Abstract
AIM: To evaluate the potential role of Nimesulide, a selective COX-2 inhibitor, in proliferation and apoptosis of gastric adenocarcinoma cells SGC-7901.
METHODS: Cell counts and MTT assay were used to quantify the influence of Nimesulide in the proliferation of SGC-7901 cells. Transmission electron microscopy and flow cytometry were used to observe the induction of Nimesulide the apoptosis of SGC-7901 cells and influence in the distribution of cell cycle. The expression of P27kip1 protein was observed by immunocytochemical staining.
RESULTS: SGC-7901 Cells treated with Nimesulide at various concentrations exhibited a profound dose- and time-dependent reduction in the proliferation rate over the 72 h test period. The highest survival rate of the cells was 78.7%, but the lowest being 22.7%. Nimesulide induced apoptosis of the cells in a dose-dependent and non-linear manner and increased the proportion of cells in the G0/G1 phase and decreased the proportion in the S and G2/M phase of the cell cycle. Meanwhile, Nimesulide could up-regulate the expression of P27kip1 protein.
CONCLUSION: The induction of apoptosis and cell cycle arrest are both anti-proliferative responses that likely contribute to the antineoplastic action of nimesulide on SGC-7901 cells. The up-regulation of P27kip1 gene may contribute to the accumulation of these cells in the G0/G1 phase following treatment with Nimesulide. Selective COX-2 inhibitor may be a new channel of the chemoprevention and chemotherapy for gastric carcinoma.
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most commonly used medications. Recently, numerous studies have shown that NSAIDs can reduce the risk of colorectal cancer. The mechanism of action of NSAIDs is principally due to the inhibition of cyclooxygenase (COX). COX exists in two isozyme forms: COX-1 is a constitutive form of the enzyme, and COX-2, a cytokine-inducible form of the enzyme. Enhanced COX-2 expression was observed in esophageal, gastric, colorectal, liver, lung, prostate, the head and neck cancers[1-10]. These findings suggest that COX-2 may play an important role in carcinogenesis. It is thought that inhibition of COX-2 activity by NSAIDs as the antineoplastic mechanism of this class of drugs and gastrointestinal complications of using NSAIDs attribute to the inhibition of COX-1. For this reason, development of selective COX-2 inhibitors was promoted. Current, studies on the relationship between COX-2 and NSAIDs with neoplasm focused on the studies of colorectal carcinomas[11-15]. Gastric carcinoma is the most common malignancy and the first leading cause of cancer deaths in our country, its pathogenesis is not completely understood and treatment for advanced tumors are relatively poor[16-21]. Therefore, an increased understanding of pathogenesis of gastric carcinoma and developing effective treatment approaches are very important. Prophylaxis and treatment of NSAIDs for gastric carcinoma are emphasized. Epidemiological studies show that NSAIDs can also reduce the incidence rate and mortality from gastric carcinoma. Nimesulide, a selective COX-2 inhibitor, was found to be between 5 and 16 fold selective that for COX-2[22]. However, effects of Nimesulide on the growth of gastric carcinoma cells have not been studied.
In this study, we have tested the effects of Nimesulide in proliferation, apoptosis and distribution of cell cycle of SGC-7901 gastric adenocarcinoma cells, In order to seek theoretical evidences for chemoprevention action of selective COX-2 inhibitor on gastric carcinoma.
MATERIALS AND METHODS
Cell culture
Human gastric adenocarcinoma cell line SGC-7901, purchased from Shanghai Insititute of Cell Biology, Chinese Academy of Sciences, was routinely maintained in RPMI 1640 containing 10% fetal bovine serum (FBS), 100 U·ml-1 penicillin, 100 U·ml-1 streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
Cell viability assay
SGC-7901 cells were plated at 3 × 104 cells/well in 24 well plates for 24 h. At day 0, the medium was changed, incubations were continued without or with increasing concentrations of Nimesulide(50, 100, 200, 400 μmol·L-1) for 72 h. For the time course experiments, cells were incubated with or without Nimesulide and harvested at various intervals. Dead cells were removed by gentle washing with PBS, and the number of living cells after treatment was counted by a hemocytometer.
