Abstract
AIM: To investigate how cholesterol (Ch) can affect the phenotype of bile duct fibroblasts of New Zealand rabbits.
METHODS: 16 rabbits were divided randomly into two groups: the control group and the experiment group. The rabbits in experiment group were fed with hypercholesterol diet for 8 wk. Bile duct was dissociated from rabbits and prepared for transmission electron microscopy. The purified bile duct fibroblasts were cultured and divided randomly into there groups: control group, Ch smiddle concentration group (0.6 g/L), Ch high concentration group (1.2 g/L). After incubated for 72 h, the fibroblasts were made into specimens for transmission electron microscopy. The expression of α-actin in bile duct fibroblasts was measured by means of laser scanning confocal microscopy.
RESULTS: With the transmission electron microscopy, the normal bile duct fibroblasts were shuttle-shaped, and there were abundant rough endoplasmic reticulums (RER), but few mitochondria or microfilaments in cytoplasm. This is the typical phenotype of fibroblasts. Bile duct fibroblasts of hypercholesterolemic rabbits were observed. by the transmission electron microscopy Rough endoplasmic reticulums were significantly reduced, with a lot of microfilament bundles or stress fibers appeared in cytoplasm, especially under plasma membrane. Dense bodies were scattered within these bundles. Macula densas and discontinuous sarcolemma were found under plasma membrane. It suggested that the bile duct fibroblasts of hypercholesterolemic rabbits presented the phenotype of smooth muscle cell. The cultured bile duct fibroblasts also had typical phenotype of fibroblasts. After stimulated by middle concentration cholesterol (0.6 g/L) for 72 h, there appeared lots of microfilaments in cytoplasm, but without dense body, macula densa and discontinuous sarcolemma. Observed with confocal microscopy, there were many regular bundles of microfilaments in fibroblasts treated with middle concentration ch (0.6 g/L) and the expression of α-actin was signifiantly increased. The average fluorescence value of middle concentration group was 1628 ± 189 (P < 0.01 versus control group). Microfilaments and the expression of α-actin were greatly decreased in fibroblastes of high concentration group (1.2 g/L). The average fluorescence value of high concentration group was 1427 ± 153 (P < 0.05 vs middle concentration group). There were a lower expression of α-actin and few microfilaments in bile duct fibroblasts of control group with an average fluorescence value of 1224 ± 138.
CONCLUSION: Cholesterol can make bile duct fibroblasts have the phenotypic characteristics of smooth muscle cell both in vitro and in vivo and this effect is more significant in vivo. The effect is probably associated with some other factors besides cholesterol.
INTRODUCTION
The disorder of cholesterol metabolism is an important cause of biliary diseases. Previous studies suggest that cholesterol can change the motility of cholecyst[1,2], gallbladder contraction in the patients with cholecyst and in the animals with hypercholesterolemia decreases[3-6]. Weak contraction may be a reason of cholesterol calculus. Researchers consider that cholesterol metabolism disorder has an effect on the structure and function of bile duct and sphincter of bile duct (SBD)[7-10]. We found that cholesterol liposome (CL) affected not only the configuration and quantity of cytoskeleton in rabbit SBD smooth muscle cells but also the proliferation of cells[11-15]. There are many fibroblasts in biliary system except that SBD is formed with smooth muscle cells[16-18], but it is still unknown if cholesterol has any effect on bile duct fibroblasts and on the configuration variation of bile duct. Using cultured fibroblasts of bile duct, we studied the effect of cholesterol on the transformation of fibroblasts and its mechanism. We tried to find out the effect of fibroblast in bile duct remodeling.
