Abstract
AIM: To identify the differentially secreted proteins or polypeptides associated with tumorigenesis of esophageal squamous cell carcinoma (ESCC) from serum and to find potential tumor secreted biomarkers.
METHODS: Proteins from human ESCC tissue and its matched adjacent normal tissue; pre-surgery and post-surgery serum; and pre-surgery and normal control serum were separated by two-dimensional electrophoresis (2-DE) to identify differentially expressed proteins. The silver-stained 2-DE were scanned with digital ImageScanner and analyzed with ImageMaster 2D Elite 3.10 software. A cluster of protein spots differentially expressed were selected and identified with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). One of the differentially expressed proteins, clusterin, was down-regulated in cancer tissue and pre-surgery serum, but it was reversed in post-surgery serum. The results were confirmed by semi-quantitative reverse-transcription (RT)-PCR and western blot.
RESULTS: Comparisons of the protein spots identified on the 2-DE maps from human matched sera showed that some proteins were differentially expressed, with most of them showing no differences in composition, shape or density. Being analyzed by MALDI-TOF-MS and database searching, clusterin was differentially expressed and down-regulated in both cancer tissue and pre-surgery serum compared with their counterparts. The results were also validated by RT-PCR and western blot.
CONCLUSION: The differentially expressed clusterin may play a key role during tumorigenesis of ESCC. The 2DE-MS based proteomic approach is one of the powerful tools for discovery of secreted markers from peripheral.
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC), the major histological form of esophageal cancer, is one of the most common malignant tumors in China especially in the north part of the country. Human ESCC carcinogenesis is a multistage process involving multifactorial etiology and genetic-environment interactions[1-3]. Patients with ESCC have a poor prognosis, with 5-year survival rates of less than 10%, because of the rapid spread and of the cancer associated malnutrition due to dysphagia and cachexia[4]. The molecular mechanisms that underlie the tumor formation and progression are still not completely perspicuous, although several progresses based on alterations of gene expression[5] and disregulated proteins, such as annexin I[6] and tumor rejection antigen[7] in esophageal cancer via proteomic approaches have been reported recently. Discovery of new markers to discriminate tumorigenic from normal cells, as well as the different stage is critical important for early detection and diagnosis of ESCC. The success of the Human Genome Project and the initiation of human proteome are strongly facilitating these efforts as tremendous information of genes and proteins are currently available[8,9]. It would be possible to undertake comprehensive profiling of tumor at the proteomics level to identify protein alterations that are unpredictable at either the genomics or transcriptomics levels[10]. However there is no comprehensive study of esophageal cancer protein profiling or protein expression patterns have been generated, especially the tumor-associated serum protein biomarkers. To identify specific protein tumor markers both in serum and tissue by proteomics approaches is a currently critical issue[8,11,12].
Tumor associated proteins as well as post-translational modifications can be identified via proteomic methodologies. In the present study, we used the 2-DE/mass spectrometry (MS)-based proteomic analysis to profile the proteins in serum of ESCC patients. We analyzed 17 pairs of patient-matched pre- and post-surgery as well as normal and tumor sera from ESCC patients to discover the alterations of expression profiling. We first found the clusterin is loss in both serum and tissue of ESCC.
MATERIALS AND METHODS
Specimens and preparation
The esophageal specimens were from patients diagnosed with esophageal cancer by the pathologists in Cancer Hospital of Chinese Academy of Medical Sciences (CAMS) (Beijing, China). The study was approved by the Institutional Review Board of Cancer Institute of CAMS. The pre-surgery serum were obtained from the first physical examination after the patients presented to the hospital, ESCC tumor tissues were obtained immediately after surgical resection and the post-surgery serum were obtained from the matched patients during the day 8 to day 10 after surgery. Sera described in Table 1, were centrifuged at 3000 g at 4 °C for 15 min. Both tissue and serum samples were snap-frozen in liquid nitrogen immediately and then stored at -80 °C. The tissues were homogenized in five volumes of lysis buffer [8 M urea, 4% CHAPS, 2% Pharmalyte, pH 3-10, 10 mM DTT] and centrifuged at 12000 g at 4 °C for 40 minutes. The supernatant was removed and protein concentration was determined using the Bradford assay.
