Abstract
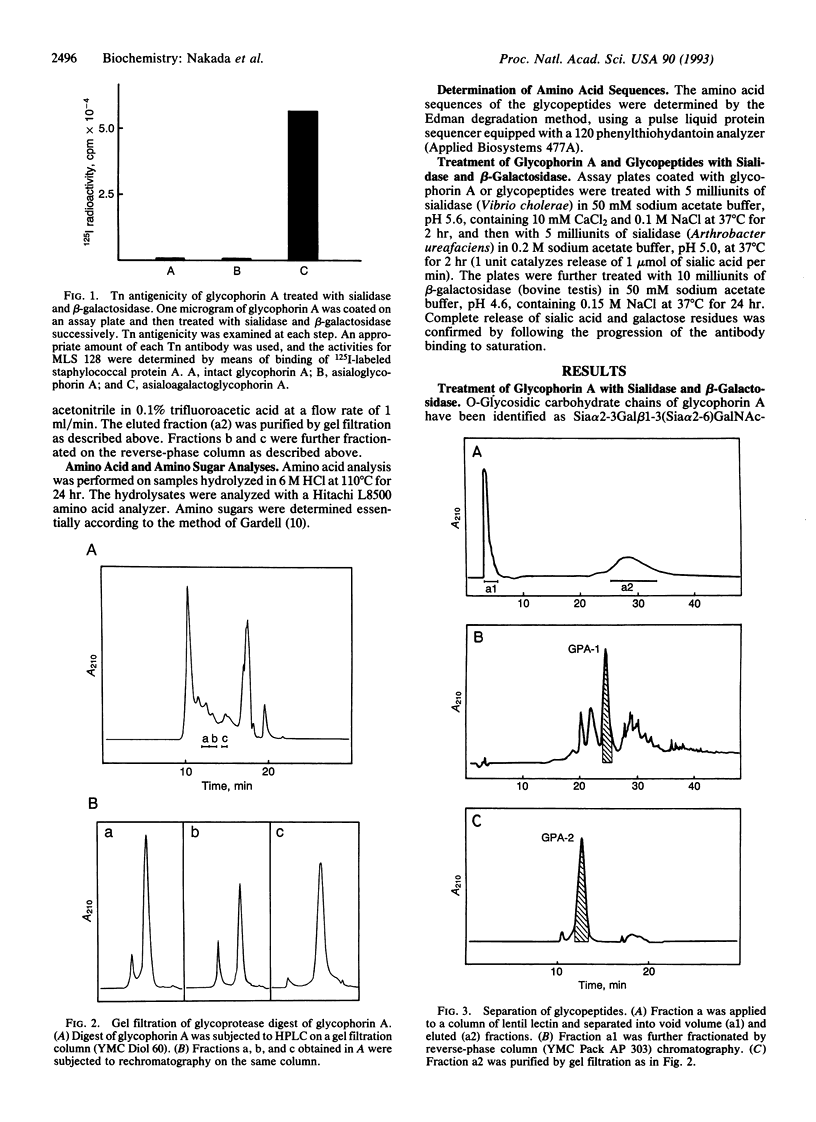
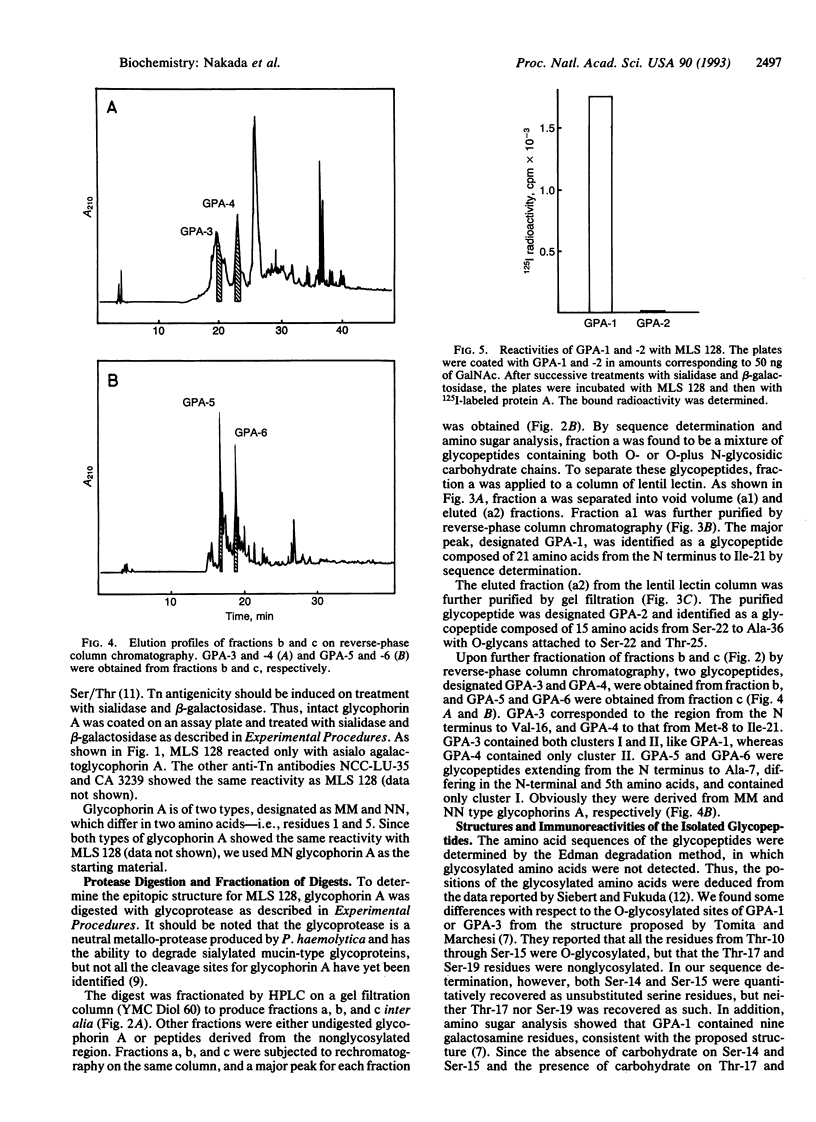
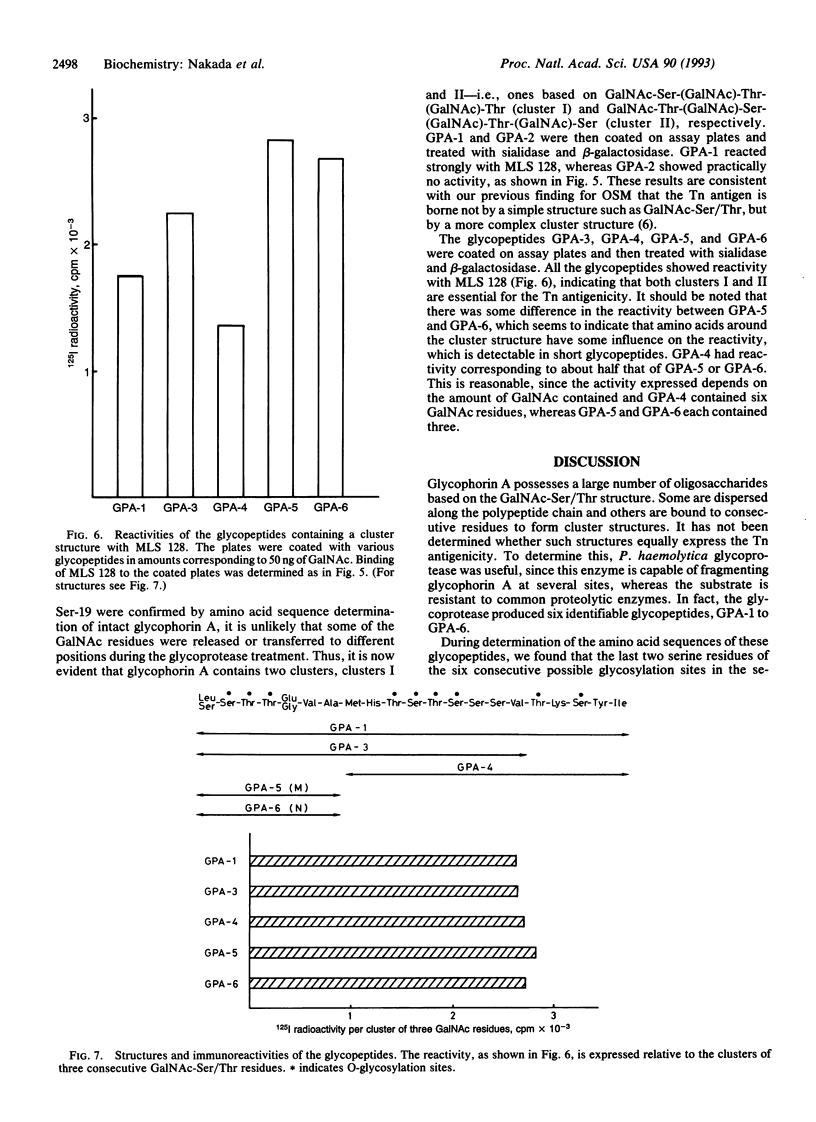
Glycophorin A was digested with glycoprotease (Pasteurella haemolytica) and the digest was fractionated by a combination of high-pressure column chromatographies to produce the glycopeptides GPA-1 to GPA-6. Sequence analysis of the glycopeptides revealed that two serine residues (Ser-14 and Ser-15) are not glycosylated, Thr-17 and Ser-19 being glycosylated instead, in disagreement with the accepted structure. The glycopeptides thus obtained were treated with sialidase and beta-galactosidase. The Tn antigenicity, as assayed by the binding to a monoclonal anti-Tn antibody (MLS 128), was found exclusively in the glycopeptides including three (cluster I) or four (cluster II) consecutive residues of GalNAc-Ser/Thr, whereas the glycopeptide (GPA-2) containing two nonconsecutive GalNAc-Ser/Thr residues had practically no Tn antigenicity. The immunoreactivities of GPA-1 and GPA-3, containing both clusters I and II, and GPA-4, containing cluster II, were 63% (calcd. 67%), 81% (calcd. 86%), and 50% (calcd. 50%), respectively, of the immunoreactivity of GPA-5 or GPA-6, containing cluster I (the average being taken as the basis), based on the reactivity per GalNAc residue. These results indicate that clusters I and II react with the antibody to the same extent. The structure consisting of three consecutive glycosylated Ser/Thr residues may be essential for Tn antigenicity in the light of previous results