MATERIALS AND METHODS
Materials
New zealand immature rabbits aged 2-3months and weighed 2.0-2.5 kg were provided by the Animal Center of the Fourth Military Medical University. Trysin (Gibco), DMEM medium (Gibco), fetal calf serum (Qinghu Institute of Foetus Bovine Utilizationin in Jinhua Zhejiang), water soluble cholesterol (Sigma), cholesterol (purity for analysis, Shanghai Chemical Co), antibody against α-actin (Gene Co), antibody of vimentin and desmin (Dako Co), ABC immunohistochemical kit (VectorCo), Fluo-3/AM (Molecular Probe Co), CO2 incubater (Forma Scientific Co), IMT-2 inverted biological microscope (Olympus Co), YJ-875 ultra-clean operating boar (Suhang Experimental Animal Technology Development Co), LD4-2 centrifugal machine (Beijing Medical Centrifugal Machine Factory), JEM-2000EX transmission electron microscope (JEOL Co), and laser confocal scanning microscope (Bio-Rad MRC-1024) were commercially obstained.
Methods
Establishment of rabbit model with hypercholesterolemia Sixteen pure breed New Zealand female rabbits were randomly divided into 2 groups (n = 8). The rabbits in experiment group were fed with cholesterol at the dose of 10 g per day and 6 d per week for 8 wk, the control rabbits were regularly fed. The rabbits with serium concentration of total cholesterol > 10 mmol/L were considered to have hypercholesterolemia It was normal that the serium concentration of total cholesterol ≤ 3.0 mmol/L. Before and during experiment, venous blood was used to measure the serium concentration of total cholesterol. Before experiment the rabbits with serium concentration of total cholesterol > 3.0 mmol/L were rejected.
Transmission electron microscope (TEM) observation of the specimen of rabbit bile duct Rabbits of control and experiment group were killed by injecting 10 mL air through ear-margin vein. The bile ducts were removed quickly and milieu connective tissue of bile ducts was curetted carefully, then samples were put into 4 °C 30 mL/L glutaral for two hours, processed conventionally for TEM examination and observed by JEM-2000EX transmission electron microscope.
Culture of rabbit bile duct fibroblasts Bile ducts of the control rabbits were dissociated by the means of aseptic technique and broken by shears. The tissue was digested to become single cell suspension by trypsin (1.25 g/L). Cells were washed and resuspended with DMEM (containing 100 mL/L FCS) and incubated for 75-90 min. Then the cells were collected and transferred into culture bottles. The cells of 2-4 passages were used for experiments.
Identification of rabbit bile duct fibroblasts Three glass cover slips (18 ± 18 mm) placed into each of 6 cm diameter culture dishes, then cell suspension was added and incubated for 48 h. The slips covered with cells were washed twice by PBS (pH7.4). There slips were fixed by cold acetone for 15 (4 °C) minutes and were used for HE staining, another 3 slips were fixed for 20 min by citromint (40 g/L) for immunohistochemical ABC staining to exam vimentin and desmin expression.
Treatment of bile duct fibroblasts with cholesterol The cells of passage 2-4 were trypsinized and seeded in culture bottles or on glass cover slips in culture dishes, after overnight preincubation with cholesterol diluted with 20 g/L DMEM was added at the final concentration of 1.2 g/L (group of high concertration, HC) or 0.6 g/L (group of middle concentration, MC). The cells were exposed to cholesterol for 72 h, then prepared for the studies with TEM and lasers caning confocal microscope (LSCM) respectively, the cells cultured without cholesterol were used as the control.
TEM and LSCM observation of rabbit bile duct fibroblasts The cells grown in culture bottles and treated with 0.6 g/L of cholesterol were trypsinized, washed, fixed and then processed for TEM observation with a JEM-2000EX transmission electron microscope. The cells grown on cover slips and treated with 0.6 g/L or 1.2 g/L of cholesterol were fixed with cold acetone for 15 min (4 °C), washed three times with PBS and incubated for 30 min in normal caprine blood serum to block antibody nonspecific binding site. Rat anti rabbit α-actin antibody (1:50) diluted by BSA/NaN3/PBS was added on the slips and the cells were incubated for 48 h (4 °C). After the slips had been washed three times with PBS, FITC tagged caprine anti rat IgG (1:30) was added on the slips and the slips were incubated at room temperature for 3 h. The amortization glycerinin without fluorescence was used to seal slips. The slips were observed by LSCM. The excitation wave-length was of 488 nm.The software was provided by Bio-Rad Co. controled LSCM. The object glass for picture collection was Planneofluar 40 × object glass. 7% light filter was used. More than 200 cells in each group were scanned.
