Abstract
AIM: The present study was aimed to investigate the developmental patterns of growth hormone receptor (GHr) and somatostatin (SS) mRNA expression in porcine gastric tissue and its relationship with gastric growth and gastric functional development.
METHODS: Erhualian and Large White boars were selected randomly and sampled at birth (D0), 3, 20, 30, 90, 120 and 180 days of age respectively, meanwhile the bodyweight and gastric weight were recorded. The single tube semi-quantitative RT-PCR was applied in this experiment to investigate the developmental patterns of gastric GHr and SS mRNA expression, the correlations between the patterns of mRNA expression and the relative gastric weight (ratio of gastric weight to bodyweight) and the pepsin contents in gastric mucous membrane were analyzed. In order to further elucidate the effect of GH on gastric function, the primary cultures of gastric fundic mucosal cells were treated with 2 ng/ml, 20 ng/ml and 200 ng/ml of rpGH for 18 hrs respectively, and the pepsin contents in culture medium were measured.
RESULTS: The expression of GHr mRNA was high at birth, followed by a significant decrease at 3 days of age (D3) in both breeds. In Large White boars, the expression of GHr mRNA reached a peak at D90 and remained a plateau afterward. In Erhualian pigs, however, a slight yet significant increase occurred at D30 reaching a level that was kept constant thereafter. From birth to D30, the expression of GHr mRNA in gastric tissue was higher in Erhualian boars than that in Large White boars, but from D90 to D180, the higher expression of GHr mRNA was found in Large White boars. The gastric GHr mRNA expression was significantly correlated with the relative gastric weight (r = 0.541, P < 0.05) and pepsin content in gastric mucosa (r = 0.625, P < 0.05) respectively.
The gastric SS mRNA expression was high at birth (Erhualian 1.59, Large White 0.80), but dropped significantly at D3 (Erhualian 0.95, Large White 0.19, P < 0.05), a stepwise increase was followed thereafter until a peak at D30 (Erhualian 1.71, Large White 0.95) In general, Erhualian pigs expressed higher levels of SS mRNA in gastric tissue as compared with Large White pigs at the same age (P < 0.05). No significant correlations between SS mRNA and relative gastric weight or pepsin content were found.
In vitro, 2 ng/ml of rpGH elicited significant increase in pepsin secretion from primary cultures of gastric mucosal cells (P < 0.05), and no responses were observed at 20 ng/ml and 200 ng/ml.
CONCLUSION: The results suggested that: 1, GHr and SS mRNA in porcine stomach are expressed according to strain specific developmental patterns; 2, GH acts directly at the gastric tissue and regulates the structural and functional growth of stomach.
INTRODUCTION
The growth hormone (GH) regulates numerous cellular functions by direct binding to its receptors (growth hormone receptor, GHr) in many different tissues, such as liver, muscle, lymph and adipose tissue, etc. It has been reported that GHr was expressed in the stomach of rat[1,2], rabbit[3] and human[4], respectively, suggesting that stomach is the target organ of GH. Somatostatin (SS) is a typical brain-gut-peptide, and it is expressed both in the brain and gastrointestinal system. Hypothalamic SS serves as an inhibitory factor to regulate GH secretion from anterior pituitary[5], while gastric SS works to inhibit the secretion of pepsin, gastric acid, as well as gastrin, hence plays an important role in the regulation of gastric function[6].
The retardation of gastric functional development, such as insufficient gastric acid and pepsin secretion, results in dyspepsia, diarrhea, poor growth and even death in newborn piglets. It is postulated that GH and gastric SS might be involved in the regulation of porcine stomach development, but no much data available to date. Therefore, the present study employed two breeds of pig with different genetic backgrounds, Large White and Erhualian pigs, as animal model to investigate the developmental patterns of growth hormone receptor (GHr) and somatostatin (SS) mRNA expression in porcine gastric tissue. The correlation between the patterns of GHr and SS mRNA expression and the gastric growth as well as the gastric functional development were analyzed. In addition, an in vitro cell culture trial was conducted to study the effect of GH on gastric pepsin secretion.
