Abstract
There is limited information on the exposure to pesticides experienced by UK residents living near agricultural land. This study aimed to investigate their pesticide exposure in relation to spray events. Farmers treating crops with captan, chlormequat, chlorpyrifos or cypermethrin provided spray event information. Adults and children residing ≤100 m from sprayed fields provided first-morning void urine samples during and outwith the spray season. Selected samples (1–2 days after a spray event and at other times (background samples)) were analysed and creatinine adjusted. Generalised Linear Mixed Models were used to investigate if urinary biomarkers of these pesticides were elevated after spray events. The final data set for statistical analysis contained 1518 urine samples from 140 participants, consisting of 523 spray event and 995 background samples which were analysed for pesticide urinary biomarkers. For captan and cypermethrin, the proportion of values below the limit of detection was greater than 80%, with no difference between spray event and background samples. For chlormequat and chlorpyrifos, the geometric mean urinary biomarker concentrations following spray events were 15.4 μg/g creatinine and 2.5 μg/g creatinine, respectively, compared with 16.5 μg/g creatinine and 3.0 μg/g creatinine for background samples within the spraying season. Outwith the spraying season, concentrations for chlorpyrifos were the same as those within spraying season backgrounds, but for chlormequat, lower concentrations were observed outwith the spraying season (12.3 μg/g creatinine). Overall, we observed no evidence indicative of additional urinary pesticide biomarker excretion as a result of spray events, suggesting that sources other than local spraying are responsible for the relatively low urinary pesticide biomarkers detected in the study population.
Keywords: exposure, pesticides, residents
INTRODUCTION
The use of pesticides can give rise to public concern. The Royal Commission on Environmental Pollution (RCEP) published a report on bystander and resident exposure to pesticides and recognised that the epidemiological literature showing associations between chronic fatigue syndrome and multiple chemical sensitivity and pesticide exposure was plausible but equivocal.1 The RCEP report and the responses to it have ensured that the issue has remained in the public eye.2, 3, 4
In the UK, Government Ministers must approve all pesticides before they can be marketed or used, including those used in agriculture, horticulture, forestry, food storage and the home or garden, to ensure that they do not present health risks to the general public, consumers, people who apply them or work in or near areas that have been treated with pesticides, the environment and animals. The Chemicals Regulation Directorate of the UK Health and Safety Executive acts as the Regulator for pesticide products and authorises their sale, supply, use and storage in the UK and represents the UK in the European Union process for the registration of new active substances and for the renewal (review) of active substances already approved. The health regulatory risk assessment (RRA) underpinning the approval of pesticides involves the comparison of estimated potential human exposures with toxicological reference values levels, for example, acceptable operator exposure level and acceptable daily intake (ADI), at and below which there is considered to be high confidence that there will be no adverse health effects. The RRA is therefore generally considered to be a conservative estimate of exposure.
There are no specific studies on the exposures of people living near agricultural land to pesticides in the UK. There are a number of reported studies from elsewhere in the world which have explored residents' exposures. For example, Koureas et al.5 found no difference in urinary dialkylphosphates (generic biomarkers of organophosphate pesticides) between urban and rural residents in Thessaly, Greece, whilst pesticide sprayers had significantly increased levels. A Canadian study6 showed a significant difference in urban and rural residents for exposure to pyrethroids, whereas in Japan7 no differences were seen between rural and suburban residents. Rural residents in Nicaragua showed significantly lower acetylcholinesterase activity which were associated with aerial pesticide spraying from planes.8 Crop maps were used by Ward et al.9 to predict that increasing acreage of corn and soybean fields within 750 m of homes in Iowa, USA, was associated with significantly elevated odds of detecting agricultural herbicides inside homes compared with homes with no crops within 750 m. These studies present an unclear picture of pesticide exposure in rural residents and are difficult to translate to the UK situation.
This manuscript reports on a study (‘Biological monitoring of pesticide exposure in residents') funded by the UK Government Department of Environment, Food and Rural Affairs (DEFRA), which aimed to assess exposure to pesticides for adults and children living within 100 m from the edge of agricultural land and to investigate whether exposures were elevated following spray events.
METHODS
The study received ethical approval from the South East Scotland Research Ethics Committee 3 (study number 10/S1103/63). Our study protocol provides details of the methodology employed and is briefly summarised below.10 To obtain an estimate of the number of subjects required for the study, a range of conservative power calculations were carried out a priori for a number of pesticides identified as being most likely to be applied during the spray seasons in the target areas.10
Recruitment of Study Participants
Sample and data collection took place in three locations in the UK: East Lothian, Kent and Norfolk. Data in East Lothian and Kent were collected in 2011 and 2012, while in Norfolk data collection took place in 2012 only. East Lothian and Norfolk are major arable crop growing areas, while most of the orchards in the UK are located in Kent. Recruitment of, and liaison with, farmers and residents participating in the study were carried out by community researchers who had local knowledge of the study areas and communities.11
Farmers were identified through publicly available resources and contacted via letter explaining the aims and objectives of the study. This was then followed by telephone contact. The pesticides of interest, the crops on which they were likely to be applied were discussed during this contact as was the proximity of residential areas to these fields. If the farmer was willing to participate and considered a suitable candidate for inclusion in the study, an in-person meeting was arranged at the farm. The community researchers recruited owners/managers of farms and orchards (hereafter both referred to as farms) that reported when they were likely to spray their agricultural crops with certain specified pesticides and which had residential areas within 100 m of these fields. Informed consenting farmers were asked to provide details of their pesticide usage throughout the spray season. The spray information included information on the start and finish times of spraying, product and active ingredients used, spray method and weather conditions. In instances where farmers demonstrated that they already maintained comprehensive records of their pesticide usage, the researcher requested copies of these to be made available. Where detailed records were not already maintained, participating farmers were asked to record the relevant information using an adaptation of the spray record form recommended by the Department of Environment, Food and Rural Affairs.12
Table 1 provides details of the pesticides considered in the study and reported in this manuscript. Inclusion was restricted to those approved pesticides where analytical methods for the associated urinary biomarkers were available to the project team. No validated method was available for determining penconazole or its metabolites in urine, and so a novel method was developed during this project, details of which, as well as the penaconazole results from this study are reported elsewhere.
Table 1. Pesticides of interest.
| Pesticide | Class | Function | Relevant crops approveda for use |
|---|---|---|---|
| Captan | Phthalimide | Fungicide | Apple, pear |
| Chlormequat | Chlorocholine | Growth regulator | Cereals |
| Chlorpyrifos | Organophosphate | Insecticide | Apple, cereals, vegetables including potato |
| Cypermethrin | Pyrethroid | Insecticide | Apple, various arable crops including potato |
| Penconazoleb | Triazole | Fungicide | Apple, blackcurrent, hops |
Approval in 2011.
Results pertaining to penconazole are presented elsewhere.
Residents (adults aged 18 years and over and children in their care aged 4–12 years) living within 100 m of the edge of a field belonging to a recruited farm were approached to participate in the study. Farmers and their family members residing within 100 m of the fields were also invited to participate providing they were not directly involved in pesticide spray or crop re-entry activities and they fulfilled the other eligibility criterion.
