Abstract
AIM: To determine the association of hOGG1 (8-oxoguanine glycosylase I, OGG1) polymorphism of Ser326Cys substitution with colon cancer risk and possible interaction with known environmental risk factors.
METHODS: A case-control study with 125 colon cancer cases and 247 controls was conducted.
RESULTS: There was no major difference in Ser326Cys genotype distribution between cases and controls. The meat intake tended to increase the odds ratio for colon cancer with an OR of 1.72 (95% confidence interval; CI = 1.12-2.76). Such tendency was more prominent in Cys/Cys carriers (OR = 4.31, 95%CI = 1.64-11.48), but meat intake was not a significant risk factor for colon cancer in Ser/Ser or Ser/Cys carriers. The OR for colon cancer was elevated with marginal significance in smokers who were Cys/Cys carriers (OR = 2.75, 95%CI = 1.07-7.53) but not in Ser/Ser or Ser/Cys carriers.
CONCLUSION: These results suggest that the hOGG1 Ser326Cys polymorphism is probably not a major contributor to individual colon cancer susceptibility overall, but the Cys/Cys genotype may alter the impact of some environmental factors on colon cancer development.
INTRODUCTION
Reactive oxygen species are formed continuously in living cells through both endogenous and exogenous processes. The reaction of reactive oxygen species results in various forms of both cellular and DNA damage[1]. Oxidative damage to DNA is thought to cause mutations, which in turn can activate oncogenes or inactivate tumor suppressor genes and may finally lead to carcinogenesis[2-4]. 7,8-Dihydro-8-oxoguanine (8oxoG) is one of the most important lesions produced in DNA by oxygen radical forming agents. Due to its mispairing with deoxyadenosine, it causes mutagenic transversion of G:C to T:A in vitro and in vivo[5,6]. The hOGG1 gene encodes a DNA glycosylase/AP-lyase that catalyzes the removal of 8oxoG adducts as part of the base excision repair pathway. The hOGG1 gene is expressed as multiple alternatively-spliced isoforms with only the 1α-form containing a nuclear localization signal[7,8]. Previous studies have revealed the presence of several polymorphisms at the hOGG1 locus. A C/G polymorphism at position of 1245 in the 1a-specific exon 7 of the hOGG1 gene results in an amino acid substitution from serine to cysteine in codon 326[9]. Although no difference in catalytic activity was observed between Ser326 and Cys326 variants in some studies[10,11], the hOGG1 protein encoded by the Ser326 allele exhibited substantially higher activities than the Cys326 variants in an in vitro Escherichia coli complementation activities assay[9].
Several studies have suggested that Cys326 type allele is associated with increased risk for lung[12], esophageal[13] and a subset of stomach cancer[14]. The possible causal relationship between oxygen free radicals and cancer development has been reported mainly in organs under a high burden of oxygen free radicals, including the lung and oro-laryngeal cancer associated with smoking history. Among the smokers, the Cys/Cys carriers who are supposed to have decreased capability in coping with the oxidative DNA damage tended to be more susceptible to lung cancer than the Ser/Ser or Ser/Cys carriers. The colon may be continuously exposed to the attack of oxygen free radicals. The relatively high concentrations of iron in feces, together with the ability of bile pigments to act as iron chelators that support Fenton chemistry, may very well permit efficient hydroxyl radical (-OH) generation from superoxide and hydrogen peroxide produced by bacterial metabolism[15]. The abundant free radical generation in colon supports the free radical related colon carcinogenesis.
We previously demonstrated that the impact of genetic defect on carcinogenesis is modulated by an environmental risk factor in hereditary nonpolyposis colorectal cancer patients[16]. In addition, we tried to delineate the relationship between hOGG1 enzyme activity and the allele type harbored in Ser326Cys polymorphism in vivo[17]. However, any clear conclusion was not made despite of possible relevance. The available information makes it plausible to hypothesize that the Ser326Cys polymorphism may alter colon cancer risk, particularly in association with the environmental risk factors. We will conduct a case control study to examine the hypothesis.