for ovine submaxillary mucin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdullah K. M., Udoh E. A., Shewen P. E., Mellors A. A neutral glycoprotease of Pasteurella haemolytica A1 specifically cleaves O-sialoglycoproteins. Infect Immun. 1992 Jan;60(1):56–62. doi: 10.1128/iai.60.1.56-62.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAUSSET J., MOULLEC J., BERNARD J. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (Anti-Tn). Blood. 1959 Oct;14:1079–1093. [PubMed] [Google Scholar]
- Dahr W., Uhlenbruck G., Bird G. W. Cryptic A-like receptor sites in human erythrocyte glycoproteins: proposed nature of Tn-antigen. Vox Sang. 1974;27(1):29–42. doi: 10.1111/j.1423-0410.1974.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Fukui S., Numata Y., Kurosaka A., Kitagawa H., Nakada H., Funakoshi I., Kawasaki T., Takahashi Y., Hayashi K., Yamashina I. Production of monoclonal antibodies directed against carbohydrate moieties of cell surface glycoproteins. Jpn J Cancer Res. 1988 Oct;79(10):1119–1129. doi: 10.1111/j.1349-7006.1988.tb01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. E., Nielsen C., Arendrup M., Olofsson S., Mathiesen L., Nielsen J. O., Clausen H. Broadly neutralizing antibodies targeted to mucin-type carbohydrate epitopes of human immunodeficiency virus. J Virol. 1991 Dec;65(12):6461–6467. doi: 10.1128/jvi.65.12.6461-6467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada H., Inoue M., Tanaka N., Numata Y., Kitagawa H., Fukui S., Yamashina I. Expression of the Tn antigen on T-lymphoid cell line Jurkat. Biochem Biophys Res Commun. 1991 Sep 16;179(2):762–767. doi: 10.1016/0006-291x(91)91882-d. [DOI] [PubMed] [Google Scholar]