Informed consenting participants completed a background questionnaire. This included questions concerning their age, sex, weight, typical consumption of organic food and home-grown produce, occupation/education, occupational and para-occupational exposure to pesticides and typical pesticide usage around the home or garden. The children's questionnaire was shorter and was completed by the consenting adult.
Urine Sample Collection and Accompanying Activity Questionnaire
Following due consideration of preliminary modelling (not presented), the peer-reviewed literature13 and the need to obtain samples over a sustained period of time, first-morning void samples were chosen as the most appropriate urine sample to request from participants. First-morning void urine samples and accompanying sample-related questionnaires were collected using two strategies.
Residents were asked to provide first-morning void urine samples and complete accompanying questionnaire once a week on a designated day during the spraying season and also for 3 weeks after the spraying season.
In instances when a community researcher was advised of a relevant spray event occurring on a given field, participants living within 100 m of the field were contacted and asked to collect additional first-morning void urine samples one and two days following the day of the spray event as well as complete a sample-related questionnaires on both days.
Each sample-related questionnaire completed by adult participants requested information on their time spent indoors and outdoors at home and other locations, domestic use and para-occupational exposure to pesticides, as well as their dietary consumption of home-grown produce within the previous 48-hour period. The sample-related questionnaire completed by the adult on behalf of a child participant was shorter and included questions concerning the time spent by the child in both outdoor and indoor environments.
The spraying season was considered to be between the months of March–August, coinciding with the main crop growing season, and stopping before harvest. First-morning void samples were requested and the time the urine sample collection was recorded by the participant.
Urine samples (~70 ml) were collected in polypropylene containers (Starplex Scientific, Canada) along with the completed sample-related questionnaire were collected at agreed times by the community researcher on the day of sample provision. The urine samples were stored in a cool bag before collection by the community researcher. The samples were frozen as soon as possible (<6 h after collection) and couriered to the laboratory, being stored at a temperature ranging from −15 to −20 °C before analysis. The community researchers checked the completeness of the sample-related questionnaires. Farmers' spray records were reviewed alongside the dates that participants' urine samples were provided.
Urine Sample Analysis
Urine samples were selected for analysis as follows, with a relevant spray event referring to those involving the application of pesticides including the active ingredients captan, chlormequat, chlorpyrifos and cypermethrin:
Urine samples collected within 2 days of relevant pesticide spray events taking place in fields within 100 m of the participant's residence (spray event-related samples).
For each participant providing at least one relevant pesticide spray event sample, up to three randomly selected background samples obtained within the spraying season which did not coincide with a relevant spray event (within spray season background samples).
For each participant providing at least one relevant spray event sample, up to three randomly selected background samples collected outwith the spraying season (during November–December) (outwith spray season background samples).
Urine samples collected within 2 days of a relevant spraying event were analysed only for the relevant pesticide(s) sprayed during the event. Background samples, both within and outwith the spray season, were analysed for all the relevant pesticides of interest to the study.
Samples were analysed according to established methods by the Health and Safety Laboratory (HSL), which participates in external quality assurance schemes for chlorpyrifos and cypermethrin (G-EQUAS, www.g-equas.de). The analysts were blind to whether the urine samples were related to spray events or were background samples. Detection limits were comparable to previous reported studies looking at general population levels. All analytes were quantified using multi-point matrix-matched calibration curves and quality control samples were run every five samples. Samples were analysed in duplicate and the mean value reported. Aliquots of positive samples were reanalysed throughout the project to evaluate sample stability. There was no evidence of sample degradation for any biomarker studied throughout the assessment period (see Supplementary Information).
The analytical method for chlormequat was based on that reported by Lindh et al.14 measuring chlormequat itself. The analytical method for captan was based on that reported by Berthet et al.15 measuring cis-1,2,3,6-tetrahydrophthalimide (THPI). The analytical method for chlorpyrifos was based on that reported by Sams et al.16 measuring 3,5,6-trichlorpyridinol (TCP). The analytical method for cypermethrin measured cis- and trans- 2,2-dichlorovinyl-3,3-dimethylcyclopropane-1-carboxylic acid (DCVA).17
Further analytical methodology details are provided as Supplementary Information.
Data Analysis
Urine samples with a creatinine concentration below 2 or >30 mmol/l (0.23 g/l and 3.39 g/l, respectively) were excluded from the analyses.18, 19 Next, participants for which either no spray event-related samples or no background (either within or outwith) samples were available were excluded.
The descriptive characteristics of the participants were summarised, along with summaries of their responses to the background questionnaire, in terms of percentage in each category or mean and range for continuous variables (Table 2).
Table 2. Description of the participants (background questionnaire responses).
| Descriptor | N | % |
|---|---|---|
| Adult | 118 | 84 |
| Child | 22 | 16 |
| Sex | ||
| Male | 54 | 39 |
| Female | 86 | 61 |
| Location | ||
| East Lothian | 29 | 21 |
| Kent | 45 | 32 |
| Norfolk | 66 | 47 |
| Employment (adults) | ||
| Full time employment | 29 | 25 |
| Part time employment | 37 | 32 |
| Full time education | 1 | 1 |
| Part time education | 39 | 34 |
| Retired | 8 | 7 |
| Non-paid employment | 1 | 1 |
| Missing or no answer | 29 | 25 |
| Education (children) | ||
| Secondary | 2 | 9 |
| Primary | 18 | 82 |
| Full time nursery | 0 | 0 |
| Part time nursery | 2 | 9 |
| Smoke | 5 | 4 |
| Own a pet | 85 | 61 |
| Job involves travelling around the local area | 22 | 16 |
| Use pesticides at work | 6 | 4 |
| Family use pesticides at work | 7 | 5 |
| Use pesticides in the home at least once a year | 112 | 80 |
| Mean | Range | |
| Age (adult) | 55 | (18–83) |
| Age (child) | 8 | (4–12) |
Urinary measurements were corrected for creatinine by dividing the urinary biomarker concentration by the creatinine concentration and are reported as μg biomarker per g creatinine. A very high proportion of samples were below the analytical limit of detection (LOD) for captan (89%) (LOD=0.1 μg/l) and cypermethrin (93%) (LOD=1.0 μg/l) and for these two pesticides only the proportion of detects, 95th percentile and the maximum levels are reported.
For chlormequat and chlorpyrifos a random imputation procedure was used to replace the values below the LOD (0.6 μg/l and 0.8 μg/l, respectively). The geometric mean (GM) and geometric standard deviation (GSD) of concentrations above the LOD were determined, assuming a log-normal distribution. Each of the values below LOD was then replaced by a value between 0 and the LOD which was randomly generated from this distribution. As is typical of data of this nature, concentrations were not normally distributed. Transforming by taking the natural logarithm improves the normality of the data significantly and therefore the sample results are summarised in terms of GM and GSD.20
For each individual the GM of their samples was determined separately for samples provided after a spray event and for within and outwith spray season backgrounds. The GM ratio of the spray event sample to the backgrounds was then calculated by taking the log of each individual ratio, averaging and exponentiating. Results are reported as GM and associated 95% confidence interval. A confidence interval containing the value 1 means that the ratio is not significantly different from 1, that is, spray event samples are not significantly higher or lower than backgrounds.