MATERIALS AND METHODS
Study populations and sample processing
Case subjects consisted of 125 patients operated with primary colorectal cancer. All the patients agreed to participate in this study and consented. Community-based controls consisted of subjects who were visited the health-screening center of Ilsan-Paik hospital. At this center, screenings of common cancers as well as evaluation on the general status of health were performed. All control subjects were recruited after an initial verbal screening to determine whether they had no previous diagnosis of cancer, and none of the controls recruited into this study were diagnosed with any form of cancer after screening. The eligible pool of control subjects was restricted to those individuals of the same age (± 5 years) and sex as case subjects. The controls pair-matched into a 2 to 1 ratio in terms of those cases studied. Ninety-five percent of the control subjects (n = 247) consented. A trained interviewer conducted in-person interviews. Information was collected on life-long smoking, alcohol consumption, diet habits, physical activity and family history of colorectal cancer.
Genotypic assays
The genotypes of hOGG1 alleles were determined by a polymerase chain reaction-restriction endonuclease length polymorphism (PCR-RELP) analysis. Briefly, a 251 bp fragment was amplified by PCR in a 25 μL reaction volume that contained 50 ng of genomic DNA, 10 mmol/L Tris-HCl, 50 mmol/L KCl, 1 mmol/L MgCl2, 0.2 mmol/L of each dNTP, 10 pmol of the hOGG1 sense (hOGG1F; 5’-AGTGGATTCTCATTGCCTTCG-3’, corresponding to nucleotide 8919 through 8939 of hOGG1 intron 6 DNA sequences; Genbank accession #HSA131341) and antisense (h OGG1R; 5’-GGTGCTTGGGGAATTTCTTT-3’, corresponding to nucleotide 9150 through 9169 of hOGG1 exon 7 sequences) primers and 2 units of Taq DNA polymerase. Cycling conditions were as follows: initial denaturation at 94 °C for 5 min following 30 cycles of denaturation at 94 °C for 30 sec, annealing at 57 °C for 30 and elongation at 72 °C for 40. Ten μL of each PCR sample was digested with 2 units of Fsp4HI (Bioneer, Taejeon, Korea) at 37 °C for 12 h and resolved on 30 g/L agarose gels to detect differences in RFLP patterns.
Statistical analysis
The association between the hOGG1 polymorphism and colorectal cancer risk was estimated by the odds ratio (OR), using the unconditional logistic regression model. We calculated not only crude ORs, but also values adjusted for age, sex, drinking habits, smoking and diet habits to control the effect of potential confounding environmental factors. Because the Ser/Ser genotype was thought to have the highest effective enzymatic activity, we used this genotype as reference for colorectal cancer risk. Thus, the ORs for the Ser/Cys and Cys/Cys types relative to the Ser/Ser were calculated. The ORs for the Cys/Cys type versus other types combined were also calculated because only the Cys/Cys type could potentially influence the individual repair activity. Statistical differences of the categorical comparison and the probability of the Hardy-Weinberg equilibrium were tested using χ2 test.
RESULTS
The mean age of the case and control groups were 57.2 and 56.1 years, respectively, and the case and control groups consisted of 36.5% and 37.2% females, respectively. There were no significant statistical differences in age and gender between the case and control groups.
Three banding patterns were observed depending on the genotypes; a single 251 bp band corresponding to the Ser326/Ser326 genotype, a 251, 153 and 98 bp bands that corresponded to the Ser326/Cys326 genotype, and 153 and 98 bp bands corresponding to the 326Cys/Cys326 genotype (Figure 1A). The specificity of the amplification products was verified by sequencing (Figure 1B).
Figure 1.