Statistical analyses
The data came into Kinetics-Imaga Scan Analysis program and were analyzed. Results were expressed as -x ± s. Student's test or an analysis of variance (ANOVA) was performed to test the statistical significance as necessary, P < 0.05 was regarded as significant.
RESULTS
Character and Identification of cultured rabbit bile duct fibroblasts
Under phase-contrast microscope, cultured rabbit bile duct fibroblasts showed shuttle-shaped or multiangular. Cytoplasm was clear and nucleus was large and ellipse. Nucleolus was obvious. Isolated bile duct fibroblasts were free of smooth muscle cell contamination as determined by positive staining with vimentin and negative staining with desmin by the means of immunocytochemical ABC staining.
TEM observation of rabbit bile duct and cultured rabbit bile duct fibroblasts
The normal bile duct fibroblasts were shuttle-shaped and there were abundant rough endoplasmic reticulums (RER), but few mitochondria and microfilament in cytoplasm. That was typical phenotype of fibroblasts (Figure 1). Rough endoplasmic reticulums (RER) was significantly reduced in the bile duct fibroblasts of hypercholesterolemic rabbits, but a lot of microfilament bundles or stress fibers appeared in cytoplasm, especially under plasma membrane. Dense bodies were scattered within these bundles. Macula densas and discontinuous sarcolemma were found under splasma membrane. It suggested that the bile duct fibroblasts of hypercholesterolemic rabbits showed the phenotype of smooth muscle cell (Figure 2).
Figure 1.
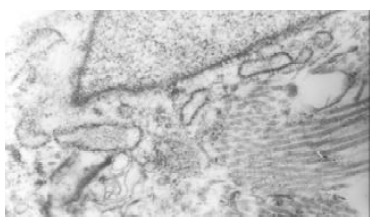
Bile duct fibroblasts of normal rabbit presented shuttle-shaped with abundant rough endoplasmic reticulums (RER), but few mitochondria and microfilament in cytoplasm.
Figure 2.
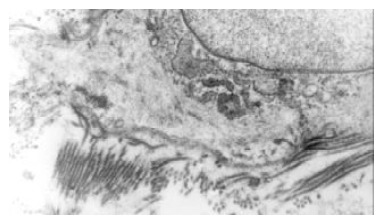
The bile duct fibroblasts of hypercholesterolemic rabbits showed the phenotype of smooth muscle cell. Rough endoplasmic reticulums (RER) was significantly reduced, but a lot of microfilament bundles or stress fibers appeared in cytoplasm. Dense bodies were scattered within these bundles. Macula densas and discontinuous sarcolemma were found under splasma membrane.
In vitro experiment the cultured bile duct fibroblasts also had typical phenotype of fibroblasts (Figure 3). After stimulated by middle concentration cholesterol (0.6 g/L) for 72 h, there appeared lots of microfilament in cytoplasm,but without dense body. macula densa and discontinuous sarcolemma (Figure 4).
Figure 3.
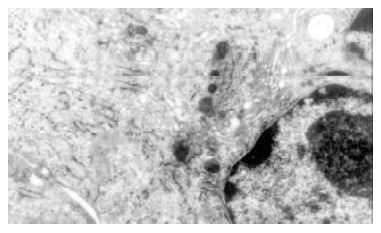
Cultured normal rabbit bile duct fibroblasts characterized typical phenotype of fibroblasts with abundant rough endoplasmic reticulums (RER) and golgi bodies, but few mitochondria and microfilament.
Figure 4.
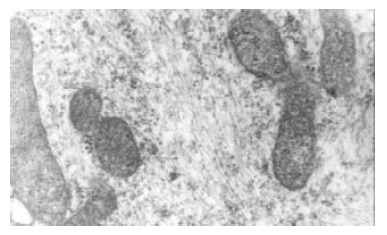
After stimulated by middle concentration cholesterol (0.6 g/L) for 72 h, the cultured bile duct fibroblasts showed some phenotype of fibroblasts. and there appeared lots of microfilament in cytoplasm, but without dense body. macula densa and discontinuous sarcolemma.