The data were further examined to investigate factors from either the background questionnaire (person-specific) or the sample-related questionnaires (sample-specific) that might explain any differences between the biomarker concentrations. General linear mixed models were used with the log of the biomarker level as the response. The participant was treated as a random variable, to account for repeated measures from the same individuals. Sample type was treated as a fixed effect of a priori interest, where the levels of sample type were defined as; outwith and within spray season backgrounds, samples provided the day after spraying (24 h), samples provided the second day after a spray event (48 h) or sample was provided after 2 consecutive days of spraying (24 and 48 h). In a step-wise manner variables were considered for inclusion in the model where they significantly improved the fit of the model, based on the likelihood ratio test or affected the coefficients of terms already in the model (by >10%).
Imputation was carried out using R v3.1.0,21 while all statistical analyses were carried out using Genstat v16 (ref. 22) and plots were prepared using Sigmaplot v10).23
RESULTS
A total of 13 participating farms during 2011 and 17 farms during 2012 reported sprayed events using at least one of the relevant pesticides. The breakdown by region is provided in Table 3, along with details of the number of relevant spray events after which urine samples were collected. All participating Kent farms were orchards while the farms in East Lothian and Norfolk were arable farms.
Table 3. Participating farms and relevant spray events by pesticide and geographical area.
| Area | No. farms | Pesticide | No. spray eventsa |
|---|---|---|---|
| East Lothian | 7 | Chlormequat | 57 |
| Cypermethrin | 2 | ||
| Kent | 9 | Captan | 214 |
| Chlorpyrifos | 51 | ||
| Norfolk | 4 | Chlormequat | 15 |
| Cypermethrin | 3 |
Spray event numbers derived by multiplying the number of farms applying the pesticide by the number of relevant fields on which the pesticide was applied. For example if farm A sprayed captan in six fields on four occasions, the number of spray events for this farm would be 24.
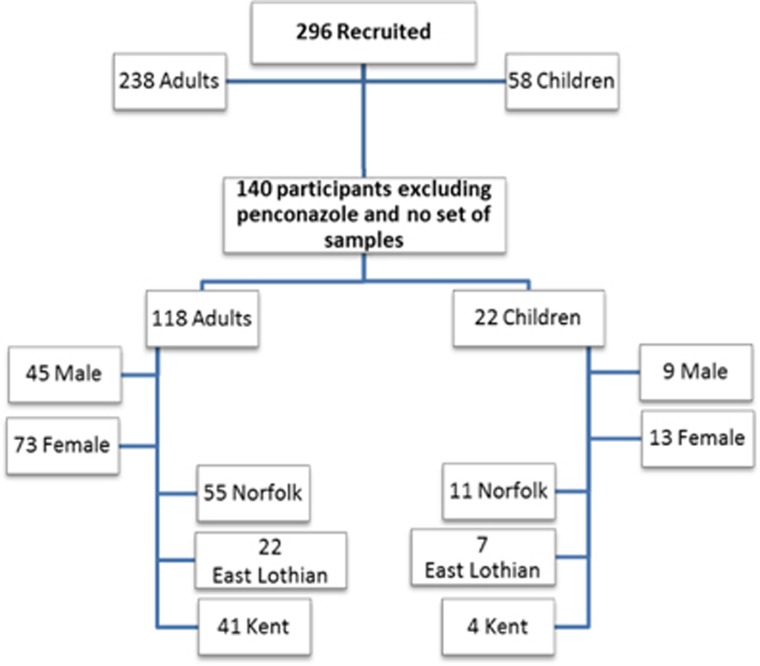
A total of 296 participants were recruited to the study (238 adults, 58 children), providing 3275 urine samples. One hundred forty-one people provided at least one sample that was related to a relevant spray event involving captan, chlormequat, chlorprifos or cypermethrin and had at least one background, resulting in 1565 samples results. Altogether, 73% of participants had the full complement of outwith spray season backgrounds and 76% had the full complement of within spray season backgrounds.
Application of the creatinine exclusion criterion resulted in 40 samples being excluded from the analysis (12 sprays and 28 backgrounds). Following the application of this exclusion criterion, one further person was removed due to no spray event-related samples remaining and as such were no longer eligible to be included in the data analysis (six backgrounds).
The final data set for statistical analysis contained 1518 sample results for 140 people, consisting of 995 background samples (440 outwith and 555 within season) (Figure 1) and 523 spray event-related samples. The spray samples included 255 captan, 63 chlorpyrifos, 46 cypermethrin and 197 chlormequat spray event results, respectively (some spray events involved multiple relevant pesticides).
Figure 1.
Participant numbers by area, sex.
Table 4 summarises the urine sample results for spray events and backgrounds (within and outwith spray season). For captan and cypermethrin, the proportion of spray event values below the LOD was 91 and 98%, respectively, whereas the proportion of background results below the LOD was 88% for captan and in excess of 90% for cypermethrin. The captan biomarker concentrations ranged from below the LOD to almost 4 μg/g creatinine. There is a slightly higher proportion of non-detects in the spray event-related samples with the largest individual result being associated with a background sample collected within the spray season. The cypermethrin biomarker concentrations follow a similar pattern, with the maximum concentration (15.4 μg/g creatinine) found to be in a background sample obtained outwith the spray season.
Table 4. Urinary biomarker concentrations (μg/g creatinine).
| Pesticide | N | N<LOD | %<LOD | Max | GM | GSD | 95th % ile |
|---|---|---|---|---|---|---|---|
| Captan | |||||||
| Outwith | 440 | 387 | 88 | 3.5 | a | a | 0.4 |
| Within | 553 | 489 | 88 | 3.9 | a | a | 0.5 |
| Spray | 255 | 232 | 91 | 1.2 | a | a | 0.2 |
| Cypermethrin | |||||||
| Outwith | 344 | 329 | 96 | 15.4 | a | a | b |
| Within | 349 | 312 | 89 | 10.8 | a | a | 5.1 |
| Spray | 46 | 45 | 98 | 7.0 | a | a | b |
| Chlormequat | |||||||
| Outwith | 440 | 17 | 4 | 281.6 | 12.3 | 3.1 | 65.6 |
| Within | 555 | 4 | 1 | 388.2 | 16.5 | 2.7 | 89.7 |
| Spray | 197 | 3 | 2 | 248.1 | 15.4 | 2.7 | 72.4 |
| Chlorpyrifos | |||||||
| Outwith | 440 | 54 | 12 | 22.7 | 3.0 | 2.2 | 9.6 |
| Within | 554 | 69 | 12 | 76.4 | 3.0 | 2.4 | 10.8 |
| Spray | 63 | 7 | 11 | 14.8 | 2.5 | 2.1 | 7.9 |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; LOD, limit of detection; Max, maximum; N, number; 95% ile, 95th percentile.