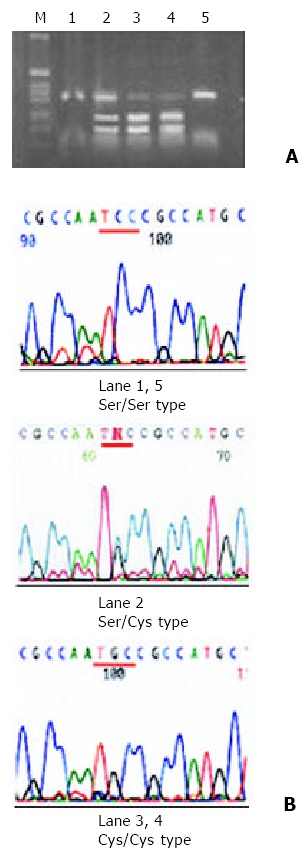
A: The representative PCR-RFLP analysis of hOGG1 Ser326Cys polymorphism. Lane 1, 5, undigested PCR-ampli-fied product observed in homozygous Ser326 carriers; Lane 2, Fsp4HI-digested PCR product from subject with heterozygous hOGG1 genotype; Lane 3, 4, Fsp4HI-digested PCR product from subject with homozygous Cys326 genotype. B: DNA se-quence histogram revealing the indicated sequence changes (underlined). In the 2nd histogram, both the cytosine (blue line) and guanine (black line) are observed in the codon 326 site (marked as N), indicating the heterozygous genotype.
The allelic frequency was not significantly different between cases and controls, slightly more homozygous Cys326 alleles were found in the case group than that in the controls (Table 1). The prevalence of the hOGG1 Ser/Cys polymorphism followed theHardy-Weinberg equilibrium.
Table 1.
Odds ratios for colon cancer according to the geno-types of hOGG1 Ser326Cys polymorphism
|
Number (%) |
OR (95%CI) | P for trend | ||
| Controls | Cases | |||
| Ser/Ser | 52 (21.1) | 24 (19.2) | 1.00 | |
| Ser/Cys | 131 (53.0) | 66 (52.8) | 1.09 (0.60-2.00) | P = 0.871 |
| Cys/Cys | 64 (25.9) | 35 (28.0) | 1.18 (0.60-2.35) | |
| Total | 247 (100) | 125 (100) | ||
To examine the relationship between the hOGG1 genotype and colon can cer risk in the presence of selected environmental risk factors, study subjects were stratified by hOGG1 genotype and several known colon cancer risk factors. Table 2 represented the frequency distribution of the hOGG1 genotype stratified with the risk factors and ORs with a 95% confidence interval (95%CI) for colon cancer cases compared with controls. None of the risk factors analyzed in the present study significantly elevated the ORs for colon cancer in the total number of subjects studied. Only frequent meat intake increased the OR with marginal statistical significance.
Table 2.
ORs for colon cancer according to individual habits and familial history of colon cancer with reference to the hOGG1 Ser326Cys polymorphism
|
Total |
hOGG1 Ser326Cys polymorphi sm |
||||||||
| Ca/Co | OR | 95%CI |
Ser/Ser + ser/Cys |
Cys/Cys |
|||||
| Ca/Co | OR | 95%CI | Ca/Co | OR | 95%CI | ||||
| Smoking | |||||||||
| No | 44/106 | 1 | - | 35/75 | 1 | - | 9/31 | 1 | - |
| Yes | 81/141 | 1.53 | 0.94-2.52 | 55/108 | 1.08 | 0.62-1.84 | 26/33 | 2.75 | 1.07-7.53 |
| Drinking | |||||||||
| < 2times/wk | 73/161 | 1 | - | 53/122 | 1 | - | 20/39 | 1 | - |
| > 2times/wk | 52/86 | 1.37 | 0.87-2.32 | 37/61 | 1.49 | 0.87-2.77 | 15/25 | 1.21 | 0.49-3.02 |
| Meat | |||||||||
| < 2time/week | 66/159 | 1 | - | 52/112 | - | 14/47 | 1 | - | |
| > 2time/week | 59/88 | 1.72 | 1.12-2.76 | 38/71 | 1.27 | 0.73-2.13 | 21/17 | 4.31 | 1.64-11.48 |
| Vegetable intake | |||||||||
| Low | 50/89 | 1 | - | 36/70 | 1 | - | 14/19 | 1 | - |
| High | 75/158 | 0.8 | 0.49-1.31 | 54/113 | 0.9 | 0.46-1.67 | 21/45 | 1.55 | 0.50-4.19 |
| Soybean product | |||||||||
| < 3/wk | 55/115 | 1 | 37/87 | 1 | - | 18/28 | 1 | - | |
| > 3/wk | 70/132 | 1.11 | 0.70-1.75 | 53/96 | 1.3 | 0.76-2.23 | 17/36 | 0.73 | 0.30-1.82 |
