Abstract
AIM: To compare the effect of nimesulide or/and 5-fluorouracil (5-FU) on tumor growth inhibition and apoptosis in mice with the implanted hepatoma and to observe their possible interactions.
METHODS: The inhibitory effects on tumor growth was evaluated by inhibition rate. Apoptosis was assessed by the ultrastructural, flow cytometry features and the DNA ladder demonstrated by agarose gel electrophoresis. PGE2 level was determined by radioimmunoassay. Expression levels of c-jun, c-fos and p53 were evaluated by western blotting.
RESULTS: Nimesulide or 5-FU alone inhibited the growth of hepatoma, while a synergistic effect was observed for a combined use of both. More pronounced morphologic changes for tumor cell apoptosis and the DNA ladder were found for the latter treatment. Expression levels of c-jun and p53 were found to be elevated for the tumors from mice treated with nimesulide and 5-FU comparing to those with either of them, but a reduced PGE2 level was observed only for the treatment with nimesulide. No change was detected on c-fos expression.
CONCLUSION: Nimesulide and 5-FU appear to have synergistic effects for the growth inhibition and apoptosis induction. Both were found to be overexpressed in p53 and c-jun proteins, rather than that of c-fos, associations with the resulted apoptosis.
INTRODUCTION
5-Fluorouracil (5-FU) is widely used in the chemotherapies for many malignancies including gastrointestinal, breast and head and neck cancers. Its intravenous or intra-arterial delivery was often used as a monotherapy or in combination with other chemotherapeutic agents. It can cause DNA damage and induces apoptosis in some cancers[1-3]. Further works are being done to potentiate 5-FU cytotoxicity by improving the dosing schedule and biochemical modulation of 5-FU. However, one of the major hindrances for its clinical application is the development of resistance by neoplastic cells, being innate or acquired for 5-FU[4]. 5-FU, therefore is often used in combination with other anti-cancer therapies in the treatment of solid tumors. Experimental have indicated that pre-exposure of MCF-7 breast cancer cells to paclitaxel followed by 5-FU was preferable[5].
During the last 20 years, accumulating data have shown an anti-proliferative effect of non-steroidal anti-inflammatory drugs (NSAIDs) in a variety of malignant cell lines[6-12]. PGs and their synthesizing enzyme COX-2 was suggested to be involved in carcinogenesis[13]. Reducing the COX-2 and PGE2 expression proved to be an alteration approach to inhibit tumor growth. Some selective COX-2 inhibitors may be of the therapeutic significance. Nimesulide, a sulfonanilide class COX-2 inhibitor, can bind specifically to the large catalytic moiety of COX-2, with much less adverse effects on the gastrointestinal tract compared to the non-specific NSAIDs[14]. In our previous studies, nimesulide was found to reduce the COX-2 and PGE2 levels in association with the resulted apoptosis in mice implanted Hepatoma. In the present study a synergistic effect was observed for nimesulide and 5-FU on the growth and apoptosis of mouse hepatoma, and its possible molecular mechanism(s) was also investigated.
MATERIALS AND METHODS
Drugs and reagents
5-FU was purchased from Dongrui Pharmaceutical Co (Jiangsu, China). Nimesulide and other chemicals were purchased from Sigma Chemical Co (St. Louis, MO, USA) and suspended in PBS (pH7.2). Monoclonal anti-mouse antibodies were supplied from Santa Cruz.
Animals and tumor model
Kunming breed mice, with their body weights ranging from 18 g to 22 g, were used. Subcutaneous inoculation was conducted in the flank with 1 × 107/ml mouse hepatoma cell line HepA[15]. The mice were bred on standard mouse chow and tap water under standard conditions. Nimesulide was given ig daily in a volume of 0.2 ml. 5-FU was injected ip every three days. Mice were randomly separated into five groups, 10 mice each: vehicle control, nimesulide 20 mg/kg, 5-FU 10 mg/kg, 5-FU 20 mg/kg, and nimesulide 20 mg/kg plus 5-FU 10 mg/kg. Throughout the experimentation period, food and water was available to animals ad libitum. After 21d test period animals were killed by cervical dislocation. Tumor was weighed, and fixed or minced using a mortar and pestle.