GM and GSD were not calculated due to the high proportion of values below LOD.
95th percentile not calculated as over 95% of the samples were below LOD.
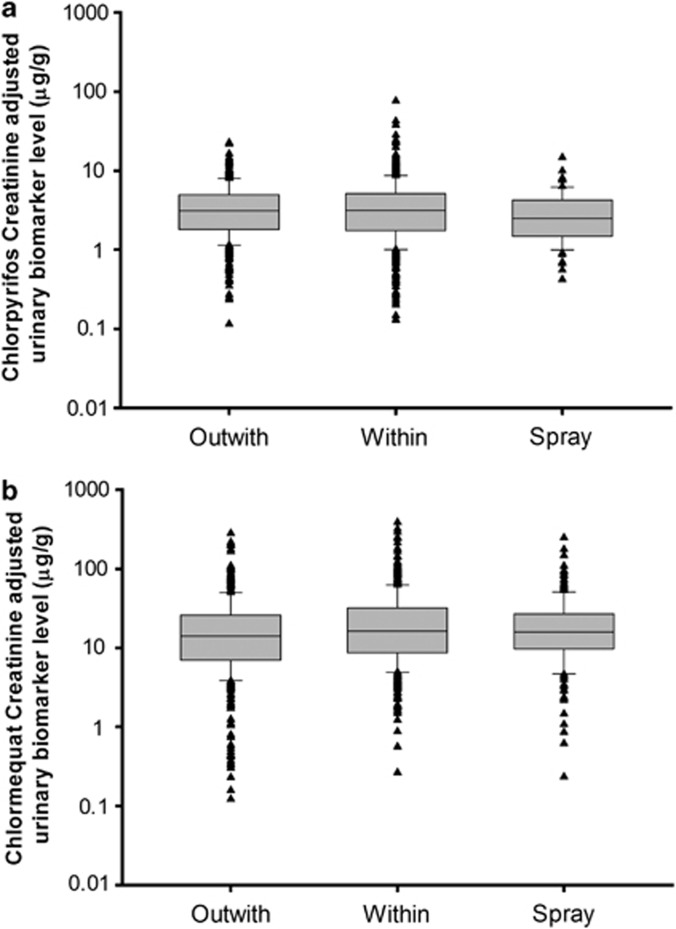
Figure 2 shows boxplots of the creatinine adjusted urinary biomarker concentrations (μg/g) for chlorpyrifos and chlormequat for the spray event-related samples as well as the within and outwith spray season background samples. Both figures show that spray event-related samples did not appear to contain elevated concentrations of urinary biomarkers compared with the background levels.
Figure 2.
Creatinine adjusted urinary biomarker levels for spray event and within and outwith spray season background samples for (a) chlorpyrifos and (b) chlormequat.
Spray event sample concentrations for chlormequat are statistically significantly higher than outwith spray season background, with the GM of the individual ratios being 1.22 (95% CI: 1.09, 1.38) but there is no significant difference between spray event-related sample concentrations and within spray season backgrounds (GM ratio 0.96 (95% CI: 0.87, 1.06)) (Table 5). Interestingly, the average spray event-related chlorpyrifos urinary biomarker concentrations are lower than both the outwith and within spray season background sample results (GM ratio: 0.80, 95% CI: 0.74, 0.87 and 1.01; 95% CI: 0.92, 1.11, respectively).
Table 5. GM ratio of creatinine corrected concentrations following spray events to the GM of backgrounds.
|
Outwith spray season |
Within spray season |
|||||
|---|---|---|---|---|---|---|
| Pesticide | GM ratio | 95% CI | GM ratio | 95% CI | ||
| Chlormequat | 1.22 | 1.09 | 1.38 | 0.96 | 0.87 | 1.06 |
| Chlorpyrifos | 0.80 | 0.74 | 0.87 | 1.01 | 0.92 | 1.11 |
Abbreviations: CI, confidence Interval; GM, geometric mean.
There were minor differences in chlormequat biomarker concentrations measured for males and females, with males having slightly higher urinary biomarker levels than females (Table 6). This difference was not significant for outwith season backgrounds and spray event-related samples but was significant for within spray season backgrounds (P=0.036). There were no statistically significant differences in the chlorpyrifos concentrations measured in samples provided by males and females (Table 6). The levels of chlormequat and chlorpyrifos were higher in samples provided by children than adults, although not significantly so; the variation in the children's sample results also tended to be greater. To ensure these patterns were not the result of variation in creatinine levels, which tend to be higher in males than females and in adults than children, the uncorrected creatinine urinary biomarker concentrations were analysed and similar patterns were observed.
Table 6. Urinary biomarker concentrations (μg/g creatinine) for males and females and for adults and children for chlormequat and chlorpyrifos.
| Pesticide |
Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|
| N | N<LOD | GM | GSD | N | N<LOD | GM | GSD | |
| Chlormequat | ||||||||
| Outwith | 262 | 11 | 11.8 | 3.2 | 178 | 6 | 13.0 | 2.9 |
| Within | 335 | 4 | 15.4 | 2.8 | 220 | 0 | 18.3 | 2.7 |
| Spray | 120 | 1 | 14.4 | 2.6 | 77 | 2 | 17.1 | 2.7 |
| Chlorpyrifos | ||||||||
| Outwith | 262 | 32 | 3.3 | 2.1 | 178 | 22 | 2.7 | 2.3 |
| Within | 334 | 42 | 3.0 | 2.4 | 220 | 27 | 2.8 | 2.4 |
| Spray | 41 | 7 | 2.5 | 2.2 | 22 | 0 | 2.5 | 1.9 |
|
Child |
Adult |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | N<LOD | GM | GSD | N | N<LOD | GM | GSD | |
| Chlormequat | ||||||||
| Outwith | 59 | 3 | 11.4 | 4.2 | 381 | 14 | 12.4 | 3.0 |
| Within | 79 | 3 | 19.3 | 3.4 | 476 | 1 | 16.1 | 2.7 |
| Spray | 40 | 0 | 17.7 | 2.8 | 157 | 3 | 14.9 | 2.6 |
| Chlorpyrifos | ||||||||
| Outwith | 59 | 4 | 3.7 | 2.2 | 381 | 50 | 2.9 | 2.2 |
| Within | 79 | 4 | 3.8 | 2.5 | 475 | 65 | 2.8 | 2.4 |
| Spray | 8 | 1 | 2.2 | 2.3 | 55 | 6 | 2.6 | 2.0 |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; LOD, limit of detection; N, number.
There were no statistically significant differences in chlormequat or chlorpyrifos concentration for the samples collected at 1 and 2 days after the spray events (Table 7). In addition, we investigated if there is any relationship between biomarker concentrations and timing of spray event and no significant relationship was found.