Table 1.
The matched sera for 2-DE
| No. of matched-sera | Gender | Age | Histopathological diagnosis |
| M51Q-M51H | M | 59 | MDSCC |
| M88Q-M88H | F | 56 | MDSCC |
| M88Q-M88H | M | 62 | MDSCC |
| M92Q-M92H | M | 65 | MDSCC |
| M93Q-M93H | M | 65 | MDSCC |
| M141Q-Nor86 | F | 62 | MDSCC |
| M149Q-Nor67 | M | 67 | PDSCC |
| M151Q-Nor24 | M | 64 | MDSCC |
| M156Q-Nor87 | F | 70 | PDSCC |
| M160Q-Nor15 | M | 68 | MDSCC |
| M162Q-Nor34 | M | 72 | MDSCC |
| M168Q-Nor16 | M | 70 | MDSCC |
| M180Q-Nor66 | F | 65 | HDSCC |
| M180Q-Nor10 | F | 65 | HDSCC |
| M181Q-Nor97 | M | 57 | MDSCC |
| M192Q-Nor36 | M | 68 | MDSCC |
| M193Q-Nor19 | F | 66 | HDSCC |
The abbreviations used are: Q, pre-surgery; H, post-surgery; Nor, age and gender matched normal serum; MDSCC, moder-ate differentiated squamous Cell Carcinoma; PDSCC, poorly differentiated squamous Cell Carcinoma; HDSCC, Highly Dif-ferentiated squamous Cell Carcinoma.
Reagents
Electrophoresis reagents including acrylamide solution (40%), N, N-methylenebisacrylamide, N, N, N’, N’-tetramethylethylenediamine, tris base, glycine, SDS, DTT, CHAPS, Immobiline Drystrips, IPG buffer, IPG cover fluid, LMW protein marker were from Amersham Pharmacia Biotechnology Inc. (Uppsala, Sweden); Iodoacetamide was from Acros (New Jersey, USA); Sequence grade Trypsin was from Washington Biochemical Corporation; Trifluoroacetic acid (TFA) was from Fluka (Switzerland); TrizolTM Reagent and Transcriptase SuperScript IITM were from Gibco BRL; PVDF membrane was from Bio-Rad; Taq DNA polymerase and dNTPs were from TaKaRa; All other reagents were of analytical grade.
Analytical 2-DE
All sera or cell lysates were quantitated by Bradford assay. 2-DE was performed by standard procedures as described[8,12] using precast IPG strips (pH3-10 linear, 18 cm, Amersham Pharmacia Biotechnology Inc.) in the first dimension, isoelectric focusing (IEF). Briefly, 180 µg proteins were diluted to a total volume of 350 µl with the buffer [8 M urea, 2% CHAPS, 0.5% IPG buffer 3-10, 20 mM DTT and a trace of bromophenol blue]. After loaded on IPG strips, IEF was carried out according to the following protocol: 6 hours of rehydration at 0 V; 6 hours at 30 V; 1 hour at 500 V; 1 hour at 1000 V and 5 hours at 8000 V. The current was limited to 50 µA per gel. After IEF separation, the strips were immediately equilibrated 2 × 15 min with equilibration solution [50 mM Tris-HCl, pH6.8, 6 M urea, 30% glycerol and 2% SDS]. 20 mM DTT was included in the first equilibration solution, and 2% (w/v) iodoacetamide was added in the second equilibration step to alkylate thiols. SDS-polyacrylamide gel electrophoresis (PAGE) was performed using 1 mm thick, 13% SDS-PAGE gels. The strips were held in place with 0.5% agarose dissolved in SDS/Tris running buffer and electrophoresis was carried out at constant power (2.5 W/gel for 40 min and 15 W/gel for 6 hours) and temperature (20 °C) using Ettan Dalt II system (Amersham Pharmacia Biotechnology Inc.). Gels were stained with silver nitrate according to the instructions of the silver-staining kit (Amersham Pharmacia Biotechnology Inc.).
Gel scanning and image analysis
Silver stained 2-DE gels were scanned with ImageScanner and analyzed including spots detection, quantification and normalization with ImageMaster 2D Elite 3.10 (Amersham Pharmacia Biotechnology Inc.). Statistical analysis was performed using SPSS statistical software late.