MTT assay
Cells were seeded at a density of 4 × 103 per well in 96-well plates in RPMI-1640 containing 10% FBS. After 24 h, fresh medium was added, containing Nimesulide at concentrations of 0 to 400 μmol·L-1. During 72 h incubation, MTT assay was performed every 24 h. Twenty μl of stock MTT (5 mg·ml-1) was added to each well, and the cells were further incubated at 37 °C for 4 h. The supernatant was removed and 150 μl DMSO was added to each well. A ELISA reader measured the absorbance at a wavelength of 570nm.
Transmission electron microscopy
SGC-7901 cells were treated with Nimesulide at various concentrations for 72 h. Then the cells were centrifugated and fixed in glutaraldehyde for observation of transmission electron microscopy.
Flow cytometry
SGC-7901 cells were treated with Nimesulide at a concentration of 0 to 400 μmol·L-1 for 72 h. Cells were digested by 2.5 g·L-1 trpsin, washed by 0.01M PBS, fixed by cold alcohol at 4 °C and dyed with PI (propidium iodide), and then were analyzed by flow cytometry.
Immunocytochemistry
SGC-7901 cells were added to 24-well plates with cover glass-slides at 3 × 104 cells/well, after being incubated with Nimesulide at the different concentrations of 0 to 400 μmol·L-1 for 72 h and fixed in 95% alcohol. After washing in 0.01M PBS, the cells were incubated in 0.5% H2O2 solution to inactive endogenous peroxidase, nonspecific binding was blocked with normal goat serum at room temperature for ten minutes. The cells were incubated with anti-P27kip1 protein monoclonal antibody (impromptu type, Zhong Shan Co) 2 h at 37 °C. Immunocytochemical staining for P27kip1 was performed using SP technique according to SP immunostain kit instructions (Zhong Shan Co). PBS was used as substitutes of protein antibody for negative controls. The positive signal of immunocytochemical staining showed brown particles, and distributed in the cytoplasm or nuclei. The absorbance value (A value) of positive cells was detected by image analysis software. The mean value under five random fields was regarded as the relative quantity of P27kip1 protein expression.
Statistical analysis
Data were presented as -x ± s. Statistical analysis of the data was performed using the Student’s t test, P < 0.05 was considered statistically significant.
RESULTS
Effect of Nimesulide on cell growth
Cell counts The number of viable cells following incubation with either Nimesulide or control medium, is shown in Figure 1. This figure demonstrates that control SGC-7901 cells entered a line growth and increased by approximately 7-fold within 72 h. Compared with control cultures, cultures treated with Nimesulide displayed a dose-dependent reduction of viable cell number in their proliferation rate over the 72 h test period. At 200 μmol·L-1 and 400 μmol·L-1, Nimesulide strongly inhibited the proliferation of these cells. At 400 μmol·L-1, the proliferation curve was flat over the entire 72 h.
Figure 1.
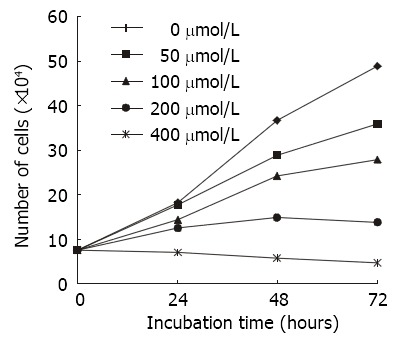
Effects of various concentration of nimesulide on the proliferation of SGC-7901 cells for different time periods analysing by cell counts (n = 3).
MTT assay In order to further investigate the growth inhibition of Nimesulide on SGC-7901 cells, cell growth was determined by MTT assay. The result was similar to cell counts. The survival rate of the control groups is regarded as 100%, and the survival rate of Nimesulide group is expressed as the% absorbance for treated wells/controls. As shown in Figure 2, the highest survival rate of the cells was 78.7%, but the lowest was 22.7%.