Table 7. Description of spray sample results by whether the sample was the day after a spray event (24 h) or 2 days after (48 h).
|
24 h Spray samples |
48 h Spray samples |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | N<LOD | GM | GSD | 95th % | N | N<LOD | GM | GSD | 95th % | |
| Chlormequat | 100 | 1 | 15.9 | 2.7 | 72.8 | 97 | 2 | 15.0 | 2.6 | 63.2 |
| Chlorpyrifos | 27 | 4 | 2.4 | 2.2 | 8.1 | 33 | 2 | 2.7 | 2.0 | 8.0 |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; LOD, limit of detection; N, number; 95%, 95th percentile.
We investigated whether other factors such as age, sex, location, smoking status, typical consumption of organic food and typical use of pesticides (as answered in background questionnaire), whether the subject had used pesticides in the previous 48 h, time spent indoors and outdoors in the previous 48 h (based on the sample-related questionnaires) might explain any differences in the biomarker concentrations between when the sample was collected. For chlormequat, after including sample type (i.e., spray event or background) in the model, the only significant factor, based on Wald test for addition to the model, was the level of organic food consumption reported, where higher levels of organic food consumption were associated with higher levels of chlormequat (where organic food consumption was recorded by participants as; none, little, some and most). However this factor did not significantly improve the fit of the model. As shown before, the statistically significant differences in the urinary biomarker levels between sample type is driven by the fact that outwith season background levels are lower than spray event-related sample levels and within season backgrounds. The results of the modelling are very similar for chlorpyrifos. Although some of the factors were significant (including age, sex and time spent outdoors), according to the Wald test, they did not significantly improve the fit of the model and therefore were not included in the final model. The significance of the sample type variable in the model for chlorpyrifos is likely being driven by the levels in the spray event-related samples being lower than background levels, both outwith and within.
DISCUSSION
We report on a study that aimed to assess exposure to pesticides for UK adults and children living within 100 m from the edge of agricultural land, and identify whether or not exposures were elevated following spray events. A distance of 100 m was selected as a balance between proximity to agricultural land and available potential study participants. In addition, researchers modelling vapour dispersion reported that vapour concentration is significantly reduced after a distance of 100 m (dependent on field size).24
Level of Urinary Biomarker Levels
The urinary biomarker levels for captan and cypermethrin were very low in this population with ~90% of samples having undetectable concentrations. For chlormequat and chlorpyrifos, the GM urinary biomarker concentrations following spray events were 15.4 μg/g creatinine and 2.5 μg/g creatinine, respectively, compared with 16.5 μg/g creatinine and 3.0 μg/g creatinine for within season background.
For chlormequat, we have only been able to identify one other, relevant, study. Lindh et al.14 reported a general population study in southern Sweden where the chlormequat concentrations ranged from 0.4–30.2 μg/g creatinine (median 2.9, 95th percentile <17.3 μg/g creatinine, N=100). These values are somewhat lower than our reported concentrations for all the chlormequat spray event and background samples combined (median 15.1, 95th percentile 79.8 μg/g creatinine). Despite these higher concentrations, UK exposures are two orders of magnitude below the values obtained from an oral dose at half the ADI (Lindh et al.).14 The only other report of measuring chlormequat in human urine was in an accidental poisoning case,25 but levels were not quantified. Excluding the different time periods of sample collection in our and Lindh et al.'s14 study, it is possible that we observed higher chlormequat urinary concentrations due to different farming practices for cereal crops and consumption of foods and beverages derived from cereal crops for which this growth regulator was applied. For example, the mean daily per capita consumption for bread and rolls, bakery products, cereal and products in the UK was reported as 103, 44 and 36 g, respectively, compared with 96, 21 and 25 g for Sweden.26
Approximately 90% of the captan urinary biomarker concentrations in this study were below the LOD, with the maximum value of 3.9 μg/g creatinine detected in a background sample. There are very few data available on environmental exposures to captan. Berthet et al.27 reported mean “pre-season” urine biomarker concentrations of 0.2 μg/l (~0.15 μg/g creatinine) but this was based on only four samples. Verberk et al.28 reported results below the LOD (8, ~5.9 μg/g creatinine) for six unexposed controls. Recent occupational studies have determined post-exposure THPI levels of <5 μg/l (~3.1 creatinine).27 No other published general population studies could be found.
There is an extensive literature on the use of urinary trichloro-2-pyridinol (TCP) as a biomarker of chlorpyrifos exposure. The US CDC NHANES study reports 95th percentiles for adults as 6.4 μg/g creatinine, N=832 and 7.4 μg/g creatinine, N=1,113 for 1999/2000 and 2001/2002, respectively.29 A study of 100 pregnant women in the Netherlands found a 95th percentile of 6.4 μg/l (~4.7 μg/g creatinine) with a maximum of 158 μg/l (~116 μg/g creatinine).30 Studies in Germany31 and Italy32 showed similar values. For Germany, the 95th percentile was 11.3 μg/l (~8.3 μg/g creatinine, N=50) and for Italy, the estimated 95th percentile was 6.5 μg/g creatinine (N=42). Our data for all the spray and background samples combined (95th percentile 10.1 μg/g creatinine) are comparable with these data from other general population studies, despite the varied geographical sources of the data sets. A study of residents' exposure to chlorpyrifos after treatment inside the home showed that potential exposures to the adult residents, as indicated by urinary 3,5,6-TCP biomonitoring, did not increase as a result of the application.33 Another study reported urinary TCP concentrations of 0.1–7.8 μg/g creatinine in 41 residents from houses where chlorpyrifos had been detected in collected indoor air samples.34 Alexander et al.35 reported a study of farm family members (spouses, and children aged 4–17 years) from Minnesota and South Carolina. Five consecutive 24-h urine samples were obtained from 34 families of licensed pesticide applicators from one day before to three days after a chlorpyrifos application. The spouses' GM exposure was reported as being 3.6 μg/g creatinine pre-application, 3.8 μg/g creatinine on the day of application and then 4.2 μg/g μg/g creatinine on days 1 and 2 post application. The reported children's exposure was 5.1 μg/g μg/g creatinine pre-application, 6.0 μg/g creatinine on day of application and 5.0 and 5.9 μg/g creatinine for days 1 and 2 post application. These are all higher than the GMs that we observed.
A number of general population studies have been reported for cypermethrin exposure. The US CDC NHANES study reports 95th percentiles for adults as 0.9 μg/g creatinine (N=1128) and 2.5 μg/g creatinine (N=1123) for cis-DCVA and trans-DCVA respectively in 2001/02.29 Detection rates were reported as less than 50% for cis-DCVA and <25% for trans-DCVA. A study of 1149 pregnant women in China found median levels of 0.7 μg/g creatinine for cis-DCVA and 1.9 μg/g creatinine for trans-DCVA.36 In the UK the 95th percentiles for adults (on the voting register) in the general population were reported as 0.7 μg/g creatinine (N=405) and 1.8 μg/g creatinine (N=404) for cis-DCVA and trans-DCVA, respectively and 2.3 μg/g creatinine combined as total-DCVA.37 In comparison, the 95th percentile was 5.8 μg/g creatinine for the spray event samples and 5.2 μg/g creatinine for our within spray season samples (which includes both adults and children). Although the 95th percentile results for our data are greater than reported by Bevan et al.,37 the number of samples above the LOD was far lower despite the same detection limit. These differences may reflect regional differences in exposure (our study was conducted in three regions compared with across the whole of the UK for Bevan et al.37) or temporal differences in pesticide use and food residues of pyrethroids (our samples were collected in 2011 and 2012 whereas those of Bevan et al.37 were collected in 2005/06).