MATERIALS AND METHODS
Animals and Sampling
Eight litters newborn purebred piglets respectively from 2nd or 3rd farrowing Large White and Erhualian sows were kept for experiment, and water and feed were available ad libitum. The diet was formulated according to the requirement of each breed. Large White piglets weaned at D30 of age, while Erhualian piglets at D45. Erhualian boars (n = 4) and Large White boars (n = 4) were selected randomly and sacrificed at birth, 3, 20, 30, 90, 120 and 180 days of age respectively, meanwhile the bodyweight and gastric weight were recorded. The fundic tissue were sampled and frozen in liquid nitrogen immediately, then stored at -70 °C. The fundic mucosa were taken and stored at -20 °C.
Pepsin detection
One gram fundic mucosa was homogenized with 3 ml 0.85% NaCl, then the homogenate was centrifuged at 4 °C for 30 min at 5000 rpm. The supernatant was collected for pepsin activity analysis according to the procedure modified by Robinson (1972), using hemoglobin as the substrate. Since hemoglobin was diluted with hydrochloric acid (pH= 1.6), at such acidic condition, all pepsinogen were activated, so the gastric pepsin activity measured accounted for the total pepsin content of gastric mucosa. The protein content in the same supernatant of the homogenate was measured for normalizing the pepsin content as follows:
Gastric mucosa pepsin activity (U/g) = [Pepsin (U)/homogenate liquid (ml)]/[Protein (g)/homogenate liquid (ml)]
Semi-quantitative RT-PCR
Total RNA was isolated from porcine gastric tissue using guanidinium thiocyanate-phenol-chloroform one-step method. RNA was reverse transcribed into cDNA with random hexamer primers. An RT- control tube containing all RT reagents except reverse transcriptase was included to monitor genomic DNA contamination.
The primers for GHr were designed according to the cDNA sequence published on Gene Bank (X54429): sense, 5’-CTCGATATTGATGACCCTGA-3’, and anti-sense, 5’-GATGAGTTGAGTCAGTTCCA-3’, and the predicted PCR product was 341 bp. SS primers were achieved according to the code region of porcine genomic DNA (Gene Bank, U36385): sense, 5’-AGCTGCTGTCTGAACCCAAC-3’, and anti-sense, 5’-GAAATTCTTGCAGCCAGCTT-3’. Classic 18S rRNA internal standards (Ambion Inc. USA) was used to adjust the variation in pipetting and amplification.
PCR conditions were optimized for both GHr and SS. The reaction volume for GHr was 50 µl, including 5 µl 10 × PCR buffer, 1.3 mmol/L MgCl2, 10 mmol/L dNTP, 0.66 mmol/L GHr sense and anti-sense primer, 2 µl 18S primer pair, 2 µl 18S competimer, 2 µl RT product, 1.0 U Tag DNA polymerase (Promega, Shanghai). Amplification conditions included: denaturation at 94 °C for 5 min, denaturation at 94 °C for 30 sec, annealing at 52 °C for 1 min, extension at 72 °C for 1 min, total in 32 cycles, finally an additional extension step at 72 °C for 5 min was done. The SS PCR reaction mix contained 5 µl 10 × PCR buffer, 1.5 mmol/L MgCl2, 10 mmol/L dNTP, 1 mmol/L SS sense and anti-sense primer, 2 µl 18S primer pair, 2 µl 18S competimer, 2 µl RT product, and 1.0 U Tag DNA polymerase (Promega, Shanghai). Total volume was 50 µl. Amplification conditions included: denaturation at 94 °C for 5 min; denaturation at 94 °C for 30 sec, annealing at 55 °C for 30 sec, extension at 72 °C for 1 min, total in 32 cycles, finally it was finished by an extension step at 72 °C for 5 min. All samples were amplified in the same PCR run together with quality and contamination controls on GeneAmp PCR system 9600 (Perkin Elmer, USA).
Twenty µl PCR products were separated through 2% agarose gel electrophoresis. The band intensities were analyzed by Kodak ID Electrophoresis Documentation and Analysis System 120 (Kodak Photo Film Co., Ltd., U.S.). The ratio of target gene intensity to 18S intensity was used to represent the abundance of GHr and SS mRNA expression.
Cell culture
Animal and reagent Four Landrace X Large White crossbred gilts at the age of D35 were used in this trial. HEPES and DMEM/F-12 were products of Hyclone, collagenase type I was bought from Sigma and the fetal bovine serum was purchased from Hangzhou Sijiqing Company, China.