Figure 2.
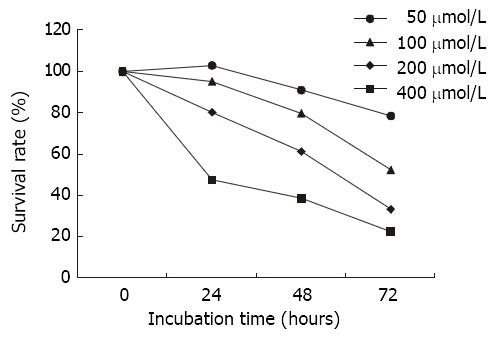
Effects of nimesulide on SGC-7901 cells analyzing the cell survival using the MTT assay.
Apoptosis of SGC-7901 cells induced by nimesulide
The TEM images of the normal SGC-7901 cells: Microvilli of SGC-7901 cells were abundant, nucleus was large and round, chromatin was disperse, and nucleolus was distinct. After treated with various concentrations of nimesulide for 72 h, disappearance of microvilli, margination and condensation of nuclear chromatin, nuclear shrinkage, the swollen organelle and cytoplasmic blebbing with maintenance of the integrity of the cell membrane (Figure 3). SGC-7901 cells were treated with various concentrations of nimesulide for 72 h, DNA content of cells was measured by PI staining and flow cytometry analysis was made to detect apoptotic cells. The apoptotic cells can be observed on a DNA histogram as subdiploid or pre-G1 peak, which was especially remarkable at the 400 μmol·L-1 concentration, and the percentage of apoptotic cells increased to 22.02 ± 1.27% of the total cell population. Nimesulide induced apoptosis of the cells in a dose-dependent and non-linear manner (Figure 4).
Figure 3.
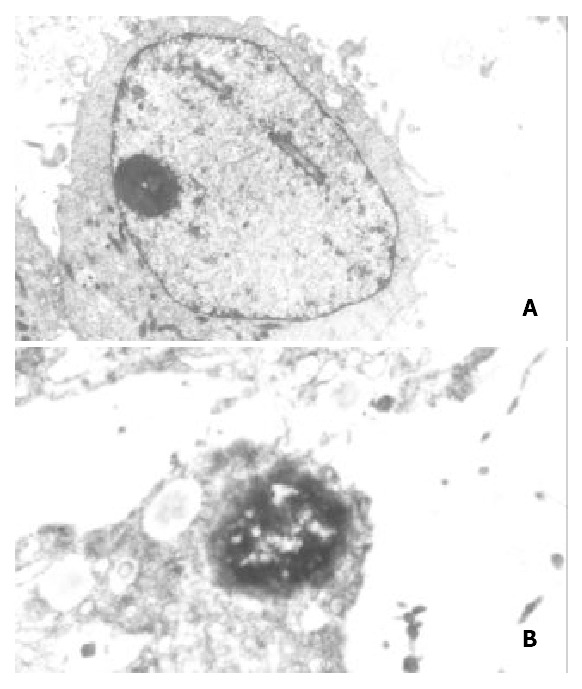
(A) control group: Microvilli of SGC-7901 cells were enrichment, nucleus was large and round, chromatin was disperse, nucleolus was distinct. TEM × 6000. (B) Treated with nimesulide at the concentration of 400 μmol/L for 72 h: Disap-pearance of microvilli, margination of nuclear chromatin, the organelle swelled up and physallization. TEM × 8000.
Figure 4.