Do Spray Events Result in Elevated Exposures to Residents Living Near Agricultural Land?
The results presented in this paper provide no evidence that, in this study population, the spray events resulted in elevated exposures compared with background samples taken within the season. For chlormequat, the biomarker levels (both spray event and background) were higher within the spray season compared with outwith the season. Chlormequat is a plant growth regulator, which acts by inhibiting cell elongation hence shortening and strengthening the stem producing a sturdier plant. It also influences the developmental cycle, leading to increased flowering and harvest.38 Growth regulating products containing chlormequat are widely used by the UK. The Pesticide Usage Survey Teams of the Food & Environment Research Agency (FERA) conducted surveys of pesticide usage in arable crops in 2011/12 by visiting holdings throughout the UK. In this survey they reported that chlormequat, applied alone or in mixtures, accounted for 59% of the area of arable crops treated with specific growth regulators: in addition there was a 13% increase in area treated using chlormequat since the previous survey conducted in 2010.39
Although not the primary aim of the study and reporting on different pesticides, Jones et al.40 reported a statistically significant difference for dialkylphosphate levels (organophosphate) in young children (<5 years) during different seasons, with autumn resulting in the highest levels. No seasonal effect was however observed for pirimicarb or carbaryl. Our chlormequat findings could be due to other sources of exposure. The most recent publication presenting results of pesticide residues in food commodities (including both raw and processed) sampled during the calendar year 2010 in the 27 European Union Member States and two European Free Trade Association countries (Iceland and Norway) reported chlormequat/oats to be the pesticide/crop combination for which residue concentrations were most frequently above the reporting level (64.6% of the samples). In addition, the highest percentage of maximum residue limit (MRL) exceedances in foods was found for chlormequat in oats (8.1% of all samples). In rye, the most frequently found pesticide residue was also chlormequat (35.9%).41 Of the 178 pesticides included in the 2010 EU-coordinated programme, the most frequent MRL exceedances were detected for chlormequat (3.6% of the samples). Chlormequat was also detected in a small number of organic food samples analysed (13 out of the 3571 samples), with measured residue levels ranging from 0.127–0.0011 mg/kg.41 Whilst in most instances these data relate to unprocessed food commodities and residue levels may decrease during food production, it is considered that diet is the primary source of exposure. For chlorpyrifos, the biomarker levels were very similar for the various sample types. Finally, for captan and cypermethrin a very large proportion of the measurements were below the LOD, whether or not these samples were collected following spray events.
The relatively short biological half-life of pesticide compounds and their biomarkers in the human body presents a major challenge to linking biological monitoring data to specific spray events and so urine samples have to be collected ideally within 24 h, and no later than 48 h following the spray events (depending on the urinary biomarker half-life). Farming activities, and in particular spaying with pesticides, are inherently unpredictable because of the changing weather and the presence of insects or other potentially damaging infestations which influence pesticide selection and application. Along with the planned weekly urine sample collections, there was a need for effective engagement with farmers to elicit this spray information at short notice and with residents to obtain the required spray event samples reactively. Through the use of community researchers, located in and knowledgeable of the geographical areas and familiar with local farming practices, effective engagement was achieved and over 3000 urine samples were collected.11
The methodology applied in this study is robust but is not without some limitations. The participating farmers may not be representative of all farmers within the study areas although there is no reason to suggest that their spraying practices are any different to those of the wider farming communities. In the UK, Government Ministers must approve all pesticides before they can be marketed or used and everyone who uses pesticides must have adequate guidance, instruction or training for their correct use and must ensure that all reasonable precautions are taken to prevent spray drift.12 It was clear from the farmers' spray records that a number of pesticide products were applied throughout the spraying season (dependent on crops, infestation, weather conditions and other factors) and so the number of relevant spray events observed may differ from year to year. Spray event start and finish times were obtained and residents were asked to provide details of their activities in the 48 h period before provision of each urine sample. However, it was not possible to establish from this when, where or how the residents' potential exposure to the assessed pesticides may have occurred. Following due consideration of preliminary modelling and the peer-reviewed literature,13 first-morning void samples were requested from participants. There is the possibility that the urine void with the highest biomarker concentration was missed however no significant relationship between biomarker concentrations and timing of spray event was found for any of the pesticides. Weather information, whilst obtained by both the farmer and the research team, was typically reported from weather stations located in some instances several miles away from where the spray took place. It is possible that the available wind speed and direction information may not reflect what actually occurred during the given spray event and so was not considered further. Good spray event information was obtained from the farmers participating in the study. However it is not possible to establish whether residents were potentially exposed to any additional relevant spray applied by non-participating farmers in the locality. Given the scope of the study, the number of dietary-related questions was kept to a minimum, restricted to the consumption of food from the residents' garden and organic food (as reported in the background questionnaire). In retrospect, the inclusion of additional food-related questions may have been useful in explaining some of the results observed. Residents (adults aged 18 years and over and children in their care aged 4–12 years) were recruited, with infants (<4 years old) and adolescents (13–17 years) being excluded from the study. This pragmatic decision was taken due to likely difficulties being encountered with obtaining relevant urine samples from babies and toddlers and adolescents potentially being less engaged with the study and so the exposures of these sub groups was not determined.
All laboratory methods for the urine samples showed good day-to-day repeatability and were based, where possible, on validated methods (see Supplementary Information) with comparable LODs to those already reported and used in environmental exposure studies. Where known, the primary or most abundant biomarker was measured; measuring multiple biomarkers for the same pesticide would not have increased the sensitivity of measurement unless all metabolites are converted to a single biomarker. All chlorpyrifos and chlormequat samples were analysed within the timeframe of the stability trials. The length of each analyte stability trial was determined by available samples within the data set. Some samples for captan and cypermethrin were analysed outside of the evaluated stability time frame. These samples have not necessarily degraded but extended sample stability was not evaluated due to insufficient sample volume. It is considered that the biomarkers and methodology used were demonstrably fit for purpose.