Tumor inhibition rate
Tumor growth was evaluated by the inhibition rate as assessed by the formula: IR = (1 - T/C) × 100%. IR represents inhibition rate, T and C indicate the mean tumor weights in the treatment and control groups, respectively.
Morphological analysis of apoptosis
Morphological changes indicative of apoptosis were detected by electron microscopy (EM). Briefly, dissected tumor samples were fixed with 20 ml/L glutaraldehyde in PBS for 1 h. After being washed with buffer for 3 times, the samples were post-fixed in OsO4 in cacodylate buffer for 1 h. Subsequently, the samples were dehydrated in ethanol and embedded in epoxy resin (Agar 100). Thin sections (70nm) were stained in uranyl acetate and Reynolds lead citrate and viewed at 75 kV in an electron microscope (JEM-100CX 11/T).
Flow cytometry
Cell suspension was fixed in ice-cold 70% ethanol in PBS, and stored at -20 °C. Prior to analysis, the cells were washed and resuspended in PBS and incubated with RNase I 1 g/L and propidium iodide 20 mg/mL at 37 °C for 30 min. The analysis of samples was performed using a flow cytometer.
DNA ladder visualication
Pulverized tumors were lysed with 150 μl hypotonic lysis buffer (10 mmol/L EDTA, 0.5% Triton X-100 in 1 mmol/L Tris-HCl, pH7.4) for 15 min on ice and were precipited with 2.5% polyethyleneglycol and 1 mol/L NaCl for 15 min at 4%. After centrifugation at 16000 g for 10 min at room temperature, the supernatant was incubated in the presence of proteinase K (0.3 g/L) at 37 °C for one hour and precipitated with isopropanol at -20 °C. After centrifugation, each pellet was dissolved in 10 μl of Tris-EDTA (pH7.6) and electrophoresed on a 1.5% agarose gel containing ethidium bromide 2 mg/mL. DNA fragments were visualized by ultraviolet transillumination.
Detection of prostaglandin E2 (PGE2) by radioimmunoassay (RIA)
The amounts of immunoreactive PGE2 in samples of solid tumor from mice were determined by RIA using a kit (Institute of Blood, Suzhou Medical university, China) following to the manufacturer’s instructions. Briefly, to each polypropylene RIA tube, 100 μl of anti-PGE2, 125I-PGE2, and PGE2 or a sample were added. Immune complexes were precipitated 24 h later with 1 ml of polyethylene glycol solution, and the radioactivity in the precipitate was determined by a gamma counter. There was no nonspecific interference of the assay by the components of the sample. Assays were carried out in triplicate and the mean and standard deviations were obtained.
Western blotting for c-jun, c-fos, and p53
Samples were extracted with a lysis buffer (1% Triton-100, 50 mM NaCl, 50 mM NaF, 20 mM Tris pH7.4, 1 mM EDTA, 1 mM EGTA, 1 mM sodium vanadate, 0.2 mM phenylmethylsulfonyl fluoride, and 0.5% Nonidet P-40). The cell lysates, 60 mg each, were solubilized in sample buffer by boiling for 5 min, and then subjected to 10% SDS-PAGE. The resolved proteins were electrotransfered onto a nitrocellulose filter. The filter was incubated consecutively with a primary antibody and with peroxidase-conjugated anti-mouse immunoglobulin G (IgG). The reactions were visualized using the ECL detection system.
RESULTS
Tumor inhibition rate
Administrated nimesulide and 5-FU suppressed tumor growth of the implanted hepatoma. The growth inhibitory rate was about 30% after treatment with nimesulide (20 mg/kg). Application of 5-FU (20 mg/kg) also resulted in a marked inhibitory effect on the growth of the implanted tumors, while no significant effect was observed with the lower dose (10 mg/kg). It showed a greater inhibitory effect with a combined use of nimesulide and 5-FU than with either of them (Table 1).
Table 1.