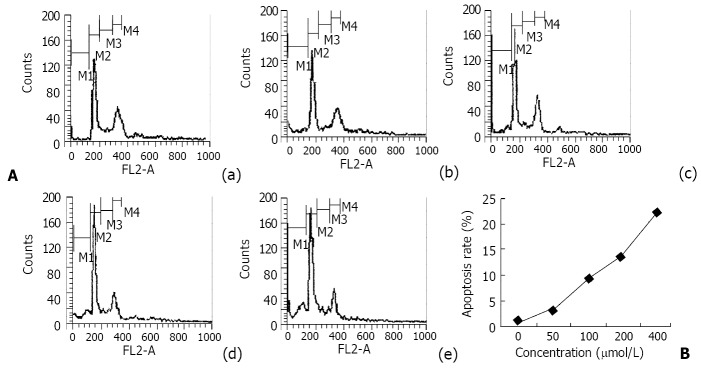
The results of flow cytometry analysis of SGC-7901 cells treated with nimesulide for 72 h and their DNA content was determined by flow cytometry. A: DNA histogram: (a) control, (b) 50 μmol/L nimesulide, (c) 100 μmol/L nimesulide, (d) 200 μmol/L nimesulide, (e) 400 μmol/L nimesulide. B: The apoptosis percentage of nimesulde-treated SGC-7901 cells.
Effect of Nimesulide on cell cycle phase distribution
We assessed the effect of Nimesulide in cell cycle phase distribution of SGC-7901 cells using flow cytometry analysis. The SGC-7901 cells incubated for 72 h in control or nimesulide medium is shown in Table 1. After 72 h treatment, Nimesulide caused a dose-dependent alteration in the cell cycle distribution of SGC-7901 cells, it increased the proportion of cells in the G0/G1 phase and decreased the proportion in the S and G2/M phases of the cell cycle.
Table 1.
Effect of Nimesulide in cell cycle progression of gastric cancer cells (-x ± s)
| Concentration (μmol/L) |
Percentage of cell cycle (%) |
||
| G0/G1 | S | G2/M | |
| 0 | 48.24 ± 2.12 | 23.51 ± 1.75 | 28.10 ± 2.17 |
| 50 | 51.14 ± 2.28 | 22.67 ± 2.30 | 26.15+1.56 |
| 100 | 57.71 ± 1.51b | 20.76 ± 1.45 | 21.52 ± 2.07a |
| 200 | 64.82 ± 2.16b | 17.06 ± 1.72b | 18.12 ± 1.71b |
| 400 | 75.89 ± 2.61b | 10.16 ± 0.79b | 13.45 ± 1.20b |
P < 0.05,
P < 0.01, vs the control.
Expression of P27kip1 protein
Before treatment with Nimesulide, the expression of P27kip1 protein was mainly located in the cytoplasm of SGC-7901 cells. After Incubated with different concentrations of Nimesulide for 72 h, P27kip1 immunoreactivity in cytoplasm was significantly increased, meanwhile nucleus exhibited positive staining. Compared with controls, absorbance value of P27kip1 positive cells was increased after Nimesulide treatment (Figure 5, Figure 6).
Figure 5.
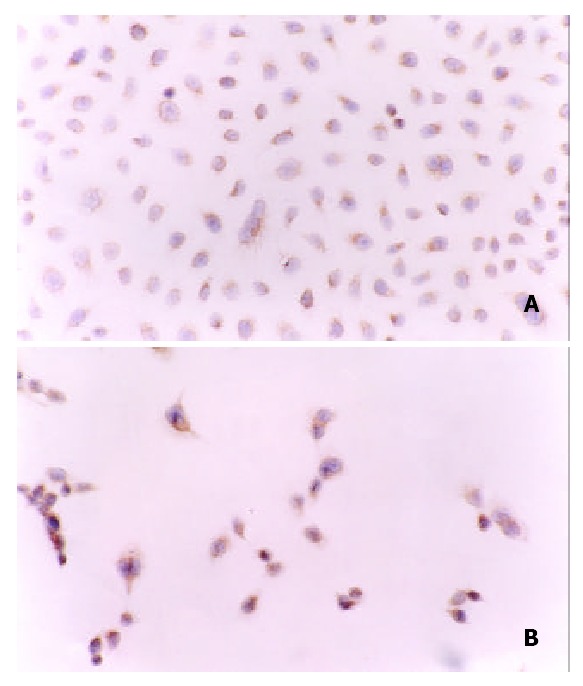
(A) Positive staing of P27kip1 protein was mainly in cytoplasm of SGC-7901 cells before treated with nimesulide. (B) Strong positive staing of P27kip1 protein was in cytoplasm and in nuclei of of SGC-7901 cells after treated with nimesulide at the concentration of 400 μmol/L. SP × 200.