Sample and culture Piglets were sacrificed and the fundic mucosa was isolated. The tissue was washed in Hank’s solution thoroughly and dipped in Hank’s solution containing 500 U/ml of antibiotics for 30 min. Then the fundic mucosa were dispersed by 600 U/ml of collagenase I at 37 °C for 2 hrs, filtrated and centrifuged (1000 r/min, 5 min). Thereafter cells at the density of 1 × 105 per cm2 were cultured (37 °C, 5% CO2) in DEME/F-12 supplemented with 10% fetal bovine serum, 5 mM HEPES buffer, and 100 U/ml antibiotics. After 24 hrs, the culture medium was refreshed by new medium containing 2 ng/ml, 20 ng/ml and 200 ng/ml GH respectively. The GH challenge lasted for 18 hrs before the medium were collected for pepsin analysis.
Statistical analysis
All data were analyzed by SPSS10. Significant difference analysis were conducted by t-test, one-way ANOVA and LSD. The correlation between the GHr, SS mRNA expression and the relative gastric weight, as well as the gastric mucosa pepsin content were determined.
RESULTS
Gastric growth
As shown in Figure 1, the gastric growth rate is low before weaning both in Large White and Erhualian pigs, especially during the period from D3 to D20. Highest growth rate is seen between D30 and D90. From D90 onwards, the gastric weight of Large White Boars keeps increase while that of Erhualian boars maintains a plateau, which results in a higher gastric growth rate in Large White boars than that of Erhualian boars. Compared to the body weight changes shown in Figure 2, the stomach weight changes display different patterns, which is more obvious in Erhualian pigs. Figure 3 shows the developmental pattern of relative gastric weight, it seems that the gastric growth lags behind the body growth during the period from D20 to D30.
Figure 1.
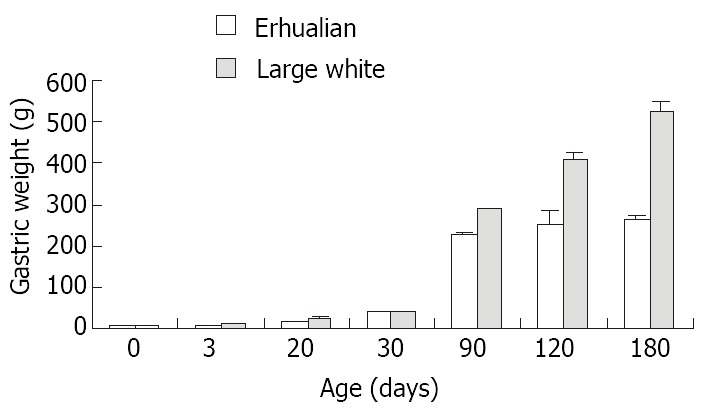
The developmental patterns of gastric weight in Erhualian and Large White boars.
Figure 2.
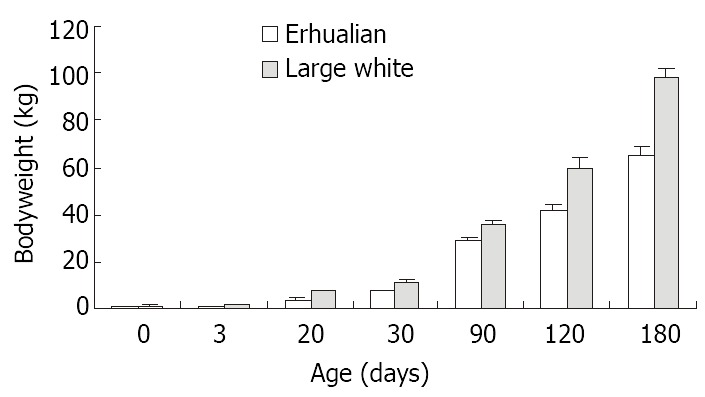
The developmental patterns of body weight in Erhualian and Large White boars.
Figure 3.