In-gel protein digestion
Individual protein spot was excised from the gel by Ettan Spot Picker (Amersham Pharmacia Biotechnology Inc.), destained with the solution [15 mM potassium ferricyanide, 50 mM sodium thiosulfate] and washed till opaque and colorless with 25 mM ammonium bicarbonate/50% acetonitrile. After dried with vacuum concentrator (SpeedVac Plus, USA) the gel was rehydrated with 3-10 µl trypsin solution (10 ng/µl) at 4 °C for 30 min and then incubated at 37 °C overnight. Tryptic peptides were eluted and dried on SpeedVac vacuum concentrator.
Protein identification by MALDI mass spectrometry
Digested peptides were dissolved with 0.5% TFA, with saturated CHCA solution in 0.1% TFA/50% acetic acid as matrix and analyzed by M@LDI R (Micromass, Manchester, UK). Spectrum acquisition was externally calibrated with lock mass 2465.199 Da and internally calibrated with autodigested peaks of trypsin (MH+: 2211.105 Da). The protein identification was performed by searching protein databases of Swiss-prot/trEMBL (http://www.expasy.ch/tools/peptident.html) and Mascot (http://www.matrixscience.com/). The error for peptide mass was set as 50 ppm and possible missed cleavage of trypsin was set as 1. The proteins with more than 4 matched peptides were thought significant.
RT-PCR
Total RNAs were isolated from esophageal cancer tissues using TRIzol reagent (Gibco BRL) according to the manufactures’ instructions. First strand cDNA was reversely transcribed from 5 µg total RNAs using SuperScript II kit (Life Technologies) at 42 °C for 50 min. Clusterin was amplified by the primers (left: 5’ACCTCACGCAAGGCGAAGAC3’, right: 5’TCTCACTCCTCCCGGTGCTT3’) and a product with 232 bp was generated.
Western blot
Total cells or tissues were lysated with the buffer [1% SDS, 10 Mm Tris-Cl, pH7.6, 20 µg/mL aprotenin, 20 µg/mL leupeptin and 1 mM AEBSF]. The protein concentrations were determined using Bradford method. Five micrograms of protein were separated on 12% of SDS-PAGE gels and transferred to PVDF membranes. After blocked with 10% non-fat milk, the membranes were incubated with anti-clusterin monoclonal antibody (Santa Cruz Bitechnology Inc.) (1:1000 dilution) at 4 °C overnight. After washing for three times the membranes were incubated with rabbit anti-mouse IgG at room temperature for 1 hour. The signals were developed with the ECL kit (Amersham Pharmacia Biotechnology Inc.) and using anti-α-tubulin antibody (Santa Cruz Biotechnology Inc.) as an internal control.
RESULTS
Clusterin was identified down-regulated in pre-surgery serum
A proteomic approach was used to determine the differentiated proteins profiling between the pre-surgery and post-surgery sera of ESCC. The proteins from five pairs of matched sera from pre- and post-surgery of ESCC patients were separated by 2DE. Figure 1 illustrates the proteomic profilings of the pre- and post-surgery sera from same individual and more than 600 proteins and polypeptides were detected on each gel. The matched rate of the five pairs gel was more than 87.2% and the spots localized in pI 3-10 with the molecular mass range around 20-200 kDa. All the identified spots can be considered as abundant proteins since they are detectable with Coomassie Blue staining on the preparative 2-DE gel. After computer analysis for spots detection, background subtraction and volume normalization, we were able to identify 20 spots (corresponding to 5 different proteins) from the tryptic digestion via MALDI-TOF-MS analysis. The isoforms of clusterin were identified from 8 spots as multipeptides components, which were dramatically down-regulated in tumor sera (Figure 2A and Figure 3).
Figure 1.