Figure 6.
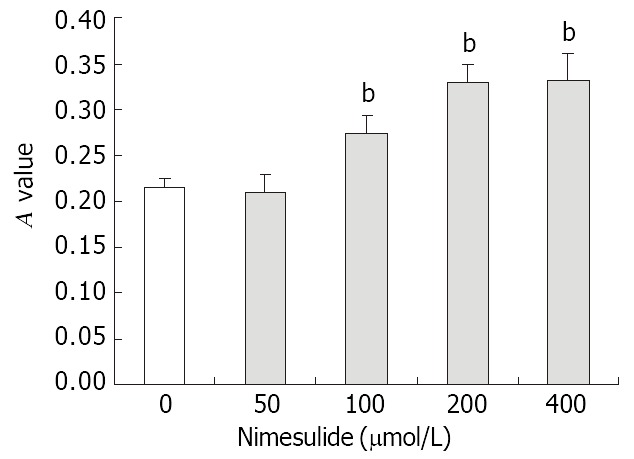
Effects of nimesulide on P27kip1 proteins expression in SGC-7901 cells. The data present -x ± s, n = 3. bP < 0.01 vs control.
DISCUSSION
Accumulating evidence indicates that NSAIDs can lower the incidence of colorectal carcinomas. The anti-neoplastic property of NSAIDs has been shown in epidemiological studies with human, clinical studies of the human disease familial adenomatous polyposis (FAP) and in experimental carcinogenesis studies with animals[23-26]. NSAIDs may be active on epithelial cancer other than colon cancer, such as esophageal[27], pancreas[28], lung[29] and breast cancer[30].
However, long-term uses of non-selective NSAIDs can lead to gastrointestinal toxicity from sustained inhibition COX-1. With the recent development of highly selective COX-2 inhibitors, an evaluation of the effectiveness of these agents in chemoprevention of cancer would be very important. Several selective COX-2 inhibitors have been tested for their possible utility as chemopreventive agents in colon cancer[31,32]. However, studies on the role of selective COX-2 inhibitor in human gastric cancer are limited.
There are a lot of sensitive tests of anti-tumor medicine in vitro. However, each test method has its merits and weak points. So we detected growth inhibitive effect of Nimesulide on SGC-7901 gastric cancer cells by two methods of cell counts and MTT assay. We found that Nimesulide has anti-proliferative effect on cultured SGC-7901 gastric cancer cells in vitro in a dose- and time-dependent manner. Our findings that Nimesulide have profound anti-proliferative effects on SGC-7901 gastric adenocarcinoma cells are in agreement with previous studies demonstrating that many of the selective COX-2 inhibitors inhibit the proliferation of tumor cell line[33-35]. A number of studies have shown that NSAIDs have an effect of growth inhibition of cancer cells, but the mechanism of the antineoplastic effect of NSAIDs is unknown. These theoretically could be related to the changes of the dynamics of cell proliferation. This hypothesis is supported by the work of Bayer et al[36], who demonstrated that indomethacin reversibly inhibits the proliferation of human fibroblasts and rat hepatoma cells and arrests them in the G1 phase of cell cycle. An explanation for the antineoplastic properties of NSAIDs was first suggested by Adolphie, who reported that certain NSAIDs were capable of inhibiting the proliferation of cultured HeLa cells by causing cell cycle arrest[37]. Since NSAIDs may inhibit proliferation of non-gastric cancer cells and change the distribution of cell cycle, we guess that it may have the similar effects on gastric cancer cells. We further studied the effects of Nimesulide on cell cycle of SGC-7901 gastric cancer cells in vitro.