LSCM observation of cultured rabbit bile duct fibroblasts
Immunofluorescence display and LSCM were connected to measure the change of nucleic acid and protein in cells. The quantity of detected substance was expressed by average fluorescence value. There were many regular bundles of microfilament in fibroblasts treated with middle concentration cholesterol (0.6 g/L). Microfilament bundles were located along cytoplasmic extension (Figure 5). The expression of α-actin was signifiantly increased. The average fluorescence value of middle concentration group was 1628 ± 189 (P < 0.01 versus control group). The quantity of microfilaments and the expression of α-actin were greatly decreased in fibroblastes of high concentration cholesterol group (1.2 g/L). The average fluorescence value of high concentration group was 1427 ± 153 (P < 0.05 vs middle concentration group). There was a lower expression of α-actin and few microfilaments in bile duct fibroblasts of control group, the average fluorescence value was 1224 ± 138 (Figure 6).
Figure 5.
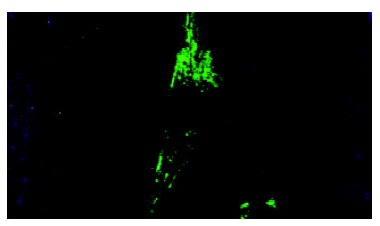
Effects of middle concentration cholesterol (0.6 g/L) on the cultured bile duct fibroblasts. There were many regular bundles of microfilament that were located along cytoplasmic extension.
Figure 6.
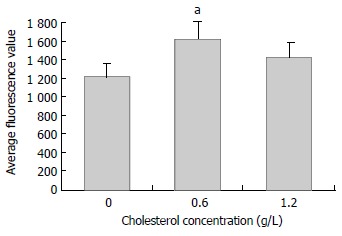
Effects of cholesterol on the α-actin expression of rabbit bile duct fibroblasts. Note aP < 0.05, versus control and high concentration group (1.2 g/L cholesterol).
DISCUSSION
Fibroblasts are derived from mesenchymal cell of embryo period. During the evolution of wound healing, fibroblasts can proliferate greatly by mitoses. At the same time fibroblasts can also synthesize and excrete collagen fibers and matrix components[19-21]. With the stimulation of trauma and other agents, some mature fibroblasts can change into naive fibroblasts and their function can be recovered. These fibroblasts take part in the tissue damage plerosis. Hypoxia can, mediated by pulmonary arterial endothelial cells (PAECs) but not directly, induce phenotype modulation of human embryonic lung fibroblasts, namely transforming to smooth muscle cell-like cells. It suggestes that the transformation of human embryonic lung fibroblasts is one of the reasons why nonmuscle lung. arteriole becomes muscle arteriole[22] With the effects of many agents, fibroblasts will proliferate, accumulate or modulate phenotype. Fibroblasts can paracrine and autocrine many cytokines (such as bFGF, KGF). The cytokines can affect fibroblasts and other cells[23]. Friedenstein A.Abdin M reported that fibroblasts can take part in heterotopic ossification under some pathologic conditions. Silent fibroblasts in the border of wood, adjusted by growth factors, can differentiate to contraction phenotype and have the special expression of α-SM actin. The cells are considered as myofibroblasts. The myofibroblasts can cause contraction of granulation tissue. It is thus clear that fibroblasts have multidifferentiation potential and take part in many kinds of physiological and pathologic responses. Fibroblasts have important effects on the occurrence and development of diseases.