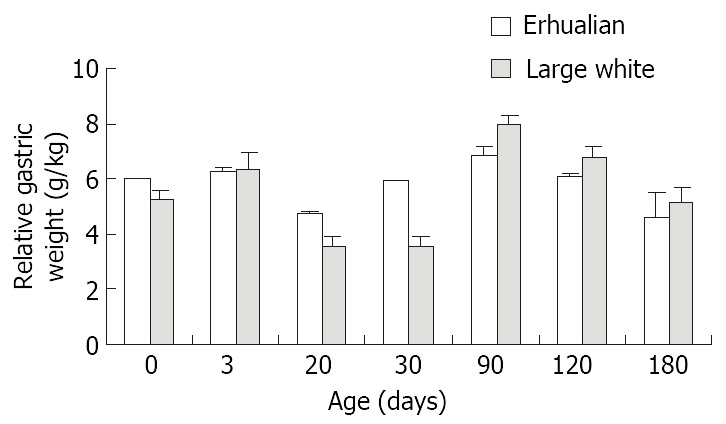
The developmental patterns of the relative gastric weight in Erhualian and Large White boars.
Developmental changes of pepsin contents in gastric mucosa
From birth to D30, no notable variation was found in gastric pepsin content in Large White boars, but after D30, the gastric pepsin content increased significantly (P < 0.05) and kept constant thereafter (Figure 4). However in Erhualian pigs, the gastric pepsin content was high at birth, but dropped significantly at D3 and D20 (P < 0.05). A significant up-regulation occurred at D30 for gastric pepsin content until a peak level was reached at D120.
Figure 4.
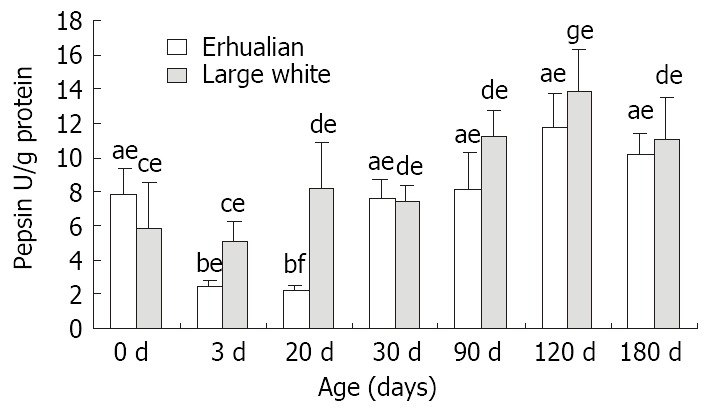
The developmental patterns of the pepsin activity (U/g protein) of fundic mucosa in Letters a and b for Erhualian boars, letters c and d and g for Large White boars, letter e and f for the different breeds at same ages, different letters differ significantly P < 0.05.
Developmental changes of gastric GHr mRNA expression (Figure 5)
Figure 5.

Representative agarose gel electrophoreses photo of RT-PCR product for gastric GHr mRNA in Erhualian and Large White boars. (M is marker pUC19, E0-E180 represents Erhualian boars at day 0-180 respectively, and L0-L180 represents Large White boars at day 0-180 respectively).
Figure 6 shows the the expression patterns of GHr mRNA in gastric tissue. The GHr mRNA was high at birth, but dropped dramatically at D3 in both breeds. In both breeds of pigs, the expression of GHr mRNA increased from D30 but followed different patterns thereafter. In Large White pigs, the GH mRNA expression continue increased till D90 when a plateau was reached. While in Erhualian pigs, the gastric GHr mRNA expression went up in a much lesser extent and reached a much lower plateau earlier at D30. As the result, the expression of GHr mRNA in gastric tissue was higher in Erhualian boars than that in Large White boars in earlier stages from birth to D30, whereas the opposite was true in later stages from D90 to D180. The statistic analysis revealed that the gastric GHr mRNA expression was positively correlated with the relative gastric weight (r = 0.541, P < 0.05) and the pepsin content in gastric mucosa (r = 0.625, P < 0.05), respectively.
Figure 6.
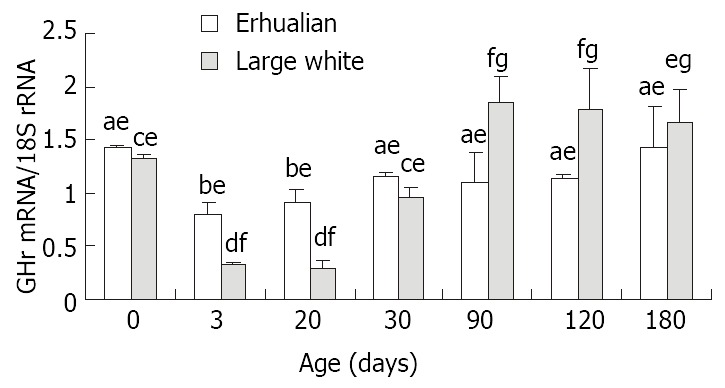
The developmental pattern of GHr mRNA expression in stomach of Erhualian and Large Letters a and b for Erhualian boars, letters c and d and g for Large White boars, letter e and f for the different breeds at same ages, different letters differ significantly P < 0.05.