- Nakada H., Numata Y., Inoue M., Tanaka N., Kitagawa H., Funakoshi I., Fukui S., Yamashina I. Elucidation of an essential structure recognized by an anti-GalNAc alpha-Ser(Thr) monoclonal antibody (MLS 128). J Biol Chem. 1991 Jul 5;266(19):12402–12405. [PubMed] [Google Scholar]
- Numata Y., Nakada H., Fukui S., Kitagawa H., Ozaki K., Inoue M., Kawasaki T., Funakoshi I., Yamashina I. A monoclonal antibody directed to Tn antigen. Biochem Biophys Res Commun. 1990 Aug 16;170(3):981–985. doi: 10.1016/0006-291x(90)90488-9. [DOI] [PubMed] [Google Scholar]
- Pallant A., Eskenazi A., Mattei M. G., Fournier R. E., Carlsson S. R., Fukuda M., Frelinger J. G. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1328–1332. doi: 10.1073/pnas.86.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller V., Piller F., Fukuda M. Biosynthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J Biol Chem. 1990 Jun 5;265(16):9264–9271. [PubMed] [Google Scholar]
- Siebert P. D., Fukuda M. Isolation and characterization of human glycophorin A cDNA clones by a synthetic oligonucleotide approach: nucleotide sequence and mRNA structure. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1665–1669. doi: 10.1073/pnas.83.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer G. F. T and Tn, general carcinoma autoantigens. Science. 1984 Jun 15;224(4654):1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]