We found that Nimesulide can result in changes in cell cycle distribution of SGC-7901 cells. There is an increase in the proportion of cells in G0/G1 and a relative decrease in the percentage of cells in S phase and G2/M. Shiff found[38] that NSAIDs caused colon cancer cells to accumulate in the G0/G1 phase of cell cycle. This effect was produced by influencing signaling pathways in these cells that control the levels and activity of certain cdks proteins. Goldberg et al[39] found that the G0/G1 block induced by sulindac and sulindac sulfide in HT-29 cells was associated with an initial rise, followed by an abrupt decrease in P34cdk2, an increase in P21WAF/cip1, and a reduction in mutant p53. We can speculate reasonably that Nimesulide may increase the fraction of cells with G0/G1 DNA content by arresting them in the G1 or G0 phase of the cell cycle through an effect on the molecular components that regulate cell cycle transitions.
The cell cycle is a complex process, regulated by many factors, which can be divided into three groups: cyclins; cyclin-dependent kinases(CDK); and CDK inhibitors(CDKI). p27Kip1 is a member of the Cip1/Kip1 family of CDKI and is a potential tumor suppressor gene. It possessed the ability to inhibit several cyclin-Cdk complexes in vitro but seem to target preferentially those containing Cdk2, which control progression through G1, and inhibit the G1/S transition[40,41]. Before treatment with Nimesulide, the expression of P27kip1 protein mainly was located in the cytoplasm of SGC-7901 cells. After treated with Nimesulide, P27kip1 immunoreactivity in cytoplasm was significantly increased, meanwhile nucleus exhibited positive staining. Interestingly, there was a change in subcellular localization of P27kip1 protein expression. Singh's studies suggested that the nuclear location of p27 was essential for its growth-inhibiting function[42]. So, the result of nuclear localization of P27kip1 protein after Nimesulide treatment indicates the anti-proliferation of Nimesulide. We found that Nimesulide could increase the proportion of SGC-7901 cells at G0/G1 phase and reduce the cells at S and G2/M phase. P27kip1 is generally associated with the G1 checkpoint. In our study, overexpression of P27kip1 may be associated with the accumulation of cells at G0/G1 phase after Nimesulide treatment.
It is also possible that mechanisms responsible for the anti-proliferative effects of the NSAIDs on cultured cells are multifactorial. Apoptotic cell death is another mechanism that could contribute to reduced cell growth. Additional recent reports have demonstrated that NSAIDs induced apoptosis in different tumor cells[43-48]. It is also conceivable that apoptosis might occur in the human gastric cancer cells in response to Nimesulide. To evaluate the antiproliferative mechanisms of Nimesulide, we tested the apoptosis of SGC-7901 cells induced by Nimesulide. Evidence that apoptosis was induced by Nimesulide was based on the detection of morphological changes of apoptosis and a sub-diploid peak of DNA content on flow cytometry analysis. The results showed Nimesulide also induced apoptosis in SGC-7901 cells at concentrations that affected their proliferation. Thus, apoptosis may be one of the mechanisms responsible for the inhibition of cell growth in Nimesulide-treated SGC-7901 cells. The intracellular molecular events involved in the role of COX-2 inhibitors in the induction of apoptosis are still by far unknown. It might be associated with the changes of expression of bcl-2[49] and c-myc[50].
In summary, we conclude that selective COX-2 inhibitor Nimesulide, has anti-proliferative effects in cultured SGC-7901 gastric cancer cells in vitro. The up-regulation of P27kip1 gene may contribute to the accumulation of these cells in the G0/G1 phase following treatment with Nimesulide. The induction of apoptosis and cell cycle arrest are both anti-proliferative responses that likely contribute to the inhibition of SGC-7901 cell proliferation treated with Nimesulide. The selective COX-2 inhibitor Nimesulide, may be useful as a novel approach to the treatment of gastric carcinoma without undesirable side effects.