| Activity | |||||||||
| < 4 hs/wk | 61/104 | 1 | - | 43/75 | - | 18/29 | 1 | - | |
| > 4hs/wk | 64/143 | 0.76 | 0.48-1.20 | 47/108 | 0.76 | 0.44-1.30 | 17/35 | 0.78 | 0.32-1.94 |
| Familial history of colon cancer | |||||||||
| No | 84/185 | 1 | - | 62/138 | - | 22/47 | 1 | - | |
| Yes | 41/62 | 1.46 | 0.80-2.40 | 28/45 | 1.38 | 0.76-2.51 | 13/17 | 1.63 | 0.62-4.32 |
Denotes: Ca/Co = Cases/Controls.
In the subgroup analysis with reference to the Ser326Cys polymorphism, the smoking habit did not increase the ORs for colon cancer in Ser/Ser or Ser/Cys carriers. In contrast, a near-significant increase in risk was observed for smokers with the Cys/Cys genotype (OR = 2.75, 95%CI = 1.07-7.53). Meat intake was associated with increased colon cancer incidence with borderline significance among the total number of subjects (OR = 1.72, 95%CI = 1.12-2.76). The OR for colon cancer with frequent meat intake was further increased in the Cys/Cys subgroup (OR = 4.31, 95%CI = 1.64-11.48), while the OR was not increased in Ser/Ser or Ser/Cys carriers. Except for the meat intake factor, the intake of selected foods including alcohol, vegetable and soybean products did not alter the ORs for colon cancer neither in the entire group of subjects studied nor in the Cys/Cys subgroup. No significant alteration in the OR for colon cancer according to the extent of physical activity was observed in either genotype carriers.
Because the presence of colon cancer in relatives, particularly in 1st or 2nd degree relatives, has been known to be associated with colon cancer risk and because the genetic polymorphism could be one of the possible causes, changing patterns of OR for colon cancer according to the hOGG1 Ser326Cys polymorphism when there was at least one colon cancer patient within a family were investigated. Although the risk was increased with the presence of colon cancer within a family among the total number of subjects studied, there was no apparent increase in the OR among subjects with Cys/Cys genotype.
DISCUSSION
It is theoretically possible that the incidence of cancer differs depending on the genotypes of hOGG1 Ser326Cys polymorphism. This view is supported by the difference in DNA repairing capability depending on the allele type[9] and increase of the incidence of cancer in certain organs in Cys326 allele carriers[12,13,18].
Colon is the most susceptible organ for cancer development in hereditary non-polyposis colorectal cancer (HNPCC), where the DNA mismatch repair activity is defective[19]. As the role of defects in DNA mismatches repair process in colon carcinogenesis is very clear, attention has also recently focused on the possible role of alternative DNA repair pathways such as base excision repair in colon cancer formation.
In the present investigation, however, the hOGG1 Ser326Sys polymorphism did not alter the overall odds ratios for colon cancers despite of the theoretical relevancies. Subgroup analysis revealed an increased tendency of ORs with smoking habit or frequent meat intaking in Cys/Cys carriers although the statistical significance of the former factor is marginal.