When considering the generalisablity of the study results to other pesticides, consideration should be given to the spray techniques used and their potential to distribute the pesticide beyond the target area and the propensity for the pesticide to redistribute post application. The spray equipment and techniques used by the participating farmers were not atypical and were reportedly used when applying other pesticide products to the given crops throughout the spray season. The range of vapour pressures for the relevant products considered in this study ranged from 0.0023 to 1.43 mPa at 25 °C, of which the highest was for chlorpyrifos. Monitoring in the USA suggests that chlorpyrifos is the worst known case for vapour concentrations42 and so we consider that our study covers both the likely spectrum and worst case vapour pressures used in modern day pesticides in the UK (and perhaps more widely). Orchard spray techniques are usually considered as potentially giving rise to higher levels of pesticide drift (and therefore potential exposure) in comparison to field crop spraying practices. For example, measurements of bystander exposure during UK field crop spraying and orchard spraying applications have been reported.43, 44 For boom sprayers the average potential dermal exposure (PDE) for a bystander, positioned 8 m downwind from the sprayer and the average amount of spray potentially inhaled in the breathing zone were 0.1 ml spray/person and 0.006 ml spray/person, respectively. For orchard sprayer applications the equivalent PDE and inhalation values were 3.7 ml spray/person and 0.002 ml spray/person. Given that we collected in excess of 300 orchard spray event samples, which also included those related to chlorpyrifos, we consider that our study adequately considers potentially higher risk spray techniques, of which the majority of the urinary biomarker results were observed to be less than the analytical LOD.
CONCLUSION
This study reports urinary biomarker concentrations for a number of active ingredients both for spray event and background samples amongst people living within 100 m of agricultural land, which has not previously been undertaken and reported on such a scale for a UK population. It has also, as far as we are aware, not been studied as systematically anywhere else. Our study did not set out to determine whether rural residents have greater exposure to pesticides than non-rural residents and so is not directly comparable to other studies that may have sought to do this.
The primary conclusion of the study is that there is no evidence of increased pesticide biomarker excretion in rural residents following a spray event within 100 m of their home, when compared with the urinary biomarker levels obtained when relevant spray events did not take place. The levels of urinary biomarkers detected in our population were generally comparable to other studies of exposure in the general population, where such data are available, and this supports the view that general population exposures to pesticides are primarily from non-spray event sources such as diet. It appears that the population recruited to this study exhibits greater exposure to chlormequat than detected in a Swedish population sub-set. Whether this is a characteristic of our particular rural residents or is more widely applicable to the entire UK general population is not known and may be an area of further work. Exposure levels for cypermethrin and captan were very low.
Acknowledgments
This project was funded by the UK Government Department for Environment Food and Rural Affairs (DEFRA), project code PS2620. We thank the following individuals (in alphabetical order) for their work as community researchers in this project: S Attwood, J Cadzow, A Carr, D Dahrendorf, T Hogg, L Jewsbury and F MacIver. Their hard work was crucial to the success of the recruitment and data collection in this project. Thanks also to the independent project Advisory Committee (Prof. David Coggon, Paul Hamey, Prof. Len Levy and Dr. Sean Semple) for their helpful comments throughout the project and on earlier manuscript drafts; Dr. Anne Sleeuwenhoek and David Todd (IOM) for their assistance with the storage of the urine samples and related administration tasks; IOM personnel (Anne Sleeuwenhoek, Shahzad Rashid, Peter Ritchie, Yvonne O'Neill, Marlyn Davis, Julie O'Neill, Selima Argoub and Araceli Sanchez Jimenez) for assistance with data storage, data input and other administrative tasks; and HSL personnel (Drs Craig Sams, Laura Kenny, Shahwaiz Iqbal and Fiona Garner) for the urine sample analysis. We express our sincere gratitude to all the farmers and householders who participated in the study.
Footnotes
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
Dr. Galea is a member of the UK Government Pesticide Incidents Appraisal Panel and Dr. Cocker was a member of the UK Government Advisory Committee on Pesticides(January 2009-December 2014). The authors declare no conflict of interest.
Supplementary Material
References
- 1RCEPCrop Spraying and The Health of Residents and Bystanders. Royal Commission on Environmental Pollution: London, UK. 2005. [Google Scholar]
- 2ACPCrop Spraying and the Health of Residents and Bystanders. A Commentary on the Report Published by the Royal Commission on Environmental Pollution in September 2005. Advisory Committee on Pesticides: York, UK. 2005. [Google Scholar]
- 3Committees on toxicity and carcinogenicity of chemicals in food, consumer products and the environment. Statement on Royal Commission on Environmental Pollution: Crop spraying and the health of residents and bystanders. Available at: http://cot.food.gov.uk/sites/default/files/cot/cotsection06.pdf (accessed on 1 September 2015).
- 4DEFRA. The Royal Commission on Environmental Pollution report on Crop Spraying and the Health of Residents and Bystanders–Government Response. Department for Environment, Food and Rural Affairs: UK. 2006. a. [Google Scholar]
- 5Koureas M, Tsakalof A, Tzatzarakis M, Vakonaki E, Tsatsakis A, Hadjichristodoulou C et al. Biomonitoring of organophosphate exposure of pesticide sprayers and comparison of exposure levels with other population groups in Thessaly (Greece). Occup Environ Med 2013; 71: 126–133. [DOI] [PubMed] [Google Scholar]
- 6Couture C, Fortin MC, Carrier G, Dumas P, Tremblay C, Bouchard M. Assessment of exposure to pyrethroids and pyrethrins in a rural population of the Montérégie area, Quebec, Canada. J Occup Envrion Hyg 2009; 6: 341–352. [DOI] [PubMed] [Google Scholar]
- 7Kimata A, Kondo T, Ueyama J, Yamamoto K, Yoshitake J, Takagi K et al. Comparison of urinary concentrations of 3-phenoxybenzoic acid among general residents in rural and suburban areas and employees of pest control firms. Int Arch Occup Environ Health 2009; 82: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 8Keifer M, Rivas F, Dong Moon J, Checkoway H. Symptoms and cholinesterase activity among rural residents living near cotton fields in Nicaragua. Occup Environ Med 1996; 53: 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Ward MH, Lubin J, Giglierano J, Colt JS, Wolter C, Bekiroglu N. Proximity to crops and residential exposure to agricultural herbicides in Iowa. Environ Health Perspect 2006; 114: 893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Galea KS, MacCalman L, Jones K, Cocker J, Teedon P, Sleeuwenhoek AJ et al. Biological monitoring of pesticide exposures in residents living near agricultural land. BMC Public Health 2011; 11: 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Teedon P, Galea KS, MacCalman L, Jones K, Cocker J, Cherrie JW et al. Engaging communities for exposure science–lessons learned from a pesticide biomonitoring study. PLOS One 2015. (doi:10.1371/journal.pone.0136347). [DOI] [PMC free article] [PubMed]
- 12DEFRAPesticides Code of Practice for Using Plant Protection Products. DEFRA: London, UK. 2006. [Google Scholar]
- 13Kissel JC, Curl CL, Kedan G, Lu C, Griffith W, Barr DB et al. Comparison of organophosphorus pesticide metabolite levels in single and multiple daily urine samples collected from preschool children in Washington State. J Expo Anal Epidemiol 2005; 15: 164–171. [DOI] [PubMed] [Google Scholar]
- 14Lindh CH, Littorin M, Johannesson G, Jonsson BAG. Analysis of chlormequat in human urine as a biomarker of exposure using liquid chromatography triple quadrupole mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 2011; 879: 1551–1556. [DOI] [PubMed] [Google Scholar]
- 15Berthet A, Bouchard M, Schüpfer P, Vernez D, Danuser B, Huynh CK. Liquid chromatography-tandem mass spectrometry (LC/APCI-MS/MS) methods for the quantification of captan and folpet phthalimide metabolites in human plasma and urine. Anal Bioanal Chem 2011; 399: 2243–2255. [DOI] [PubMed] [Google Scholar]
- 16Sams C, Jones K. Human volunteer studies investigating the potential for toxicokinetic interactions between the pesticides deltamethrin; pirimicarb and chlorpyrifos-methyl following oral exposure at the acceptable daily intake. Toxicol Lett 2011; 200: 41–45. [DOI] [PubMed] [Google Scholar]
- 17Jones K, Sams C, Patel K, Johnson P. Development of cost-effective biomarkers for herbicides and fungicides. Available at: http://www.foodbase.org.uk/results.php?f_report_id=407. (accessed on 11 June 2014).