Biliary system is the unique passage of bile ejection. It can modulate bile ejection and maintain normal pressure of biliary system[24-31]. The coordination of its anatomic structure and function makes it not only prevent regurgitation of duodenal fluid but also modulate and stabilize the pressure of bile duct[32-38]. So the structural remodeling of biliary system and the derangement of motor function coordination can become one of basic reasons of biliary system diseases occurrence. There are a great deal of fibroblasts in bile duct system[39]. At present, it is still unknown what are the effects of cholesterol on bile duct fibroblasts and whether cholesterol can change the configuration of bile duct[40]. So we use hypercholesterolemia rabbit model and cultured rabbit bile duct fibroblasts to observe the effects of cholesterol on fibroblasts and the mechanism. Our results show that there are a lot of microfilament bundles, dense bodies, macula densa and discontinuous sarcolemma appeared in the bile duct fibroblasts of hypercholesterolemia rabbit. Bile duct fibroblasts present the phenotypic characters of smooth muscle cell. Vitro experiment show that lots of microfilament bundles are found in cultured bile duct fibroblasts treated by middle concentration cholesterol (0.6 g/L) but dense bodies and macula densa are not found. Laser confocal microscopy displays that the expression of α-actin in cultured bile duct fibroblasts treated by middle concentration cholesterol (0.6 g/L) and many regular bundles of microfilament are located along cytoplasmic extension.
Cholesterol is the major lipid structural component of mammal plasma membrane. It has important effects on maintaining plasmalemma normal behavior and function. It has been proved that cholesterol could alter smooth muscle membrane and cell function by changing the physical state of the membrane phospholipid bilayer, and therefore affect the function of integral membrane proteins, such as Ca2+[41] and potassium channels, as well as transmembrane receptors[42]. Excessive cholesterol incorporation decreased membrane fluidity and subsequently restricted optimal function of membrane proteins, such as receptor binding of ligands, receptor coupling with G proteins and activation of enzymes[43]. Wei et al[8] found the abnormalities in ultrastructure of SO in rabbits with hypercholesterolemia. Therefore, hypercholesterolemia might be one of causes of SOD. Meanwhile, the effect of cholesterol on gallbladder contractility has been interpreted by many authors, who found that the cholesterol incorporated into membrane can impair the cellular signal transdution and contractility as well. Thus, we think that cholesterol can affect signal transduction and in the end change the gene expression. In the 1950s caveolae were discovered. Now It has been proposed that caveolae are important in signal transduction, forming a platform on which different signaling components can congregate. and caveolae have been linked to cholesterol regulation: caveolin binds to cholesterol, its production is controlled by cholesterol. Simons[44] found that caveolae are important for inhibiting certain signaling pathways that regulate cellular proliferation. Our experiment found that bile duct fibroblasts treated by middle concentration cholesterol presented the phenotypic characters of smooth muscle cell. We suppose that the mechanism may be that cholesterol can alter the biologic character of plasmalemma and affect signal transduction, which induces the gene expression. Whether the effects of cholesterol observed in this study were due to cytotoxicity, other authors reported that high concentration of cholesterol might have influence to cultured muscle cells. The similar procedures had been done by many reseachers, and the cholesterol was within the serum concentration range of hypercholesterolemia rabbit model. So, the cytotoxicity of cholesterol to cells in this study, if any, might be unconsiderable.
Epidemiological study shows that woman is the high-risk group of calculus and multiple birth procreation women are always accompanied with lipid metabolism disturbance. Du Fan confirmed that hypercholesterolemic rabbit presented the functional disturbance of bile duct before the occurrence of cholelithiasis. It suggested that cholesterol does affect the function of bile duct. Fibroblasts have the potential of multidifferentiation and they can differentiate or transform after being activated. Fibroblasts can differentiate to myofibroblasts or transform to smooth muscle cells because there are some similarities of form and constitution between the two kinds of cells and fibroblasts. Our experiment detected that cultured rabbit bile duct fibroblasts treated with cholesterol presented some characters of smooth muscle cell. In vivo experiment shows that bile duct fibroblasts of hypercholesterolemic rabbits present more characters of smooth muscle cell. Therefore we can conclude that cholesterol does activate bile duct fibroblasts and induce fibroblasts to transform into smooth muscle cell. But the change is affected by multiple factors and is a multistage procedure. Although cholesterol is an important factor, only with the help of other factors, can it complete the procedure. The certain mechanism has not been elucidated yet. We suspect that the differentiation and transformation of bile duct fibroblasts have an important effect on the configuration remodeling of biliary system, which provides a pathway to research the occurrence, development and treatment of biliary system diseases.