The intake of meat and animal fat was almost consistently associated with the increased risk of large-bowel cancer in the previous studies[20-22]. It was suggested that the abundant fecal iron after red meat intake, together with the ability of bile pigments to act as iron chelators, supports the superoxide-driven Fenton reaction[15]. Meanwhile, excessive fat ingestion increases the concentrations of bile acids in the colon. The increased fatty acids and secondary bile acids in the colonic lumen, which acts as cytotoxic surfactants, can damage colonic epithelial cells and thus induce a compensatory hyperproliferation of crypt cells[23]. In addition, the high intake of polyunsaturated fatty acid induces peroxidative reaction with plasma low-density lipoproteins, which in turn generate free radicals. Meat is a major source of dietary animal fat. Thus, the question is still unresolved whether the association of meat with colorectal cancer seen in some epidermiologic investigations reflects the effect of fat, or meat in general, or just particular types of meat. One prospective study showed that red meat, but not the chicken or fish could be an independent risk factor for colon cancer[24]. The result of the present investigation together with a literature review consists with the suggestion that oxidative damage after meat intake may play a role in colon carcinogenesis and that the role may be more pronounced in Cys/Cys carriers, who have a lower activity in terms of repair of DNA.
Some discussion is required on the credibility of the answer concerning the meat intake habit. The dietary environment has been rapidly changing in Korea in accordance with economic development. Meat consumption per capita has increased more than 3 times during the past 3 decades[25]. A possible problem under this circumstance is that the answers on the questionnaire might be more influenced by recent dietary habits rather than lifelong ones, although we tried to reduce such problems to a minimum during the interview.
Among the various lifestyle factors, tobacco smoking might provide the strongest oxygen radical generating environment. An array of studies demonstrated the increased oxidative DNA damage in smokers compared to non-smokers[26,27]. The carcinogenic role of tobacco in association with the oxygen free radical seems clear in airway tract tissues. Recent studies showed that an increased risk of squamous type lung cancer, orolaryngeal cancer in smokers, was particularly found in homozygous Cys 326 gene carriers. In general, the biologic role of tobacco smoke as an etiology of cancer in the gastrointestinal tract might be less prominent than that in the airway tract. Tobacco smoking history is often inconsistent with the incidence of colorectal cancers although it shows a near consistent positive relationship with the presence of colorectal adenomas[28,29]. The previous studies, which investigated the effect of smoking in gastric cancer development in connection with the Ser326Cys polymorphism, showed only a weak association[14]. In the present study, the OR for colon cancer among smokers tended to be higher in Cys/Cys carriers than that in Ser/Ser and Ser/Cys carriers, the interaction being of borderline statistical significance. The result of present findings suggested that the oxygen free radical generated from tobacco smoke might be involved in colon carcinogenesis although the role is probably limited.
The role of oxidative DNA damage in alcohol-related carcinogenesis is still controversial. Alcohol consumption was associated with increased DNA damage or induction of 8oxoG in some studies[30,31] in contrast, but not in others[32,33]. The present study showed that alcohol intake did not increase the risk of colon cancer even in Cys/Cys carriers. It suggested that the role of alcohol consumption in colon cancer formation, particularly in association with the 8oxoG adduct induction, was limited. The other factors including vegetable or soybean product intake, physical exercise and family history of colon cancer did not increase OR for colon cancer in the total number of subjects studied as well as in Cys/Cys carriers. Thus, it was difficult to interpret whether these factors play a role during colon carcinogeneis, particularly in interaction with the hOGG1 Ser326Cys polymorphism.
In summary, the hOGG1 Ser/Cys polymorphism does not significantly alter the overall risk of colon cancer. The Cys type allele, however, may exert an impact on colon carcinogenesis through an interaction with certain environmental factors such as smoking or meat consumption. There are lines of complex biologic factors involved in colon carcinogenesis. The present study suggests the possibility that an inter-individual difference in DNA repair capacity can be one such factor, however, the influence of such a role is believed to be limited. Further studies with a large number of subjects are warranted to better assess the role of hOGG1 polymorphism in colon carcinogenesis.