Effect of nimesulide and 5-FU on tumor inhibition in mouse implanted hepatomas (n = 10, -x ± s)
| Group | X1 | R1 | X2 | R2 |
| Control | 3.67 ± 1.7 | - | 3.69 ± 1.6 | - |
| Nimesulide 20 mg/kg | 2.59 ± 0.9c | 30% | 2.56 ± 0.9c | 31% |
| 5-FU 10 mg/kg | 2.63 ± 0.9c | 28% | 2.61 ± 1.0c | 29% |
| 5-FU 20 mg/kg | 1.11 ± 0.6ab | 70% | 1.01 ± 0.6ab | 73% |
| Nimesulide 20 mg/kg + 5-FU 10 mg/kg | 0.43 ± 0.2a | 88% | 0.49 ± 0.2a | 87% |
P < 0.01 vs control,
P < 0.05,
P < 0.01 vs nimesulide + 5-FU. X1: the first mean tumor weights; R1: the first mean inhibition rates; X2: the second mean tumor weights; R2: the second mean in-hibition rates.
Effect of nimesulide and 5-FU on apoptosis in HepA cells
The tumor growth-inhibiting effect was found to be associated with apoptosis as demonstrated by EM, and characterized by cell shrinkage and blebbing, condensation of unclear chromatin and nuclear fragment (Figure 1). Administration of 20 mg/kg of nimesulide or 20 mg/kg of 5-FU alone resulted in slight increases in apoptotic cell numbers, whereas the combined use of 20 mg/kg of nimesulide and 10 mg/kg of 5-FU caused markedly increased number of apoptotic cells.
Figure 1.
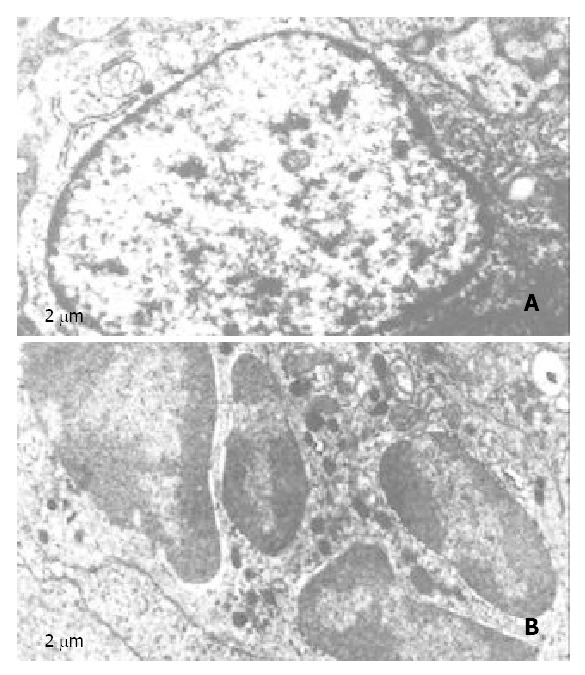
Electro micrographs of nimesulide plus 5-FU treated mice hepatoma. A, control; B, nimesulide plus 5-FU.
Agarose gel electrophoresis showed DNA ladder in the hepatoma tissue 21 d after the treatment. Compared to those caused by nimesulide or 5-FU alone, the DNA ladder appeared more pronounced for the combined the treatment with 5-FU (10 mg/kg) and nimesulide. (Figure 2).
Figure 2.
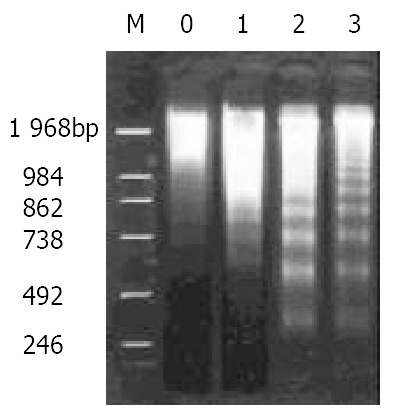
DNA ladder pattern of hepatoma tissues as demon-strated by agarose gel 1.5% electrophoresis. M, DNA markers; lanes 0-3, control, nimesulide 20 mg/kg, 5-FU 20 mg/kg, nimesulide 20 mg/kg + 5-FU 10 mg/kg.
The pro-apoptotic effect of nimesulide was further confirmed by flow cytometry. After treatment with nimesulide and/or 5-FU for 21 d, the profiles of the DNA histograms were different from control (Figure 3). With 5-FU and nimesulide administered, a striking SubG1 peak was found and the apoptotic index increased from (4.3 ± 1.5)% to (72 ± 2.5)% (Table 2).
Figure 3.