Footnotes
Edited by Zhao M
References
- 1.Chen SZ, Sha JP, Chen XC, Hou CC, Fu WH, Liu W. Dysrelaxation of Sphincter of Oddi in patients with bile reflux gastritis: study on effect of nifedipine on gallbladder emptying. Shijie Huaren Xiaohua Zazhi. 1999;7:1020–1023. [Google Scholar]
- 2.Li XP, Ouyang KQ, Cai SX. The regulation of bile secretion and eduction. Shijie Huaren Xiaohua Zazhi. 2001;9:1066–1070. [Google Scholar]
- 3.Lammert F, Südfeld S, Busch N, Matern S. Cholesterol crystal binding of biliary immunoglobulin A: visualization by fluorescence light microscopy. World J Gastroenterol. 2001;7:198–202. doi: 10.3748/wjg.v7.i2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lui P, Chen DF. The separation and primary culture of canine gallbladder epithelium. Shijie Huaren Xiaohua Zazhi. 2001;9:99–100. [Google Scholar]
- 5.Zapata R, Severín C, Manríquez M, Valdivieso V. Gallbladder motility and lithogenesis in obese patients during diet-induced weight loss. Dig Dis Sci. 2000;45:421–428. doi: 10.1023/a:1005497517854. [DOI] [PubMed] [Google Scholar]
- 6.Greaves RR, O'Donnell LJ, Farthing MJ. Differential effect of prostaglandins on gallstone-free and gallstone-containing human gallbladder. Dig Dis Sci. 2000;45:2376–2381. doi: 10.1023/a:1005624016268. [DOI] [PubMed] [Google Scholar]
- 7.Wu PJ. The motility and dysfunction of bile duct system. Shijie Huaren Xiaohua Zazhi. 1999;7:603–604. [Google Scholar]
- 8.Wei JG, Wang YC, Du F, Yu HJ. Dynamic and ultrastructural study of sphincter of Oddi in early-stage cholelithiasis in rabbits with hypercholesterolemia. World J Gastroenterol. 2000;6:102–106. doi: 10.3748/wjg.v6.i1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toouli J. Biliary Dyskinesia. Curr Treat Options Gastroenterol. 2002;5:285–291. doi: 10.1007/s11938-002-0051-9. [DOI] [PubMed] [Google Scholar]
- 10.Yang CM, Mao GP, Zhang XR, Zhang YH, Jian YP. The effects of ethanol on rabbit Oddi's sphincter. Shijie Huaren Xiaohua Zazhi. 2000;8:87. [Google Scholar]
- 11.Zhang JS, Wei JG, Wu JZ, Chen JY. Culture and morphologic observation of rabbit Oddi's sphincter cells. Shijie Huaren Xiaohua Zazhi. 1999;7:316–319. [Google Scholar]
- 12.Salam OM, Nada SA, Arbid MS. The effect of ginseng on bile-pancreatic secretion in the rat. Increase in proteins and inhibition of total lipids and cholesterol secretion. Pharmacol Res. 2002;45:349–353. doi: 10.1006/phrs.2002.0961. [DOI] [PubMed] [Google Scholar]
- 13.Shen CM, Mao SJ, Huang GS, Yang PC, Chu RM. Stimulation of smooth muscle cell proliferation by ox-LDL- and acetyl LDL-induced macrophage-derived foam cells. Life Sci. 2001;70:443–452. doi: 10.1016/s0024-3205(01)01428-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang XJ, Wei JG, Wang YC, Xu JK, Wu QZ, Wu DC, Yang XX. Effect of cholesterol liposomeon on contractility of rabbit Oddi's sphincter smooth muscle cells. Shijie Huaren Xiaohua Zazhi. 2000;8:633–637. [Google Scholar]
- 15.Ishibashi Y, Murakami G, Honma T, Sato TJ, Takahashi M. Morphometric study of the sphincter of oddi (hepatopancreatic) and configuration of the submucosal portion of the sphincteric muscle mass. Clin Anat. 2000;13:159–167. doi: 10.1002/(SICI)1098-2353(2000)13:3<159::AID-CA1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Liashchenko SN. [The microsurgical anatomy of the major duodenal papilla and of the sphincter of the hepatopancreatic ampulla] Morfologiia. 1999;116:50–53. [PubMed] [Google Scholar]