The developmental changes of porcine gastric SS mRNA expression (Figure 7)
Figure 7.
Representative agarose gel electrophoreses photo of RT-PCR product for gastric SS mRNA in Erhualian and Large White boars. (M is marker pUC19, E0-E180 represents Erhualian boars at day 0-180 respectively, and L0-L180 represents Large White boars at day 0-180 respectively).
The gastric SS mRNA expression followed a similar pattern in two breeds of pigs (Figure 8). The SS mRNA expression was high at birth, but significantly decreased at D3 (P < 0.05), soon after a stepwise increase was found reaching a peak at D30 in both breeds of pigs. In general, Erhualian pigs expressed higher levels of SS mRNA in gastric tissue as compared with Large White pigs at the same age in early stages (P < 0.05) but this difference diminished in later stages at D120 and D180. No clear correlation was found between gastric SS mRNA expression and relative gastric weight or pepsin content in gastric mucosa.
Figure 8.
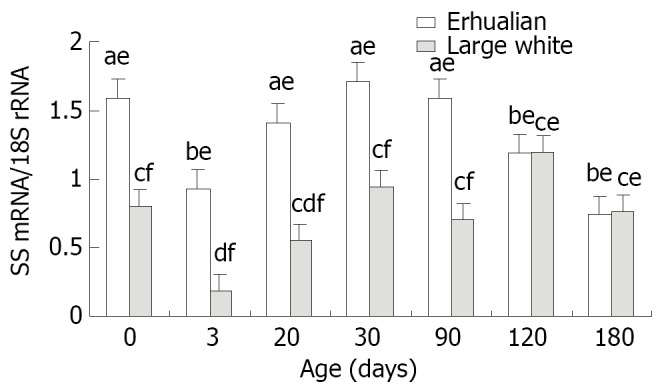
The developmental pattern of SS mRNA expression in stomach of Erhualian and Large Letters a and b for Erhualian boars, letters c and d for Large White boars, letter e and f for the different breeds at same ages, different letters differ significantly P < 0.05.
The effect of GH on pepsin secretion in vitro
As shown in Figure 9, 2 ng/ml of rpGH significantly stimulated pepsin secretion (P < 0.05), but 20 and 200 ng/ml of GH failed to show any influence in terms of pepsin secretion in vitro.
Figure 9.
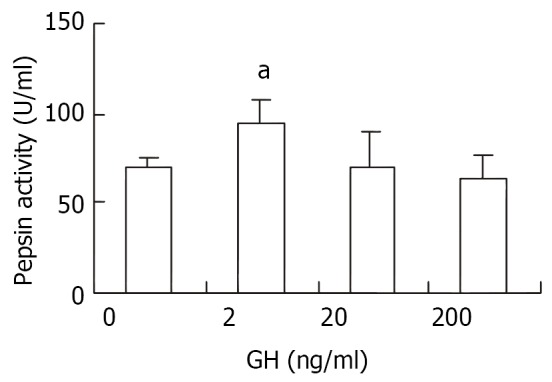
Effects of rpGH on the pepsin secretion from gastric mucous cells in vitro (a indicating significant difference between different treatment, P < 0.05).