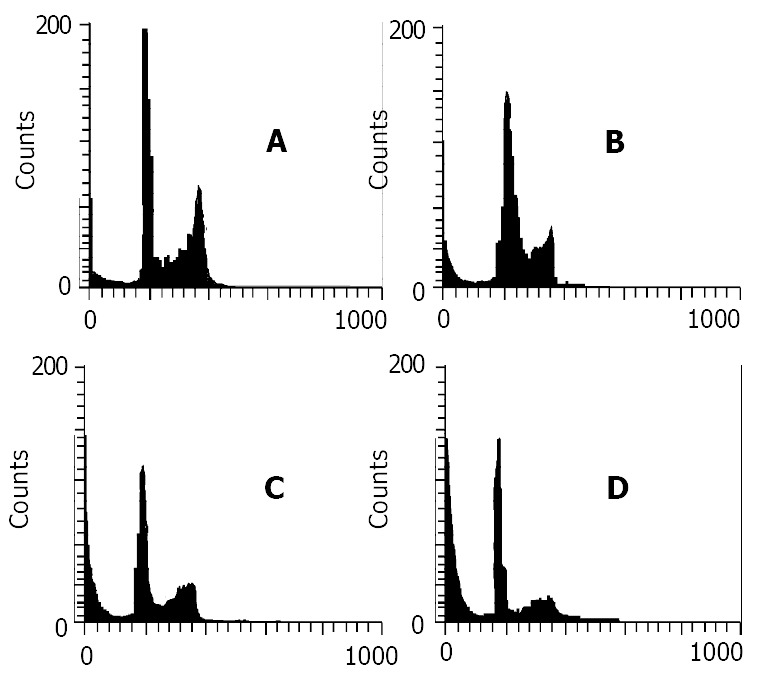
Data of flow cytometry of mouse hepatoma without any treatment as a control (A), or following 21 d treatment with nimesulide 20 mg/kg (B), 5-FU 20 mg/kg (C) and nimesulide 20 mg/kg + 5-FU 10 mg/kg (D). The sub-G1 peak to the left of the G1 peak represents apoptotic cells.
Table 2.
Apoptotic indices of nimesulide and 5-FU in mouse hepatoma tissues after the treatment with determined by FCM. n = 3, -x ± s
| Control | Nimesulide (20 mg/kg) | 5-FU (20 mg/kg) | Nimesulide (20 mg/kg) + 5-FU (10 mg/kg) | |
| Apoptotic Index | 4.3 ± 1.5 | 29.0 ± 2.6ac | 49.0 ± 2.0ac | 72.0 ± 2.5a |
P < 0.01 vs control;
P < 0.01 vs nimesulide + 5-FU.
Expression of PGE2
The PGE2 contents of the implanted hepatoma tissue was 636.67 ± 17.9 ng/ml after treatment with 20 mg/kg of 5-FU, being at the same level as in the control group. However, it was significantly reduced with nimesulide administered, alone or combined with 5-FU (10 mg/kg) (Figure 4). Apparently, the mechanisms for the growth inhibition by nimesulide and 5-FU may be different, the former being associated with, and the latter independent of, PGE2 content in tumor tissue.
Figure 4.
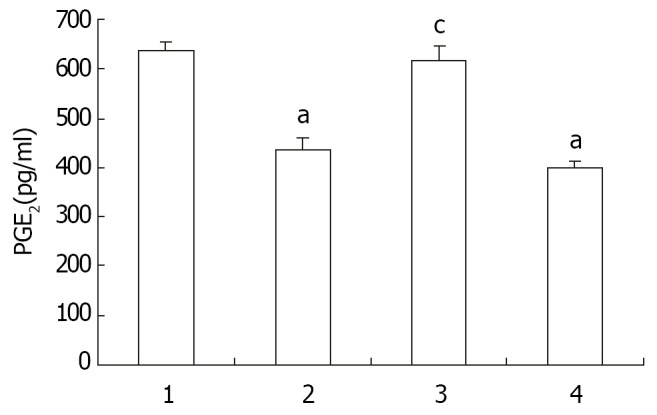
Effect of Nimesulide and 5-FU on PGE2 content in mouse hepatoma. Each column is the mean ± SD of the sample. 1-4, control, nimesulide 20 mg/kg, 5-FU 20 mg/kg, and nimesulide 20 mg/kg + 5-FU 10 mg/kg. n = 3. -x ± s. aP < 0.01 vs control, cP < 0.01 vs nimesulide + 5-FU.
