-seal whole-cell patch-clamp techniques were performed to investigate the effects of palmatine on the delayed outward potassium currents (IK), inward rectifier potassium current (IK1) and Ca2+ release-activated Ca2+ current (ICRAC) in enzymatically isolated rat hepatocytes.
RESULTS: Palmatine 0.3-100 μM reduced IK in a concentration-dependent manner with EC50 of 41.62 ± 10.11 μM and nH, 0.48 ± 0.07 (n = 8). The effect of the drug was poorly reversible after washout. When the bath solution was changed to tetraethylammonium (TEA) 8 mM, IK was inhibited. Palmatine 10 μM and 100 μM shifted the I-V curves of IK downward, and the block of IK was voltage-independent. Palmatine 0.3-100 μM also inhibited ICRAC in a concentration-dependent manner. The fitting parameters were as follows: EC50 = 51.19 ± 15.18 mM, and nH = 0.46 ± 0.07 (n = 8). The peak value of ICRAC in the I-V relationship was decreased by palmatine 10 μM and 100 μM. But the reverse potential of ICRAC occurred at Voltage = 0 mV in all cells. Palmatine 0.3-100 μM failed to have any significant effect on either inward or outward components of IK1 at any membrane potential examined.
CONCLUSION: The inhibitory effects on IK and ICRAC could be one of the mechanisms that palmatine exerts protective effect on hepatocytes.
INTRODUCTION
There are many natural drugs for liver diseases currently used in popular medicine. For example, quaternary protoberberine alkaloids from Flissitigma and Goniothalamus have been used in popular medicine for hepatomegaly and hepatosplenomegaly[1,2]. The uses of alkaloids from Berberis aristata for liver injury induced by chemical carcinogenesis and alkaloids from Enantica for disorders of bilirubin have also been reported[3,4].
Palmatine, the protoberberine class of isoquinoline alkaloids, has been found in plants of various families, and mainly presents in the rhizomes of Fibrarurea Tinctoria Lour. These medicinal plants have been used as folk medicine in treatment of jaundice, dysentery, hypertension, inflammation and liver-related diseases[5,6]. The previous studies have shown that palmatine could block the delayed rectifier potassium current and had inhibition effect on L-type calcium current (ICa,L) in guinea pig ventricular myocytes[7-11]. Pauli et al[12] reported a protoberberine alkaloids mixture from Enantia chlorantha, called Hepasor, containing palmatine, columbamine and jatrorrhizine prevented liver from chemically induced traumatization and also promoted the healing process in the hepatic injury models selected. Hepasor improved the blood flow and mitotic activity in thioacetamide-traumatized rat livers. However, the hepatoprotective mechanism of palmatine still remains unknown. And there are no data available to the relationship between ion currents in hepatocytes and the hepatoprotective effect of palmatine.
In the present study, we investigated the effects of palmatine on whole-cell currents recorded from isolated rat hepatocytes to explore its mechanisms against liver injury. We tried to develop not only an effective hepatoprotective agent but also a promising leading compound against liver injury while maintaining a low side-effect profile.
MATERIALS AND METHODS
Cell preparation
The rat hepatocytes were enzymatically isolated from Sprague Dawley (SD) rats of either sex (150 to 200 g) by slightly modified procedures described previously[13-18]. Briefly, adult animals were anesthetized with an intraperitoneal injection of pentobarbital sodium (30 mg/kg) in strict accordance to the guidelines established by the Institutional Animal Care and Use Committee, which follow all applicable state and federal laws. The portal vein and the inferior vena cava were cannulated. The liver was initially perfused at a flow rate of 25 mL·min-1 with a constant-flow system with modified oxygenated Ca2+, Mg2+-free Hanks’ solution containing (in mM): NaCl 137, KCl 5.4, NaH2PO4 0.5, Na2HPO4 0.58, NaHCO3 4.16 and Glucose 5.5 (pH7.3) for several minutes, followed by perfusion with a Ca2+, Mg2+-free Hanks'solution containing collagenase (0.3 g·L-1; type I) for 10 min. The solutions were gassed with 100% O2 and warmed to 37 °C. After these perfusions, the liver was excised and then minced in Ca2+, Mg2+-free Hanks’ solution at 0 °C. The cells were filtered through a 200 μm nylon mesh, and washed three times by centrifugation at 50 g for 2 min. The cell pellets were resuspended in Kraft-bruhe (KB) solution containing (in mM): L-glutamic acid 70, KCl 130, taurine 15, KH2PO4 10, MgCl2 0.5, Glucose 11, 4- (2-hydroxyethyl)-1-piperazine-N'-2-ethanesulfonic acid (HEPES) 10 and ethylene glycol-bis (β-aminoethyl ether)-N, N, N', N'-tetraacetic acid (EGTA) 0.5 (pH7.4) that yielded approximately 85% to 95% viable hepatocytes. A small aliquot of the medium containing single cell was transferred into a 1 mL chamber mounted on the stage of an inverted microscope (XD-1012B, Nanjing, China). The spherical, smooth cells were used for the whole-cell voltage-clamp studies. All experiments were performed at room temperature (20 to 22 °C).