DISCUSSION
The effects of GH on growth can be mediated via: (1) binding to the hepatic GHr to stimulate the IGF-1 secretion from liver into circulation, in which IGF-1 then works in an endocrine manner to promote the growth of target organs, (2) directly binding to GHr in extra-hepatic tissues to induce IGF-1 expression, in which IGF-1 works in both endocrine and paracrine manner to stimulate target tissue development, (3) directly binding to the GHr of extra-hepatic tissues to regulate the development of target organs[7]. It was reported by Lobie et al[2] (1992) that bGH (bovine GH) administration significantly increased rat gastric weight, relative gastric weight, as well as the height of mucosa. There are evidences for the localization of GHr mRNA expression in the gastric mucous membrane of rat, rabbit, human[1-4]. Using semi-quantitative RT-PCR, the present experiment proved the expression of GHr mRNA in stomach of both Large White and Erhualian pigs. Furthermore, the gastric GHr mRNA expressed in an age-dependent manner which is positively correlated to relative gastric weight. This positive correlation implies that GH may directly act on stomach to regulate its growth. However, from birth to D3, the expression of gastric GHr significantly decreased, but the stomach was in a rapid growth period, it may be contributed by maternal influences through colostrum. Xu (1996) reviewed that there are high concentrations of hormones and growth-promoting peptides, such as insulin, cortisol, epidermal growth factor (EGF) and insulin-like growth factor I (IGF-I) in the maternal colostrums and there are evidences that colostrum-borne EGF and IGF-I play a role in postnatal stomach development[8]. Many researches have proved that IGF-1 can stimulate gastric mucosal cell proliferation[9-12]. Coerper et al[13] (2001) reported that IGF-1 at low dose of 0.4 mg/kg BW and 4 mg/kg BW significantly stimulated gastric cell proliferation and ulcer healing. However, it is not clear whether the maternal growth factors in colostrum contribute to the down regulation of gastric GHr mRNA expression at 3 days of age.
The GH actions are tissue-specific, which depends on the sensitivities of target tissues to GH stimulation. The tissue sensitivity depends upon the abundance of GHr[14]. Studies of Ilkbahar. (1995)[15], Klempt et al[16] (1993), Schnoebelen-Combes et al[17] (1996) and Peng et al[18] (1998) on mice, sheep and pigs, proved that the developmental patterns of GHr mRNA expression varied among different tissues, species and breeds. Our results also showed that the developmental patterns of GHr expression in stomach differ from that in liver, spleen and muscle. This tissue-specific GHr expression may account for the different functions and regulatory mechanisms of GH in different tissues or organs.
Our results also showed that the expression of GHr mRNA in gastric tissues was higher in Erhualian boars in early stages from birth to D30, but from D90 to D180, the higher expression of GHr mRNA was found in Large White boars. This result was in accordance with the differences in stomach growth between the two breeds of pigs. The present results suggest that GH play an important role in the regulation of gastric growth through the GHr expressed in stomach.
The significant correlation between gastric GHr mRNA expression and the pepsin content in mucous membrane provided an important hint that GH may be involved in the regulation of gastric function apart from its role in gastric growth. To date, however, controversy results have been published in this regard. Drago et al[19] (1997) proved that GH stimulated the gastric acid secretion in rats but failed to restore the decreased peptic activity in hypophysectomized rats. On the contrary, however, GH did restore peptic activity in hypophysectomized dog[1,2]. In order to clarify the effect of GH on pepsin secretion from gastric mucosa membrane, we performed an in vitro trial in primary cell culture and found that 2 ng/ml of GH significant elevated pepsin secretion (P < 0.05), but 20 ng/ml and 200 ng/ml of GH did not show significant effect on pepsin secretion compared with the control. The results suggest that GH is indeed involved in the regulation of pepsin secretion in a dose-related manner.
It is well known that gastric SS located in D-cell of autrum and fundus is a key player in the gastric function regulation. SS inhibits gastric acid, pepsin, and gastrin secretion via both paracrine and endocrine pathways[21]. It was reported that SS inhibits gastrin secretion by down-regulating the gastrin mRNA expression. Sheep immunized against SS boosted gastric acid and gastrin secretion[6]. In vitro studies proved that SS antisera significantly increased gastric acid release from perfused stomach of rat, mouse and pig[22].
There have been some publications on the ontogeny of gastric SS. Yee et al[23] (1996) concluded that, in the fetal rabbit stomach, the expression of gastrin and somatostatin may regulate the onset of acid secretion from parietal cells. Read et al[24] (1992) found that in ovine, the developmental pattern of gastric SS mRNA was on the contrary to that patterns of gastrin mRNA and H+/K+-ATPase mRNA. It was proposed that the gastrin, H+/K+-ATPase and SS work in synergy to initiate gastric acid secretion. In present experiment, the developmental changes of gastric SS expression negatively correlated with the changes in gastric acid secretion in general. The gastric SS mRNA decreased significantly from birth to D3, which coincided with the increase in gastric acid secretion in the same period[20]. Before weaning, the inhibition of gastric acid secretion agreed with the up-regulation of gastric SS expression. Our results confirmed that SS is the inhibiting factor for gastric acid secretion. Gastric SS inhibits both gastric acid and gastrin secretion, and it has negative effect on pepsin secretion as well[25]. We found in present experiment significant differences in the expression of gastric SS mRNA between two breeds of pigs, which was negatively correlated with the pepsin content in fundic mucosa during the sucking period. However, the correlation between gastric SS mRNA expression and pepsin content was low, suggesting that the gastric SS is not the major regulator of pepsin secretion.