Footnotes
Supported by the grants from the Korea Research Foundation, No. 2001-003-F00117
Edited by Xu XQ
References
- 1.Poulsen HE, Prieme H, Loft S. Role of oxidative DNA damage in cancer initiation and promotion. Eur J Cancer Prev. 1998;7:9–16. [PubMed] [Google Scholar]
- 2.Fearon ER. Human cancer syndromes: clues to the origin and nature of cancer. Science. 1997;278:1043–1050. doi: 10.1126/science.278.5340.1043. [DOI] [PubMed] [Google Scholar]
- 3.Loft S, Deng XS, Tuo J, Wellejus A, Sørensen M, Poulsen HE. Experimental study of oxidative DNA damage. Free Radic Res. 1998;29:525–539. doi: 10.1080/10715769800300571. [DOI] [PubMed] [Google Scholar]
- 4.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 5.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 6.Michaels ML, Miller JH. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) J Bacteriol. 1992;174:6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishioka K, Ohtsubo T, Oda H, Fujiwara T, Kang D, Sugimachi K, Nakabeppu Y. Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Mol Biol Cell. 1999;10:1637–1652. doi: 10.1091/mbc.10.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinmura K, Kohno T, Takeuchi-Sasaki M, Maeda M, Segawa T, Kamo T, Sugimura H, Yokota J. Expression of the OGG1-type 1a (nuclear form) protein in cancerous and non-cancerous human cells. Int J Oncol. 2000;16:701–707. doi: 10.3892/ijo.16.4.701. [DOI] [PubMed] [Google Scholar]
- 9.Kohno T, Shinmura K, Tosaka M, Tani M, Kim SR, Sugimura H, Nohmi T, Kasai H, Yokota J. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16:3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- 10.Dherin C, Radicella JP, Dizdaroglu M, Boiteux S. Excision of oxidatively damaged DNA bases by the human alpha-hOgg1 protein and the polymorphic alpha-hOgg1(Ser326Cys) protein which is frequently found in human populations. Nucleic Acids Res. 1999;27:4001–4007. doi: 10.1093/nar/27.20.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen K, Schlink K, Götte W, Hippler B, Kaina B, Oesch F. DNA repair activity of 8-oxoguanine DNA glycosylase 1 (OGG1) in human lymphocytes is not dependent on genetic polymorphism Ser326/Cys326. Mutat Res. 2001;486:207–216. doi: 10.1016/s0921-8777(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 12.Sugimura H, Kohno T, Wakai K, Nagura K, Genka K, Igarashi H, Morris BJ, Baba S, Ohno Y, Gao C, et al. hOGG1 Ser326Cys polymorphism and lung cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 1999;8:669–674. [PubMed] [Google Scholar]
- 13.Xing DY, Tan W, Song N, Lin DX. Ser326Cys polymorphism in hOGG1 gene and risk of esophageal cancer in a Chinese population. Int J Cancer. 2001;95:140–143. doi: 10.1002/1097-0215(20010520)95:3<140::aid-ijc1024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Takezaki T, Gao CM, Wu JZ, Li ZY, Wang JD, Ding JH, Liu YT, Hu X, Xu TL, Tajima K, et al. hOGG1 Ser(326)Cys polymorphism and modification by environmental factors of stomach cancer risk in Chinese. Int J Cancer. 2002;99:624–627. doi: 10.1002/ijc.10400. [DOI] [PubMed] [Google Scholar]
- 15.Babbs CF. Free radicals and the etiology of colon cancer. Free Radic Biol Med. 1990;8:191–200. doi: 10.1016/0891-5849(90)90091-v. [DOI] [PubMed] [Google Scholar]