Voltage-clamp recording
A programmable vertical puller (pp-83, Narishige, Japan) was used to pull the electrodes. The resistance of the capillary glass electrodes (GG-17, Nanjing, China) used was 2 to 4 ΩW when filled with internal solution. A patch-clamp amplifier (PC-II, Wuhan, China) was used to record whole-cell currents with four-pole Bessel filter set at 1 kHz, digitized at 5 kHz. The protocols for voltage clamp and data analysis were established with routines using software (pClamp 6.0, Wuhan, China) and data were stored on computer for subsequent analysis. Drug actions were measured only after steady-state-conditions were reached, which were judged by the amplitudes and time courses of currents remaining constant with further perfusion of drug.
Drugs and solutions
Palmatine hydrochloride was obtained from Zhonglian Pharmaceutical Company of China as base powders, dissolved in distilled water and made a stock solution at 0.1 M. Palmatine was added to bath solution for extracellular application. All drugs were from Sigma Chemical Co unless otherwise indicated.
With studies of IK, the bath solution was a modified Tyrode's solution contained (in mM): NaCl 144, KCl 4.0, CaCl2 1.8, MgCl2 0.53, Na2HPO4 0.33, HEPES 5 and Glucose 5.5 (pH7.3). The patch pipette solution contained (in mM): KCl 130, K2ATP 5.0, creatine phosphate 5.0 and HEPES 5.0 (pH7.4).
For experiments on IK1, both the bath solution and the pipette solution contained (in mM): KCl 7, MgCl2 2, EGTA 1, K-glutamate 130 and HEPES 10 (pH7.4).
For ICRAC recording, the bath solution was (in mM): NaCl 140, KCl 2.8, CaCl2 10, MgCl2 0.5, Glucose 11 and HEPES 10 (pH7.4). The pipette solution used (in mM): K-glutamate 145, NaCl 8, MgCl2 1, MgATP 0.5, EGTA 10 and HEPES 10 (pH7.2).
Statistics
All values are expressed as mean ± S.E.M and error bars were plotted as S.E.M. Student's t test was used to evaluate the statistical significance of differences between means. A value of P < 0.05 was considered to be statistically significant. Concentration-response curves were fitted by the Hill equation:
Inhibition of current (%) = 100/[1+ (EC50/C)nH]
Where EC50 is the concentration of palmatine for half-maximum block, C is the concentration of palmatine, and nH, the Hill coefficient.
RESULTS
Effects of palmatine on IK
IK was evoked in isolated rat hepatocytes by depolarizing pulse to +140 mV for 900 ms from a holding potential of -50 mV. The current at the end point of the test pulse was measured as the amplitude of IK[19].
To better the concentration of palmatine necessary for half-maximal effect, six concentrations (0.3-100 μM) were studied. The percentage block of IK was defined as (IControl-Ipalmatine)/IControl and plotted as a function of logarithm [palmatine] in Figure 1B. At +140 mV, palmatine exerted a concentration-dependent inhibition of the current, which was poorly reversible after washout (Table 1). The data points are fitted according to the Hill equation with an EC50 for palmatine on IK is 41.62 ± 10.11 μM and nH, 0.48 ± 0.07 (n = 8). When the bath solution was changed to tetraethylammonium (TEA) 8 mM, IK was inhibited.