Footnotes
Edited by Ma JY
References
- 1.Wu QM, Li SB, Wang Q, Wang DH, Li XB, Liu CZ. The expression of COX-2 in esophageal carcinoma and its relation to clinicopathologic characteristics. Shijie Huaren Xiaohua Zazhi. 2001;9:11–14. [Google Scholar]
- 2.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 3.Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- 4.Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. [PubMed] [Google Scholar]
- 5.Shen ZX, Cao G, Sun J. Expression of COX-2 mRNA in colorectal carcinoma and their relation with clinical pathological characters. Shijie Huaren Xiaohua Zazhi. 2001;9:1082–1084. [Google Scholar]
- 6.Dimberg J, Samuelsson A, Hugander A, Söderkvist P. Differential expression of cyclooxygenase 2 in human colorectal cancer. Gut. 1999;45:730–732. doi: 10.1136/gut.45.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R, et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688–696. doi: 10.1002/hep.510290355. [DOI] [PubMed] [Google Scholar]
- 8.Ochiai M, Oguri T, Isobe T, Ishioka S, Yamakido M. Cyclooxygenase-2 (COX-2) mRNA expression levels in normal lung tissues and non-small cell lung cancers. Jpn J Cancer Res. 1999;90:1338–1343. doi: 10.1111/j.1349-7006.1999.tb00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 10.Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589–596. [PubMed] [Google Scholar]
- 11.Fournier DB, Gordon GB. COX-2 and colon cancer: potential targets for chemoprevention. J Cell Biochem Suppl. 2000;34:97–102. doi: 10.1002/(sici)1097-4644(2000)77:34+<97::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, DuBois RN. Par-4, a proapoptotic gene, is regulated by NSAIDs in human colon carcinoma cells. Gastroenterology. 2000;118:1012–1017. doi: 10.1016/s0016-5085(00)70352-0. [DOI] [PubMed] [Google Scholar]
- 13.Elder DJ, Halton DE, Playle LC, Paraskeva C. The MEK/ERK pathway mediates COX-2-selective NSAID-induced apoptosis and induced COX-2 protein expression in colorectal carcinoma cells. Int J Cancer. 2002;99:323–327. doi: 10.1002/ijc.10330. [DOI] [PubMed] [Google Scholar]
- 14.Konturek PC, Bielanski W, Konturek SJ, Hartwich A, Pierzchalski P, Gonciarz M, Marlicz K, Starzynska T, Zuchowicz M, Darasz Z, et al. Progastrin and cyclooxygenase-2 in colorectal cancer. Dig Dis Sci. 2002;47:1984–1991. doi: 10.1023/a:1019652224424. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Sun YJ, Half E, Kuo MT, Sinicrope F. Cyclooxygenase-2 overexpression inhibits death receptor 5 expression and confers resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human colon cancer cells. Cancer Res. 2002;62:4903–4908. [PubMed] [Google Scholar]
- 16.Lu YY. The review and the outlook of the related gene research of Chinese stomach cancer. Shijie Huaren Xiaohua Zazhi. 1998;6(Suppl 7):10–11. [Google Scholar]
- 17.Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359–361. [Google Scholar]
- 18.Zou SC, Qiu HS, Zhang CW, Tao HQ. A clinical and long-term follow-up study of peri-operative sequential triple therapy for gastric cancer. World J Gastroenterol. 2000;6:284–286. doi: 10.3748/wjg.v6.i2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He XX, Wang JL, Wu JL, Yuan SY, Ai L. Telomerase expression, Hp infection and gastric mucosal carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2000;8:505–508. [Google Scholar]
- 20.Wang GT, Zhu JS, Xu WY, Wang Y, Zhou AG. Clinical and experimental studies on fuzheng anti-cancer granula combined with chemotherapy in advanced gastric cancer. Huaren Xiaohua Zazhi. 1998;6:214–218. [Google Scholar]
- 21.Deng DJ, E Z. Overview on recent studies of gastric carcinogenesis: human exposure of N nitrosamides. Shijie Huaren Xiaohua Zazhi. 2000;8:250–252. [Google Scholar]