In conclusion, GH and gastric SS play important roles in the regulation of porcine stomach development and gastric functions, but the interaction between GH and gastric SS is still unclear. Drago et al[19] (1997) reported that the stimulation of GH on gastric acid secretion may be related to GH down-regulating gastric SS secretion. Our unpublished data indicated that GH mRNA expression in the pituitary of Large White pigs was higher than that of Erhualian pigs. It might be presumed that the lower expression of gastric SS mRNA in Large White pigs was resulted from, to some extent, the higher expression of GH in pituitary. However, the positive correlation between gastric SS and GHr mRNA expression over the period of investigation did not support this presumption. The complex interactions between GH and gastric SS are still to be illuminated.
Footnotes
Supported by National Natural Science Foundation of China, No. 39830290
Edited by Zhang JZ
References
- 1.Lobie PE, Breipohl W, Waters MJ. Growth hormone receptor expression in the rat gastrointestinal tract. Endocrinology. 1990;126:299–306. doi: 10.1210/endo-126-1-299. [DOI] [PubMed] [Google Scholar]
- 2.Lobie PE, García-Aragón J, Waters MJ. Growth hormone (GH) regulation of gastric structure and function in the GH-deficient rat: up-regulation of intrinsic factor. Endocrinology. 1992;130:3015–3024. doi: 10.1210/endo.130.5.1374019. [DOI] [PubMed] [Google Scholar]
- 3.Delehaye-Zervas MC, Mertani H, Martini JF, Nihoul-Feketé C, Morel G, Postel-Vinay MC. Expression of the growth hormone receptor gene in human digestive tissue. J Clin Endocrinol Metab. 1994;78:1473–1480. doi: 10.1210/jcem.78.6.8200952. [DOI] [PubMed] [Google Scholar]
- 4.Nagano M, Chastre E, Choquet A, Bara J, Gespach C, Kelly PA. Expression of prolactin and growth hormone receptor genes and their isoforms in the gastrointestinal tract. Am J Physiol. 1995;268:G431–G442. doi: 10.1152/ajpgi.1995.268.3.G431. [DOI] [PubMed] [Google Scholar]
- 5.Müller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- 6.Zavros Y, Fleming WR, Shulkes A. Concurrent elevation of fundic somatostatin prevents gastrin stimulation by GRP. Am J Physiol. 1999;276:G21–G27. doi: 10.1152/ajpgi.1999.276.1.G21. [DOI] [PubMed] [Google Scholar]
- 7.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- 8.Xu RJ. Development of the newborn GI tract and its relation to colostrum/milk intake: a review. Reprod Fertil Dev. 1996;8:35–48. doi: 10.1071/rd9960035. [DOI] [PubMed] [Google Scholar]
- 9.Kato S, Tanaka A, Ogawa Y, Kanatsu K, Seto K, Yoneda T, Takeuchi K. Effect of polaprezinc on impaired healing of chronic gastric ulcers in adjuvant-induced arthritic rats--role of insulin-like growth factors (IGF)-1. Med Sci Monit. 2001;7:20–25. [PubMed] [Google Scholar]
- 10.Shen WH, Xu RJ. Stability of insulin-like growth factor I in the gastrointestinal lumen in neonatal pigs. J Pediatr Gastroenterol Nutr. 2000;30:299–304. doi: 10.1097/00005176-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Korolkiewicz RP, Tashima K, Fujita A, Kato S, Takeuchi K. Exogenous insulin-like growth factor (IGF)-1 improves the impaired healing of gastric mucosal lesions in diabetic rats. Pharmacol Res. 2000;41:221–229. doi: 10.1006/phrs.1999.0581. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay E, Chailler P, Ménard D. Coordinated control of fetal gastric epithelial functions by insulin-like growth factors and their binding proteins. Endocrinology. 2001;142:1795–1803. doi: 10.1210/endo.142.5.8140. [DOI] [PubMed] [Google Scholar]