Figure 1.
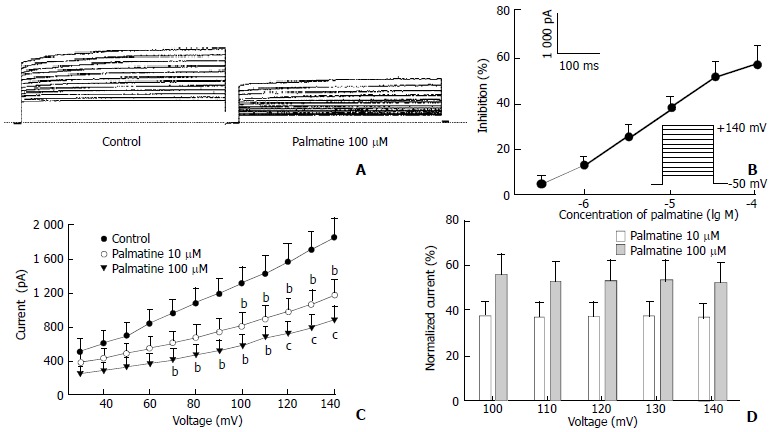
Effects of palmatine on IK. (A) Family of IK recorded with changes in the absent or present of palmatine 100 μM. Dotted line indicates zero current level; (B) Dose-response curve for the effects of palmatine on IK. The data are mean values from n = 8 cells; (C) I-V relationship of IK under control (●) and palmatine 10 μM (▼), 100 μM (▼). The voltage steps used to elicit IK are shown in the inset of panel (B). bP < 0.05, cP < 0.01 versus control (n = 8); (D) Dependence of palmatine effects on test potential. The values for the mean percentage reductions in IK induced by palmatine 10 μM (□) and 100 μM (■) are plotted against the corresponding test potential. No significant voltage-dependence was observed for the blocks induced by palmatine.
Table 1.
Effects of palmatine on IK at a test potential of +140 mV and ICRAC at -100 mV
| Concentration (μM) | Inhibition of IK (%) | Inhibition of ICRAC (%) |
| 0.3 | 3.36 ± 1.96 | 3.81 ± 0.40 |
| 1 | 11.04 ± 3.03 | 10.19 ± 2.03 |
| 3 | 23.97 ± 4.62 | 21.42 ± 3.22 |
| 10 | 36.75 ± 5.12 | 37.39 ± 3.95 |
| 30 | 49.58 ± 7.98 | 47.50 ± 4.38 |
| 100 | 55.40 ± 8.97 | 52.10 ± 5.98 |
Figure 1C shows the effects of palmatine 10 μM and 100 μM on the steady-state I-V relationship for IK generated by applying 12 depolarizing pulses from +30 mV to +140 mV for 900 ms with a 10 mV increment from a holding potential of -50 mV. In the presence of palmatine 100 μM, the amplitude of IK was significantly reduced from +70 mV through +140 mV (n = 8, P < 0.05 or P < 0.01 versus control). The currents were inhibited in a voltage-independent manner at the potentials tested. For example, at +100 mV, IK was reduced by palmatine 100 μM from 1319.52 ± 192.60 to 575.37 ± 133.29 pA (56.40% reduction), and at +140 mV, current was reduced from 1861.42 ± 215.76 to 879.37 ± 172.30 pA (52.76% reduction). The relative reductions of IK were illustrated in Figure 1D. Currents after addition of 10 μM and 100 μM palmatine were normalized to currents under control conditions at a given voltage, and it can clearly be seen that the currents were inhibited to the same degree at all potentials tested.
Effects of palmatine on IK1
Hyperpolarizing and depolarizing potentials over a range from -200 mV to +175 mV were applied from a holding level of 0 mV[20]. The absolute value at the end of test pulse was measured as the amplitude of IK1. Palmatine 0.3-100 μM failed to have any significant effect on either inward or outward components of IK1 at any membrane potential examined.