Effect on c-jun, c-fos, and p53 expressions
Western blotting showed that nimesulide or 5-FU induced expression of c-jun and p53 in mouse hepatoma. Treatment with nimesulide plus 5-FU resulted in up regulation of the expression of these genes, while it was not for the c-fos expression. Treatment with of 5-FU, 20 mg/kg in dose, also gave rise to expression of c-jun and p53. However, the effect of the combined application of nimesulide and a low dose of 5-FU (10 mg/kg) was more pronounced compared to those of 5-FU or nimesulide alone (Figure 5).
Figure 5.
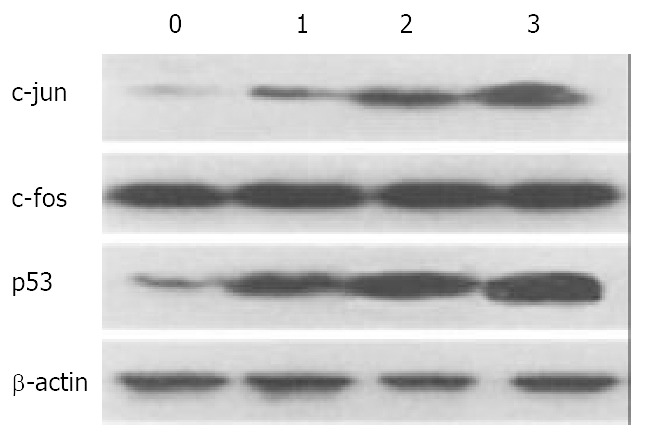
Effect of nimesulide and 5-FU on expression of c-fos, c-jun, and p53 in mouse hepatoma. 0-3, control, nimesulide 20 mg/kg, 5-FU 20 mg/kg, nimesulide 10 mg/kg + 5-Fu 10 mg/kg. The β-actin was used as an intrinsic reference molecule.
DISCUSSION
Previous observations showed that indomethacin, a non-selective COX inhibitor, improved hematopoietic recovery following 5-FU. 5-FU toxicity, determined as loss of colony-forming ability, increased with its dose, and indomethacin caused a generalized alleviation of 5-FU toxicity, but only if given concurrently with 5-FU[16]. By the subsequent treatment with interferon γ, indomethacin, and phenylbutyrate in human colon carcinoma cells, the recurrence of colon carcinomas, occurring frequently between cycles of 5-FU treatment, can be prevented or at least effectively retarded[17].
The present study demonstrated that both nimesulide and 5-FU can inhibit the tumor growth and induce apoptosis, the combination of nimesulide and 5-FU being more effective compared to that of 5-FU or nimesulide alone. Apparently, apoptosis may be associated with the growth-inhibiting effect of 5-FU combined with nimesulide, and a synergistic effect was observed for these two agents. As demonstrated in this report, 5-FU (20 mg/kg) or nimesulide alone stimulated c-jun and p53 expression in hepatoma at a low level, this effect being greatly enhanced its combination with nimesulide.
The mechanism involved in growth inhibition and cell apoptosis by combination nimesulide with 5-FU treatment remains obscure. The ultimate outcome of tumor treatment with anti-cancer agents is the effect of many intrinsic and extrinsic factors, functioning independently or cooperatively[18-20]. In the present study the possible role of c-fos and c-jun protein on tumor growth and apoptosis was evaluated[21]. The well-characterized substrate of JNK is c-Jun, a component of the AP-1 transcription factor[22,23]. Expression of c-jun was repeatedly shown to be involved in apoptosis induction and growth inhibition of many anti-cancer agents. In the present study, the level of c-jun was up-regulated by the treatment with nimesulide or 5-FU alone, and that with nimesulide plus 5-FU were even more effective. No significant change was observed in the c-fos levels in tumors after treatment with nimesulide or 5-FU alone, or their combined use, indicating that the anti-cancer effects on mouse hepatoma were associated with c-jun rather than c-fos.