- 18Cocker J, Mason HJ, Warren ND, Cotton RJ. Creatinine adjustment of biological monitoring results. Occup Med Oxf 2011; 61: 349–353. [DOI] [PubMed] [Google Scholar]
- 19EWDTS. European Laboratory Guidelines for Legally Defensible Workplace Drug Testing. 2002. Available at: www.ewdts.org (accessed on 1 September 2015).
- 20Helsel DR. Nondetects and Data Analysis: Statistics for Censored Environmental Data. John Wiley and Sons: New York, USA. 2005. [Google Scholar]
- 21R Core Team.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2014. [Google Scholar]
- 22Payne RW, Harding SA, Murray DA, Soutar DM, Baird DB, Glaser AI et alThe Guide to GenStat Release 14, Part 2: Statistics. VSN International: Hemel Hempstead, UK. 2011. [Google Scholar]
- 23Systat Software SigmaPlot version 10. Systat Software, Inc., San Jose California USA. www.sigmaplot.com.
- 24The Arable Group. PS2005. The Development and Validation of a Bystander and Resident Exposure Assessment Model (BREAM) Research Project Final Report. DEFRA: York, UK. 2010. [Google Scholar]
- 25Winek CL, Wahba WW, Edelstein JM. Sudden-death following accidental ingestion of chlormequat. J Anal Toxicol 1990; 14: 257–258. [DOI] [PubMed] [Google Scholar]
- 26DAFNE The Pan-European food bank based on household budget surveys National and Kapodistrian University of Athens: Athens. 2006. http://www.nut.uoa.gr/dafnesoftweb/. [Google Scholar]
- 27Berthet A, Heredia-Ortiz R, Vernez D, Danuser B, Bouchard M. A detailed urinary excretion time course study of captan and folpet biomarkers in workers for the estimation of dose, main route-of-entry and most appropriate sampling and analysis strategies. Ann Occup Hyg 2012; 56: 815–828. [DOI] [PubMed] [Google Scholar]
- 28Verberk MM, Brouwer DH, Brouwer EJ, Bruyzeel DP, Emmen HH, Van Hemmen JJ et al. Health effects of pesticides in the flower-bulb culture in Holland. Med Lav 1990; 81: 530–541. [PubMed] [Google Scholar]
- 29CDCThird National Report on Human Exposure to Environmental Chemicals. NCEH Pub. No. 05-0570, Atlanta, Georgia, USA. 2005. [Google Scholar]
- 30Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environ Res 2008; 108: 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Koch HM, Hardt J, Angerer J. Biological monitoring of exposure of the general population to the organophosphorus pesticides chlorpyrifos and chlorpyrifos-methyl by determination of their specific metabolite 3,5,6-trichloro-2-pyridinol. Int J Hyg Environ Health 2001; 204: 175–180. [DOI] [PubMed] [Google Scholar]
- 32Aprea C, Betta A, Catenacci G, Lotti A, Magnaghi S, Barisano A et al. Reference values of urinary 3,5,6-trichloro-2-pyridinol in the Italian population—validation of analytical method and preliminary results (Multicentric study). J AOAC Int 1999; 82: 305–312. [PubMed] [Google Scholar]
- 33Byrne SL, Shurdut BA, Saunders DG. Potential chlorpyrifos exposure to residents following standard crack and crevice treatment. Environ Health Perspect 1998; 106: 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Dai H, Asakawa F, Suna S, Hirao T, Karita T, Fukunaga I et al. Investigation of indoor air pollution by chlorpyrifos: determination of chlorpyrifos in indoor air and 3,5,6-trichloro-2-pyridinol in residents' urine as an exposure index. Environ Health Prev Med 2003; 8: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Alexander BH, Burns CJ, Bartels MJ, Acquavella JF, Mandel JS, Gustin C et al. Chlorpyrifos exposure in farm families: results from the farm family exposure study. J Expo Sci Environ Epidemiol 2006; 16: 447–456. [DOI] [PubMed] [Google Scholar]
- 36Qi X, Zheng M, Wu C, Wang G, Feng C, Zhou Z. Urinary pyrethroid metabolites among pregnant women in an agricultural area of the Province of Jiangsu, China. Int J Hyg Environ Health 2012; 215: 487–495. [DOI] [PubMed] [Google Scholar]
- 37Bevan R, Jones K, Cocker J, Assem FL, Levy LS. Reference ranges for key biomarkers of chemical exposure within the UK population. Int J Hyg Environ Health 2013; 216: 170–174. [DOI] [PubMed] [Google Scholar]
- 38Tomlin C. (ed) The Pesticide Manual: A World Compendium 15th edn British Crop Protection Council. 2009. [Google Scholar]
- 39Garthwaite DG, Hudson S, Barker I, Parrish G, Smith L, Pietravalle S. Pesticide Usage Survey Reports 250. Arable Crops in the United Kingdom 2012 (Including Aerial Applications 2012) DEFRA: York, UK. 2014. [Google Scholar]
- 40Jones K, Everard M, Harding AH. Investigation of gastrointestinal effects of organophosphate and carbamate pesticide residues on young children. Int J Hyg Environ Health 2014; 217: 392–398. [DOI] [PubMed] [Google Scholar]
- 41EFSA. Scientific report of EFSA. The 2010 European Union Report on Pesticide Residues in Food. European Food Safety Authority (EFSA). EFSA J 2013; 11: 3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42California Environmental Protection AgencyReport for the Application and Ambient Air Monitoring for Chlorpyrifos (and the Oxon Analogue) in Tulare County During Spring/Summer 1996. Air Resources Board, California Environmental Protection Agency, USA1998. [Google Scholar]
- 43Lloyd GA, Bell GJ. Hydraulic Nozzles: Comparative Spray Drift Study. MAFF/ADAS: UK. 1983. [Google Scholar]
- 44Lloyd GA, Bell GJ, Samuels SW, Cross JV, Berrie AM. Orchard Sprayers: Comparative Operator Exposure and Spray Drift Study. MAFF Report, UK Pesticide Registration and Surveillance Department, UK. 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.