Effects of palmatine on ICRAC
When the holding potential was 0 mV, and the cells were depolarized to -100 mV for 200 ms at a frequency of 0.2 Hz, the ICRAC was evoked[21]. As shown in Figure 2, ICRAC also was blocked by palmatine in a concentration-dependent fashion, and the current was less sensitive to palmatine than IK with an EC50 of 51.19 ± 15.18 μM and nH = 0.46 ± 0.07 (n = 8). Table 1 also showed the effects of palmatine on ICRAC at a test potential of -100 mV. Figure 2C showed the effects of palmatine on the steady-stated I-V relationships generated by applying a series depolarizing pulses from a holding potential of 0 mV to different membrane potentials (-100 mV to +80 mV) with a 20 mV increment. The peak value of ICRAC in the I-V relationship was decreased by palmatine 10 μM and 100 μM (n = 8, P < 0.05 or P < 0.01 versus control). But the reverse potential of ICRAC occurred at voltage = 0 mV in all cells.
Figure 2.
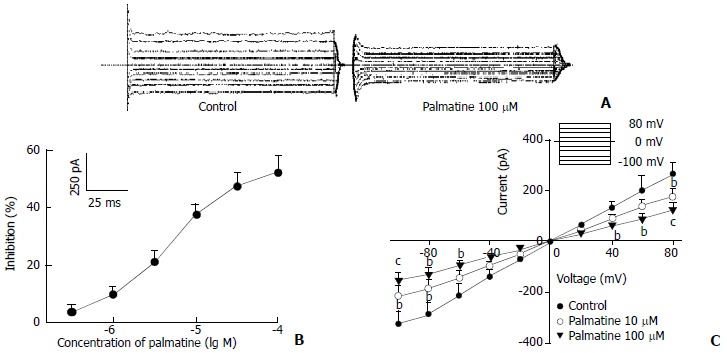
Effects of palmatine on ICRAC. (A) Family of ICRAC recorded with changes in the absent or present of palmatine 100 μM. Dotted line indicates zero current level; (B) Dose-response curve for effects of palmatine on ICRAC. The data are mean values from n = 8 cells; (C) I-V relationship of ICRAC under control (●) and palmatine 10 μM (○), 100 μM (▼). The voltage steps used to elicit IK are shown in the inset of panel (B). bP < 0.05, cP < 0.01 versus control (n = 8).
DISCUSSION
In this study we have, for the first time, characterized the effects of palmatine on the hepatocyte IK, IK1 and ICRAC by patch-clamp techniques and demonstrated that palmatine effectively inhibited IK and ICRAC in isolated rat hepatocytes.
Membrane potential is important in regulating metabolic processes in the liver, including gluconeogenesis, amino acid transport, and the rate of uptake of bile salts[22,23]. Changes in K+ permeability can affect the transmembrane potential. Transcellular bile acid transport is integrated in the regulation of intracellular pH, K+ homeostasis and membrane potential. Hepatocellular K+-depletion can result in inhibition of bile acid secretion despite rising intracellular concentration[24-26].
During ischemia and hypoxia, hepatocellular volume and K+ conductance are increased. It was reported that the extracellular K+ increase would result in hyperpolarization and hyperexcitabillity of cells. This would lead to cell death[27-29]. Nietsch et al[30,31] demonstrated membrane potential change by modulation of K+ channel activity might be involved in the mechanism of apoptosis in human hepatoma cells. The inhibition of K+ channels could delay hepatocyte apoptosis and death.
Calcium has been demonstrated to play an important role in hepatocyte damage. Elevation of intracellular Ca2+ concentration was associated with the development of cell damage and apoptosis[32-35].
Recent developments suggest that an early disturbance in hepatocellular Ca2+ homeostasis might be involved in the hepatocellular damage induced by CCl4[36-38].
Hepatocytes as the nonexcitable cells are short of the voltage-dependent Ca2+ channels but possess plasma membrane Ca2+ channels that have a high selectivity for Ca2+, and are activated by a decrease in the concentration of Ca2+ in intracellular stores, which named ICRAC[39,40]. The gating of ICRAC is independent of membrane voltage, there is, nevertheless, a strong dependence of Ca2+ influx on the driving force exerted by the membrane potential, ie, the influx rate increases with hyperpolarization and decreases with depolarization, which is different from cardiac myocytes that Ca2+ influx increases with depolarization and decreases with hyperpolarization[41].