- 17.Avisse C, Flament JB, Delattre JF. Ampulla of Vater. Anatomic, embryologic, and surgical aspects. Surg Clin North Am. 2000;80:201–212. doi: 10.1016/s0039-6109(05)70402-3. [DOI] [PubMed] [Google Scholar]
- 18.Avisse C, Delattre JF, Flament JB. The inguinofemoral area from a laparoscopic standpoint. History, anatomy, and surgical applications. Surg Clin North Am. 2000;80:35–48. doi: 10.1016/s0039-6109(05)70396-0. [DOI] [PubMed] [Google Scholar]
- 19.Laplante AF, Germain L, Auger FA, Moulin V. Mechanisms of wound reepithelialization: hints from a tissue-engineered reconstructed skin to long-standing questions. FASEB J. 2001;15:2377–2389. doi: 10.1096/fj.01-0250com. [DOI] [PubMed] [Google Scholar]
- 20.Yokohata K, Tanaka M. Cyclic motility of the sphincter of Oddi. J Hepatobiliary Pancreat Surg. 2000;7:178–182. doi: 10.1007/s005340050172. [DOI] [PubMed] [Google Scholar]
- 21.Aymerich RR, Prakash C, Aliperti G. Sphincter of oddi manometry: is it necessary to measure both biliary and pancreatic sphincter pressures. Gastrointest Endosc. 2000;52:183–186. doi: 10.1067/mge.2000.105985. [DOI] [PubMed] [Google Scholar]
- 22.Delattre JF, Avisse C, Marcus C, Flament JB. Functional anatomy of the gastroesophageal junction. Surg Clin North Am. 2000;80:241–260. doi: 10.1016/s0039-6109(05)70404-7. [DOI] [PubMed] [Google Scholar]
- 23.Li AJ, Liu JZ, Liu CY. Anatomical and functional study of localization of originating neurons of the parasympathetic nerve to gallbladder in rabbit brain stem. Chin J Physiol. 2002;45:19–24. [PubMed] [Google Scholar]
- 24.Fang CH, Yang JZ, Kang HG. A PCR study on Hp DNA of bile, mucosa and stone in gallstones patients and its relation to stone nuclear formation. Shijie Huaren Xiaohua Zazhi. 1999;7:233–235. [Google Scholar]
- 25.Gu SW, Luo KX, Zhang L, Wu AH, He HT, Weng JY. Relationship between ductule prol iferation and liver fibrosis of chronic liver disease. Shijie Huaren Xiaohua Zazhi. 1999;7:845–847. [Google Scholar]
- 26.Zhou LS, Shi JS, Wang ZR, Wang L. Tumor necrosis factor a in gallbladder and gallstone. Shijie Huaren Xiaohua Zazhi. 2000;8:426–428. [Google Scholar]
- 27.Fang CH, Yang J. A study on DNA of aerobic and anaerobic bacteria in bile, mucosa and stone in gallstone patients. Shijie Huaren Xiaohua Zazhi. 2000;8:66–68. [Google Scholar]
- 28.Li XP, Mao XZ. Effect of estrogen, cholic acid loading and bile draining on hepatobiliary functions in rats. Shijie Huaren Xiaohua Zazhi. 2000;8:1009–1012. [Google Scholar]
- 29.He XS, Huang JF, Liang LJ, Lu MD, Cao XH. Surgical resection for hepatoportal bile duct cancer. World J Gastroenterol. 1999;5:128–131. doi: 10.3748/wjg.v5.i2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang ZQ, Zhou NX, Wang DD, Lu JG, Chen MY. Changing trends of surgical treatment of hilar bile duct cancer: clinical and experimental perspectives. World J Gastroenterol. 2000;6:777–782. doi: 10.3748/wjg.v6.i6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MY, Huang ZQ, Chen LZ, Gao YB, Peng RY, Wang DW. Detection of hepatitis C virus NS5 protein and genome in Chinese carcinoma of the extrahepatic bile duct and its significance. World J Gastroenterol. 