- 13.Coerper S, Wolf S, von Kiparski S, Thomas S, Zittel TT, Ranke MB, Hunt TK, Becker HD. Insulin-like growth factor I accelerates gastric ulcer healing by stimulating cell proliferation and by inhibiting gastric acid secretion. Scand J Gastroenterol. 2001;36:921–927. doi: 10.1080/003655201750305422. [DOI] [PubMed] [Google Scholar]
- 14.Hull KL, Harvey S. Autoregulation of central and peripheral growth hormone receptor mRNA in domestic fowl. J Endocrinol. 1998;156:323–329. doi: 10.1677/joe.0.1560323. [DOI] [PubMed] [Google Scholar]
- 15.Ilkbahar YN, Wu K, Thordarson G, Talamantes F. Expression and distribution of messenger ribonucleic acids for growth hormone (GH) receptor and GH-binding protein in mice during pregnancy. Endocrinology. 1995;136:386–392. doi: 10.1210/endo.136.2.7835269. [DOI] [PubMed] [Google Scholar]
- 16.Klempt M, Bingham B, Breier BH, Baumbach WR, Gluckman PD. Tissue distribution and ontogeny of growth hormone receptor messenger ribonucleic acid and ligand binding to hepatic tissue in the midgestation sheep fetus. Endocrinology. 1993;132:1071–1077. doi: 10.1210/endo.132.3.8440172. [DOI] [PubMed] [Google Scholar]
- 17.Schnoebelen-Combes S, Louveau I, Postel-Vinay MC, Bonneau M. Ontogeny of GH receptor and GH-binding protein in the pig. J Endocrinol. 1996;148:249–255. doi: 10.1677/joe.0.1480249. [DOI] [PubMed] [Google Scholar]
- 18.Peng M, Abribat T, Calvo E, LeBel D, Palin MF, Bernatchez G, Morisset J, Pelletier G. Ontogeny of insulin-like growth factors (IGF), IGF binding proteins, IGF receptors, and growth hormone receptor mRNA levels in porcine pancreas. J Anim Sci. 1998;76:1178–1188. doi: 10.2527/1998.7641178x. [DOI] [PubMed] [Google Scholar]
- 19.Drago F, Montoneri C. Growth hormone and somatostatin interaction in the ulcerogenic effect of cysteamine in female rats. J Physiol Paris. 1997;91:127–130. doi: 10.1016/s0928-4257(97)89476-4. [DOI] [PubMed] [Google Scholar]
- 20.Xu RJ, Cranwell PD. Development of gastric acid secretion in pigs from birth to thirty six days of age: the response to pentagastrin. J Dev Physiol. 1990;13:315–326. [PubMed] [Google Scholar]
- 21.Shulkes A, Read M. Regulation of somatostatin secretion by gastrin- and acid-dependent mechanisms. Endocrinology. 1991;129:2329–2334. doi: 10.1210/endo-129-5-2329. [DOI] [PubMed] [Google Scholar]
- 22.Westbrook SL, McDowell GH, Hardy KJ, Shulkes A. Active immunization against somatostatin alters regulation of gastrin in response to gastric acid secretagogues. Am J Physiol. 1998;274:G751–G756. doi: 10.1152/ajpgi.1998.274.4.G751. [DOI] [PubMed] [Google Scholar]
- 23.Yee LF, Wong HC, Calaustro EQ, Mulvhill SJ. Roles of gastrin and somatostatin in the regulation of gastric acid secretion in the fetal rabbit. J Surg Res. 1996;63:364–368. doi: 10.1006/jsre.1996.0277. [DOI] [PubMed] [Google Scholar]
- 24.Read MA, Chick P, Hardy KJ, Shulkes A. Ontogeny of gastrin, somatostatin, and the H+/K(+)-ATPase in the ovine fetus. Endocrinology. 1992;130:1688–1697. doi: 10.1210/endo.130.3.1347009. [DOI] [PubMed] [Google Scholar]
- 25.Felley CP, O'Dorisio TM, Howe B, Coy DH, Mantey SA, Pradhan TK, Sutliff VE, Jensen RT. Chief cells possess somatostatin receptors regulated by secretagogues acting through the calcium or cAMP pathway. Am J Physiol. 1994;266:G789–G798. doi: 10.1152/ajpgi.1994.266.5.G789. [DOI] [PubMed] [Google Scholar]