Palmatine inhibits ICRAC with EC50 of 51.19 μM, which is higher than the EC50 of ICa,L in cardiac myocytes[42]. The differential drug sensitivity of the two currents also provides further support for the idea that ICRAC is different from voltage-gated Ca2+ channel.
In conclusion, palmatine blocks K+ channel and decreases the extracellular K+ to regulate the metabolic processes in the liver. Palmatine also inhibits ICRAC effectively and protects hepatocytes from calcium overload via the inhibition of ICRAC. The inhibitory effects on IK and ICRAC may partly contribute to the hepatoprotective action of palmatine.
Footnotes
Edited By Zhou YP
References
- 1.Virtanen P, Lassila V, Njimi T, Mengata DE. Natural protoberberine alkaloids from Enantia chlorantha, palmatine, columbamine and jatrorrhizine for thioacetamide-traumatized rat liver. Acta Anat (Basel) 1988;131:166–170. doi: 10.1159/000146507. [DOI] [PubMed] [Google Scholar]
- 2.Virtanen P, Lassila V, Njimi T, Ekotto Mengata D. Regeneration of D-galactosamine-traumatized rat liver with natural protoberberine alkaloids from Enantia chlorantha. Acta Anat (Basel) 1988;132:159–163. doi: 10.1159/000146568. [DOI] [PubMed] [Google Scholar]
- 3.Virtanen P, Lassila V, Njimi T, Mengata DE. Effect of splenectomy on hepasor treatment in allyl-alcohol-traumatized rat liver. Acta Anat (Basel) 1989;134:301–304. doi: 10.1159/000146706. [DOI] [PubMed] [Google Scholar]
- 4.Anis KV, Rajeshkumar NV, Kuttan R. Inhibition of chemical carcinogenesis by berberine in rats and mice. J Pharm Pharmacol. 2001;53:763–768. doi: 10.1211/0022357011775901. [DOI] [PubMed] [Google Scholar]
- 5.Niu XW, Zeng T, Qu AL, Kang HG, Dai SP, Yao WX, Jiang MX. Effects of 7-bromoethoxybenzene-tetrahydropalmatine on voltage-dependent currents in guinea pig ventricular myocytes. Zhongguo Yaoli Xuebao. 1996;17:227–229. [PubMed] [Google Scholar]
- 6.Chang YL, Usami S, Hsieh MT, Jiang MJ. Effects of palmatine on isometric force and intracellular calcium levels of arterial smooth muscle. Life Sci. 1999;64:597–606. doi: 10.1016/s0024-3205(98)00602-x. [DOI] [PubMed] [Google Scholar]
- 7.Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001;19:234–244. doi: 10.1111/j.1527-3466.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 8.Xu C, Sun MZ, Li YR, Yang BF, Wang LJ, Li JM. Inhibitory effect of tetrahydropalmatine on calcium current in isolated cardiomyocyte of guinea pig. Zhongguo Yaoli Xuebao. 1996;17:329–331. [PubMed] [Google Scholar]
- 9.Li Y, Fu LY, Yao WX, Xia GJ, Jiang MX. Effects of benzyltetrahydropalmatine on potassium currents in guinea pig and rat ventricular myocytes. Acta Pharmacol Sin. 2002;23:612–616. [PubMed] [Google Scholar]
- 10.Yang BF, Zong XG, Wang G, Yao WX, Jiang MX. [The mechanism of antiarrhythmic action of 7-bromoethoxybenzene-tetrahydropalmatine] Yaoxue Xuebao. 1990;25:481–484. [PubMed] [Google Scholar]
- 11.Li BX, Yang BF, Zhou J, Xu CQ, Li YR. Inhibitory effects of berberine on IK1, IK, and HERG channels of cardiac myocytes. Acta Pharmacol Sin. 2001;22:125–131. [PubMed] [Google Scholar]
- 12.Virtanen P, Lassila V, Söderström KO. Protoberberine alkaloids from Enantia chlorantha therapy of allyl-alcohol- and D-galactosamine-traumatized rats. Pathobiology. 1993;61:51–56. doi: 10.1159/000163761. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Sun J, Sun H, Li F, Liu F, Liu S. [Bile salt induces apoptosis of hepatocytes: the mechanism of hepatic function injury during obstructive jaundice] Zhonghua Waike Zazhi. 1998;36:624–626, 117. [PubMed] [Google Scholar]