- 16.Park JG, Park YJ, Wijnen JT, Vasen HF. Gene-environment interaction in hereditary nonpolyposis colorectal cancer with implications for diagnosis and genetic testing. Int J Cancer. 1999;82:516–519. doi: 10.1002/(sici)1097-0215(19990812)82:4<516::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Park YJ, Choi EY, Choi JY, Park JG, You HJ, Chung MH. Genetic changes of hOGG1 and the activity of oh8Gua glycosylase in colon cancer. Eur J Cancer. 2001;37:340–346. doi: 10.1016/s0959-8049(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 18.Elahi A, Zheng Z, Park J, Eyring K, McCaffrey T, Lazarus P. The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk. Carcinogenesis. 2002;23:1229–1234. doi: 10.1093/carcin/23.7.1229. [DOI] [PubMed] [Google Scholar]
- 19.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 20.Drasar BS, Irving D. Environmental factors and cancer of the colon and breast. Br J Cancer. 1973;27:167–172. doi: 10.1038/bjc.1973.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–2397. [PubMed] [Google Scholar]
- 22.Neugut AI, Garbowski GC, Lee WC, Murray T, Nieves JW, Forde KA, Treat MR, Waye JD, Fenoglio-Preiser C. Dietary risk factors for the incidence and recurrence of colorectal adenomatous polyps. A case-control study. Ann Intern Med. 1993;118:91–95. doi: 10.7326/0003-4819-118-2-199301150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Van der Meer R, Lapré JA, Govers MJ, Kleibeuker JH. Mechanisms of the intestinal effects of dietary fats and milk products on colon carcinogenesis. Cancer Lett. 1997;114:75–83. doi: 10.1016/s0304-3835(97)04629-6. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 25. Available from: http//www.nso.go.kr.
- 26.Nakayama T, Kaneko M, Kodama M, Nagata C. Cigarette smoke induces DNA single-strand breaks in human cells. Nature. 1985;314:462–464. doi: 10.1038/314462a0. [DOI] [PubMed] [Google Scholar]
- 27.Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res. 1996;56:2546–2549. [PubMed] [Google Scholar]
- 28.Kune GA, Kune S, Vitetta L, Watson LF. Smoking and colorectal cancer risk: data from the Melbourne Colorectal Cancer Study and brief review of literature. Int J Cancer. 1992;50:369–372. doi: 10.1002/ijc.2910500307. [DOI] [PubMed] [Google Scholar]
- 29.Heineman EF, Zahm SH, McLaughlin JK, Vaught JB. Increased risk of colorectal cancer among smokers: results of a 26-year follow-up of US veterans and a review. Int J Cancer. 1994;59:728–738. doi: 10.1002/ijc.2910590603. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima M, Takeuchi T, Takeshita T, Morimoto K. 8-Hydroxydeoxyguanosine in human leukocyte DNA and daily health practice factors: effects of individual alcohol sensitivity. Environ Health Perspect. 1996;104:1336–1338. doi: 10.1289/ehp.961041336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye XB, Fu H, Zhu JL, Ni WM, Lu YW, Kuang XY, Yang SL, Shu BX. A study on oxidative stress in lead-exposed workers. J Toxicol Environ Health A. 1999;57:161–172. doi: 10.1080/009841099157737. [DOI] [PubMed] [Google Scholar]
- 32.van Zeeland AA, de Groot AJ, Hall J, Donato F. 8-Hydroxydeoxyguanosine in DNA from leukocytes of healthy adults: relationship with cigarette smoking, environmental tobacco smoke, alcohol and coffee consumption. Mutat Res. 1999;439:249–257. doi: 10.1016/s1383-5718(98)00192-2. [DOI] [PubMed] [Google Scholar]
- 33.Lodovici M, Casalini C, Cariaggi R, Michelucci L, Dolara P. Levels of 8-hydroxydeoxyguanosine as a marker of DNA damage in human leukocytes. Free Radic Biol Med. 2000;28:13–17. doi: 10.1016/s0891-5849(99)00194-x. [DOI] [PubMed] [Google Scholar]