The tumor suppressor gene, p53, is a key component in regulating cell cycle progression[24]. Strikingly, many apoptotic stimuli are known to be p53-dependent[25-30]. For example, p53 is required for the bleomycin-induced cerebellar granule cell death, following c-jun protein overexpression[31]. MIF, a local proinflammatory cytokine, is capable of functionally inactivating p53. The observation provides a mechanistic link between inflammation and cancer[32]. Moreover it is well known that selective COX-2 inhibitors have anti-inflammation and anti-cancer effects through inhibition COX-2. The elevated p53 level in mouse hepatoma treated with nimesulide alone or plus 5-FU may be due to inhibit COX-2 and PGE2 level. Furthermore 5-FU induces cell apoptosis in a p53-dependent way[33]. Our results raise the possibility that p53 is required for c-jun-dependent apoptosis induced by nimesulide or/and 5-FU treatment. It is possible that theses two drugs increase p53 expression though different intracellular pathway for activating cell death processes.
In conclusion, a synergistic effect was observed between nimesulide and 5-FU on apoptosis in murine hepatoma, indicating its potential application in the management of human hepatocellular carcinomas.
Footnotes
Supported by National Natural Science Foundation of China, No. 39770300, and the Overseas Chinese Affairs Office of the State Council Foundation, No. 98-33
Edited by Su Q
References
- 1.Yoneda K, Yamamoto T, Osaki T. p53- and p21-independent apoptosis of squamous cell carcinoma cells induced by 5-fluorouracil and radiation. Oral Oncol. 1998;34:529–537. doi: 10.1016/s1368-8375(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 2.Warr JR, Bamford A, Quinn DM. The preferential induction of apoptosis in multidrug-resistant KB cells by 5-fluorouracil. Cancer Lett. 2002;175:39–44. doi: 10.1016/s0304-3835(01)00721-2. [DOI] [PubMed] [Google Scholar]
- 3.Mizutani Y, Nakanishi H, Yoshida O, Fukushima M, Bonavida B, Miki T. Potentiation of the sensitivity of renal cell carcinoma cells to TRAIL-mediated apoptosis by subtoxic concentrations of 5-fluorouracil. Eur J Cancer. 2002;38:167–176. doi: 10.1016/s0959-8049(01)00339-2. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima M, Fujioka A, Uchida J, Nakagawa F, Takechi T. Thymidylate synthase (TS) and ribonucleotide reductase (RNR) may be involved in acquired resistance to 5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur J Cancer. 2001;37:1681–1687. doi: 10.1016/s0959-8049(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 5.Grem JL, Nguyen D, Monahan BP, Kao V, Geoffroy FJ. Sequence-dependent antagonism between fluorouracil and paclitaxel in human breast cancer cells. Biochem Pharmacol. 1999;58:477–486. doi: 10.1016/s0006-2952(99)00099-4. [DOI] [PubMed] [Google Scholar]
- 6.Smith ML, Hawcroft G, Hull MA. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000;36:664–674. doi: 10.1016/s0959-8049(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 7.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HL, Chen DD, Li XH, Zhang HW, Lü JH, Ren XD, Wang CC. JTE-522-induced apoptosis in human gastric adenocarcinoma [correction of adenocarcinoma] cell line AGS cells by caspase activation accompanying cytochrome C release, membrane translocation of Bax and loss of mitochondrial membrane potential. World J Gastroenterol. 2002;8:217–223. doi: 10.3748/wjg.v8.i2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li HL, Zhang HW, Chen DD, Zhong L, Ren XD, St-Tu R. JTE-522, a selective COX-2 inhibitor, inhibits cell proliferation and induces apoptosis in RL95-2 cells. Acta Pharmacol Sin. 2002;23:631–637. [PubMed] [Google Scholar]
- 10.Zhu GH, Wong BC, Ching CK, Lai KC, Lam SK. Differential apoptosis by indomethacin in gastric epithelial cells through the constitutive expression of wild-type p53 and/or up-regulation of c-myc. Biochem Pharmacol. 1999;58:193–200. doi: 10.1016/s0006-2952(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 11.Eli Y, Przedecki F, Levin G, Kariv N, Raz A. Comparative effects of indomethacin on cell proliferation and cell cycle progression in tumor cells grown in vitro and in vivo. Biochem Pharmacol. 2001;61:565–571. doi: 10.1016/s0006-2952(00)00578-5. [DOI] [PubMed] [Google Scholar]