- 14.Serrar H, Musallam L, Haddad P. Comparative effects of UW and SLS solutions on concentrative proline uptake in cold preserved rat hepatocytes. Therapie. 1999;54:601–606. [PubMed] [Google Scholar]
- 15.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 16.Breit S, Kolb HA, Häussinger D, Lang F. Effects of tetrabutylhydroperoxide on hepatocyte ion channels. Cell Physiol Biochem. 1999;9:133–138. doi: 10.1159/000016310. [DOI] [PubMed] [Google Scholar]
- 17.Zhang GL, Wang YH, Teng HL, Lin ZB. Effects of aminoguanidine on nitric oxide production induced by inflammatory cytokines and endotoxin in cultured rat hepatocytes. World J Gastroenterol. 2001;7:331–334. doi: 10.3748/wjg.v7.i3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YJ, Li MD, Wang YM, Ding J, Nie QH. Simplified isolation and spheroidal aggregate culture of rat hepatocytes. World J Gastroenterol. 1998;4:74–76. doi: 10.3748/wjg.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JM, Cui GY, Liu DJ, Cui H, Chang TH, Wang YP, Zhang KY. Effects of N-methyl berbamine on delayed outward potassium current in isolated rat hepatocytes. Zhongguo Yaoli Xuebao. 1998;19:24–26. [PubMed] [Google Scholar]
- 20.Henderson RM, Graf J, Boyer JL. Inward-rectifying potassium channels in rat hepatocytes. Am J Physiol. 1989;256:G1028–G1035. doi: 10.1152/ajpgi.1989.256.6.G1028. [DOI] [PubMed] [Google Scholar]
- 21.Cui GY, Li JM, Cui H, Hao LY, Liu DJ, Zhang KY. Effects of calcium channel blockers on calcium release-activated calcium currents in rat hepatocytes. Zhongguo Yaoli Xuebao. 1999;20:415–418. [PubMed] [Google Scholar]
- 22.Bernardi P, Azzone GF. Regulation of Ca2+ efflux in rat liver mitochondria. Role of membrane potential. Eur J Biochem. 1983;134:377–383. doi: 10.1111/j.1432-1033.1983.tb07578.x. [DOI] [PubMed] [Google Scholar]
- 23.Einarsson C, Ellis E, Abrahamsson A, Ericzon BG, Bjorkhem I, Axelson M. Bile acid formation in primary human hepatocytes. World J Gastroenterol. 2000;6:522–525. doi: 10.3748/wjg.v6.i4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devin A, Espié P, Guérin B, Rigoulet M. Energetics of swelling in isolated hepatocytes: A comprehensive study. Mol Cell Biochem. 1998;184:107–121. [PubMed] [Google Scholar]
- 25.vom Dahl S, Hallbrucker C, Lang F, Häussinger D. Regulation of cell volume in the perfused rat liver by hormones. Biochem J. 1991;280(Pt 1):105–109. doi: 10.1042/bj2800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiss L, Immke D, LoTurco J, Korn SJ. The interaction of Na+ and K+ in voltage-gated potassium channels. Evidence for cation binding sites of different affinity. J Gen Physiol. 1998;111:195–206. doi: 10.1085/jgp.111.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill CE, Jacques JE. Cholestatic effects of the K+ channel blockers Ba2+ and TEA occur through different pathways in the rat liver. Am J Physiol. 1999;276:G43–G48. doi: 10.1152/ajpgi.1999.276.1.G43. [DOI] [PubMed] [Google Scholar]