- 22.Hawkey CJ. COX-2 inhibitors. Lancet. 1999;353:307–314. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- 23.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 24.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 25.Schmid K, Nair J, Winde G, Velic I, Bartsch H. Increased levels of promutagenic etheno-DNA adducts in colonic polyps of FAP patients. Int J Cancer. 2000;87:1–4. doi: 10.1002/1097-0215(20000701)87:1<1::aid-ijc1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733–1740. [PubMed] [Google Scholar]
- 27.Shureiqi I, Xu X, Chen D, Lotan R, Morris JS, Fischer SM, Lippman SM. Nonsteroidal anti-inflammatory drugs induce apoptosis in esophageal cancer cells by restoring 15-lipoxygenase-1 expression. Cancer Res. 2001;61:4879–4884. [PubMed] [Google Scholar]
- 28.Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356–4362. [PubMed] [Google Scholar]
- 29.Hida T, Leyton J, Makheja AN, Ben-Av P, Hla T, Martinez A, Mulshine J, Malkani S, Chung P, Moody TW. Non-small cell lung cancer cycloxygenase activity and proliferation are inhibited by non-steroidal antiinflammatory drugs. Anticancer Res. 1998;18:775–782. [PubMed] [Google Scholar]
- 30.Planchon P, Veber N, Magnien V, Prévost G, Starzec AB, Israël L. Evidence for separate mechanisms of antiproliferative action of indomethacin and prostaglandin on MCF-7 breast cancer cells. Life Sci. 1995;57:1233–1240. doi: 10.1016/0024-3205(95)02069-u. [DOI] [PubMed] [Google Scholar]
- 31.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 33.Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767–5772. [PubMed] [Google Scholar]
- 34.Tsubouchi Y, Mukai S, Kawahito Y, Yamada R, Kohno M, Inoue K, Sano H. Meloxicam inhibits the growth of non-small cell lung cancer. Anticancer Res. 2000;20:2867–2872. [PubMed] [Google Scholar]
- 35.Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483–487. doi: 10.3748/wjg.v8.i3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayer BM, Beaven MA. Evidence that indomethacin reversibly inhibits cell growth in the G1 phase of the cell cycle. Biochem Pharmacol. 1979;28:441–443. doi: 10.1016/0006-2952(79)90112-6. [DOI] [PubMed] [Google Scholar]
- 37.Adolphe M, Deysson G, Lechat P. Action of some steroid and non-steroid anti-inflammatory agents on the cell cycle: cytophotometric study of the DNA content. Rev Eur Etud Clin Biol. 1972;17:320–323. [PubMed] [Google Scholar]
- 38.Shiff SJ, Qiao L, Tsai LL, Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg Y, Nassif II, Pittas A, Tsai LL, Dynlacht BD, Rigas B, Shiff SJ. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle-regulatory proteins. Oncogene. 1996;12:893–901. [PubMed] [Google Scholar]
- 40.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 41.Peng D, Fan Z, Lu Y, DeBlasio T, Scher H, Mendelsohn J. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–3669. [PubMed] [Google Scholar]
- 42.Singh SP, Lipman J, Goldman H, Ellis FH, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- 43.Piazza GA, Rahm AL, Krutzsch M, Sperl G, Paranka NS, Gross PH, Brendel K, Burt RW, Alberts DS, Pamukcu R. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55:3110–3116. [PubMed] [Google Scholar]
- 44.Lu X, Xie W, Reed D, Bradshaw WS, Simmons DL. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92:7961–7965. doi: 10.1073/pnas.92.17.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arber N, DuBois RN. Nonsteroidal anti-inflammatory drugs and prevention of colorectal cancer. Curr Gastroenterol Rep. 1999;1:441–448. doi: 10.1007/s11894-999-0027-1. [DOI] [PubMed] [Google Scholar]
- 46.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 48.Qiao L, Hanif R, Sphicas E, Shiff SJ, Rigas B. Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem Pharmacol. 1998;55:53–64. doi: 10.1016/s0006-2952(97)00400-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhu GH, Wong BC, Eggo MC, Ching CK, Yuen ST, Chan EY, Lai KC, Lam SK. Non-steroidal anti-inflammatory drug-induced apoptosis in gastric cancer cells is blocked by protein kinase C activation through inhibition of c-myc. Br J Cancer. 1999;79:393–400. doi: 10.1038/sj.bjc.6690062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]