2000;6:800–804. doi: 10.3748/wjg.v6.i6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang HM, Wu J, Li JY, Zhou JL, He LJ, Xu XF. Optic properties of bile liquid crystals in human body. World J Gastroenterol. 2000;6:248–251. doi: 10.3748/wjg.v6.i2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig A, Toouli J. Sphincter of Oddi dysfunction: is there a role for medical therapy. Curr Gastroenterol Rep. 2002;4:172–176. doi: 10.1007/s11894-002-0056-5. [DOI] [PubMed] [Google Scholar]
- 34.Nomura T, Shirai Y, Sandoh N, Nagakura S, Hatakeyama K. Cholangiographic criteria for anomalous union of the pancreatic and biliary ducts. Gastrointest Endosc. 2002;55:204–208. doi: 10.1067/mge.2002.121341. [DOI] [PubMed] [Google Scholar]
- 35.Baroni A, Gorga F, Baldi A, Perfetto B, Paoletti I, Russo A, Lembo L, Rossano F. Histopathological features and modulation of type IV collagen expression induced by Pseudomonas aeruginosa lipopolysaccharide (LPS) and porins on mouse skin. Histol Histopathol. 2001;16:685–692. doi: 10.14670/HH-16.685. [DOI] [PubMed] [Google Scholar]
- 36.Laukkarinen J, Koobi P, Kalliovalkama J, Sand J, Mattila J, Turjanmaa V, Porsti I, Nordback I. Bile flow to the duodenum is reduced in hypothyreosis and enhanced in hyperthyreosis. Neurogastroenterol Motil. 2002;14:183–188. doi: 10.1046/j.1365-2982.2002.00316.x. [DOI] [PubMed] [Google Scholar]
- 37.Moulin V, Auger FA, Garrel D, Germain L. Role of wound healing myofibroblasts on re-epithelialization of human skin. Burns. 2000;26:3–12. doi: 10.1016/s0305-4179(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 38.Kupfahl C, Pink D, Friedrich K, Zurbrügg HR, Neuss M, Warnecke C, Fielitz J, Graf K, Fleck E, Regitz-Zagrosek V. Angiotensin II directly increases transforming growth factor beta1 and osteopontin and indirectly affects collagen mRNA expression in the human heart. Cardiovasc Res. 2000;46:463–475. doi: 10.1016/s0008-6363(00)00037-7. [DOI] [PubMed] [Google Scholar]
- 39.Schnee JM, Hsueh WA. Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc Res. 2000;46:264–268. doi: 10.1016/s0008-6363(00)00044-4. [DOI] [PubMed] [Google Scholar]
- 40.Chen BY, Wei JG, Wang YC, Yang XX, Qian JX, Yu J, Chen ZN, Xu J, Wu DC. Effects of cholesterol on the proliferation of cultured rabbit bile duct fibroblasts. Shijie Huaren Xiaohua Zazhi. 2002;10:566–570. [Google Scholar]
- 41.Wang XJ, Wei JG, Wang CM, Wang YC, Wu QZ, Xu JK, Yang XX. Effect of cholesterol liposomes on calcium mobilization in muscle cells from the rabbit sphincter of Oddi. World J Gastroenterol. 2002;8:144–149. doi: 10.3748/wjg.v8.i1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao ZL, Chen Q, Biancani P, Behar J. Abnormalities of gallbladder muscle associated with acute inflammation in guinea pigs. Am J Physiol Gastrointest Liver Physiol. 2001;281:G490–G497. doi: 10.1152/ajpgi.2001.281.2.G490. [DOI] [PubMed] [Google Scholar]
- 43.Razani B, Schlegel A, Lisanti MP. Caveolin proteins in signaling, oncogenic transformation and muscular dystrophy. J Cell Sci. 2000;113(Pt 12):2103–2109. doi: 10.1242/jcs.113.12.2103. [DOI] [PubMed] [Google Scholar]
- 44.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]