- 28.Hill CE, Briggs MM, Liu J, Magtanong L. Cloning, expression, and localization of a rat hepatocyte inwardly rectifying potassium channel. Am J Physiol Gastrointest Liver Physiol. 2002;282:G233–G240. doi: 10.1152/ajpgi.00256.2001. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Jin NG, Piao L, Hong MY, Jin ZY, Li Y, Xu WX. Hyposmotic membrane stretch potentiated muscarinic receptor agonist-induced depolarization of membrane potential in guinea-pig gastric myocytes. World J Gastroenterol. 2002;8:724–727. doi: 10.3748/wjg.v8.i4.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nietsch HH, Roe MW, Fiekers JF, Moore AL, Lidofsky SD. Activation of potassium and chloride channels by tumor necrosis factor alpha. Role in liver cell death. J Biol Chem. 2000;275:20556–20561. doi: 10.1074/jbc.M002535200. [DOI] [PubMed] [Google Scholar]
- 31.Kim JA, Kang YS, Jung MW, Kang GH, Lee SH, Lee YS. Ca2+ influx mediates apoptosis induced by 4-aminopyridine, a K+ channel blocker, in HepG2 human hepatoblastoma cells. Pharmacology. 2000;60:74–81. doi: 10.1159/000028350. [DOI] [PubMed] [Google Scholar]
- 32.Crenesse D, Hugues M, Ferre C, Poiree JC, Benoliel J, Dolisi C, Gugenheim J. Inhibition of calcium influx during hypoxia/reoxygenation in primary cultured rat hepatocytes. Pharmacology. 1999;58:160–170. doi: 10.1159/000028278. [DOI] [PubMed] [Google Scholar]
- 33.Isozaki H, Fujii K, Nomura E, Hara H. Calcium concentration in hepatocytes during liver ischaemia-reperfusion injury and the effects of diltiazem and citrate on perfused rat liver. Eur J Gastroenterol Hepatol. 2000;12:291–297. doi: 10.1097/00042737-200012030-00006. [DOI] [PubMed] [Google Scholar]
- 34.Ueda T, Takeyama Y, Hori Y, Takase K, Goshima M, Kuroda Y. Pancreatitis-associated ascitic fluid increases intracellular Ca (2+) concentration on hepatocytes. J Surg Res. 2000;93:171–176. doi: 10.1006/jsre.2000.5959. [DOI] [PubMed] [Google Scholar]
- 35.Lafuente NG. Calcium channel blockers and hepatotoxicity. Am J Gastroenterol. 2000;95:2145. doi: 10.1111/j.1572-0241.2000.02223.x. [DOI] [PubMed] [Google Scholar]
- 36.Hemmings SJ, Pulga VB, Tran ST, Uwiera RR. Differential inhibitory effects of carbon tetrachloride on the hepatic plasma membrane, mitochondrial and endoplasmic reticular calcium transport systems: implications to hepatotoxicity. Cell Biochem Funct. 2002;20:47–59. doi: 10.1002/cbf.934. [DOI] [PubMed] [Google Scholar]
- 37.Recknagel RO. A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci. 1983;33:401–408. doi: 10.1016/0024-3205(83)90787-7. [DOI] [PubMed] [Google Scholar]
- 38.Huang ZS, Wang ZW, Liu MP, Zhong SQ, Li QM, Rong XL. Protective effects of polydatin against CCl (4)-induced injury to primarily cultured rat hepatocytes. World J Gastroenterol. 1999;5:41–44. doi: 10.3748/wjg.v5.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takanashi H, Sawanobori T, Kamisaka K, Maezawa H, Hiraoka M. Ca (2+)-activated K+ channel is present in guinea-pig but lacking in rat hepatocytes. Jpn J Physiol. 1992;42:415–430. doi: 10.2170/jjphysiol.42.415. [DOI] [PubMed] [Google Scholar]
- 40.Rychkov G, Brereton HM, Harland ML, Barritt GJ. Plasma membrane Ca2+ release-activated Ca2+ channels with a high selectivity for Ca2+ identified by patch-clamp recording in rat liver cells. Hepatology. 2001;33:938–947. doi: 10.1053/jhep.2001.23051. [DOI] [PubMed] [Google Scholar]
- 41.Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang K, Dai GZ, Li XH, Fan Q, Cheng L, Feng YB, Xia GJ, Yao WX. Blocking L-calcium current by l-tetrahydropalmatine in single ventricular myocyte of guinea pigs. Zhongguo Yaoli Xuebao. 1999;20:907–911. [PubMed] [Google Scholar]