- 12.Piqué M, Barragán M, Dalmau M, Bellosillo B, Pons G, Gil J. Aspirin induces apoptosis through mitochondrial cytochrome c release. FEBS Lett. 2000;480:193–196. doi: 10.1016/s0014-5793(00)01922-0. [DOI] [PubMed] [Google Scholar]
- 13.Munkarah AR, Morris R, Baumann P, Deppe G, Malone J, Diamond MP, Saed GM. Effects of prostaglandin E(2) on proliferation and apoptosis of epithelial ovarian cancer cells. J Soc Gynecol Investig. 2002;9:168–173. [PubMed] [Google Scholar]
- 14.Denda A, Kitayama W, Murata A, Kishida H, Sasaki Y, Kusuoka O, Tsujiuchi T, Tsutsumi M, Nakae D, Takagi H, et al. Increased expression of cyclooxygenase-2 protein during rat hepatocarcinogenesis caused by a choline-deficient, L-amino acid-defined diet and chemopreventive efficacy of a specific inhibitor, nimesulide. Carcinogenesis. 2002;23:245–256. doi: 10.1093/carcin/23.2.245. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Qin SK, Chen BA, Chen HY. Experimental study on antitumor effect of arsenic trioxide in combination with cisplatin or doxorubicin on hepatocellular carcinoma. World J Gastroenterol. 2001;7:702–705. doi: 10.3748/wjg.v7.i5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djordjevic B, Lange CS, Schwartz MS, Rotman M. Clonogenic inactivation of colon cancer-derived cells treated with 5-fluorouracil and indomethacin in hybrid spheroids. Acta Oncol. 1998;37:735–739. doi: 10.1080/028418698430124. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Horvath CM, Waxman S. Regrowth of 5-fluorouracil-treated human colon cancer cells is prevented by the combination of interferon gamma, indomethacin, and phenylbutyrate. Cancer Res. 2000;60:3200–3206. [PubMed] [Google Scholar]
- 18.Xu CT, Huang LT, Pan BR. Current gene therapy for stomach carcinoma. World J Gastroenterol. 2001;7:752–759. doi: 10.3748/wjg.v7.i6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen ZY, Shen J, Li QS, Chen CY, Chen JY, Yi Z. Morphological and functional changes of mitochondria in apoptotic esophageal carcinoma cells induced by arsenic trioxide. World J Gastroenterol. 2002;8:31–35. doi: 10.3748/wjg.v8.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Li JY, Wu J, Meng L, Shou CC. Mycoplasma infections and different human carcinomas. World J Gastroenterol. 2001;7:266–269. doi: 10.3748/wjg.v7.i2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333(Pt 2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 23.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs SY, Adler V, Pincus MR, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci USA. 1998;95:10541–10546. doi: 10.1073/pnas.95.18.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajnakova A, Moochhala S, Goh PM, Ngoi S. Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer Lett. 2001;172:177–185. doi: 10.1016/s0304-3835(01)00645-0. [DOI] [PubMed] [Google Scholar]
- 26.Li HL, Ren XD, Zhang HW, Ye CL, Lv JH, Zheng PE. Synergism between heparin and adriamycin on cell proliferation and apoptosis in human nasopharyngeal carcinoma CNE2 cells. Acta Pharmacol Sin. 2002;23:167–172. [PubMed] [Google Scholar]
- 27.Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792–796. doi: 10.3748/wjg.v8.i5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779–782. doi: 10.3748/wjg.v7.i6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araki T, Enokido Y, Inamura N, Aizawa S, Reed JC, Hatanaka H. Changes in c-Jun but not Bcl-2 family proteins in p53-dependent apoptosis of mouse cerebellar granule neurons induced by DNA damaging agent bleomycin. Brain Res. 1998;794:239–247. doi: 10.1016/s0006-8993(98)00231-5. [DOI] [PubMed] [Google Scholar]
- 32.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pritchard DM, Jackman A, Potten CS, Hickman JA. Chemically-induced apoptosis: p21 and p53 as determinants of enterotoxin activity. Toxicol Lett. 1998;102-103:19–27. doi: 10.1016/s0378-4274(98)00273-2. [DOI] [PubMed] [Google Scholar]