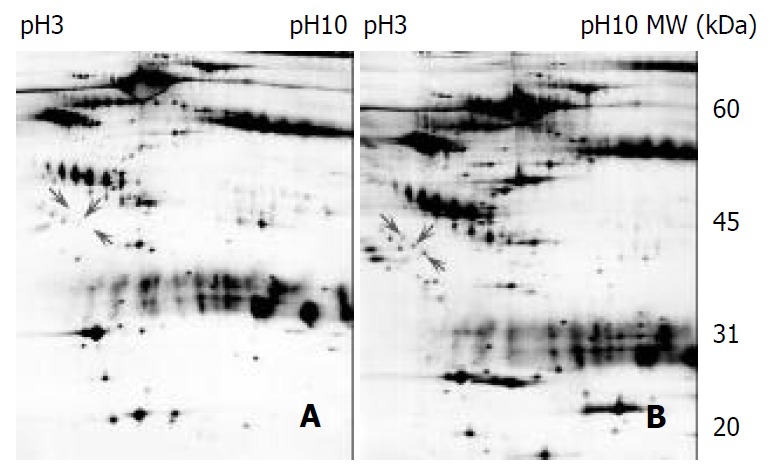
2-DE profiles of the pre- and post-surgery matched esophageal cancer sera from a same individual. One hundred and eighty microgram of protein was separated by 2-DE (IEF at pH3-10, 13% of SDS-PAGE) and stained by silver staining. LMW markers were from Amersham Pharmacia. (A) pre-sur-gery serum, (B) matched post-surgery serum.
Figure 2.
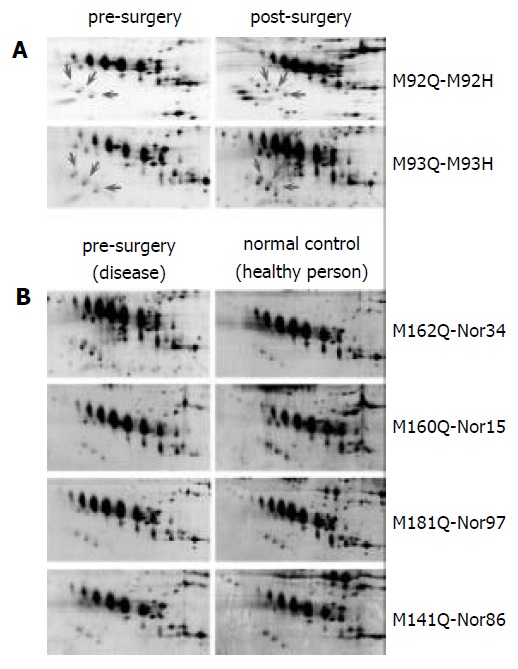
Representative regions of 2-DE patterns of more matched esophageal cancer sera. One hundred and eighty microgram of protein was separated by 2-DE (IEF at pH3-10, SDS-PAGE 13%) and stained by silver staining. LMW mark-ers were from Amersham Pharmacia. (A) pre-surgery sera vs post-surgery sera, (B) pre-surgery sera vs normal control sera.
Figure 3.

The spectra of MALDI-TOF-MS obtained from one of the differentiated polypeptide spots matched with the tryptic peptide sequences of clusterin (characters in red).
Proteomic determination of esophageal cancer serum
We asked the question whether the loss of clusterin was induced by surgical attacks? In order to get rid of the interferences of the human immunoresponse after routine surgery, we additionally selected and matched 12 pairs of pre-surgery sera with the healthy sera from the same age and gender individuals who had no any surgical attacks and infections recently. We ran 2-DE profiling again between the two groups. Comparison with the healthy reference sera, clusterin proteins were also identified as more than four times lower in esophageal cancer serum after statistical analysis (Figure 2B).
Loss of clusterin in tumor epithelium
Immunoblot analysis of clusterin expressed in patient-matched normal and tumor epithelium from different ESCC individuals, whose sera had been found with low level of clusterin was performed. Using a commercially available antibody, clusterin has also been found down-regulated at different ESCC tissues (20/24), but absent in two kinds of cell lines of esophageal squamous cell carcinoma, EC1 and EC2 (Figure 4A, B). At the transcriptional level, clusterin was also found lower-expressed in 88% of esophageal cancer tissues (22/25) by semi-quantitative RT-PCR (Figure 4C). The findings of tumor tissue and cell lines were consistent with the findings of ESCC serum.
Figure 4.
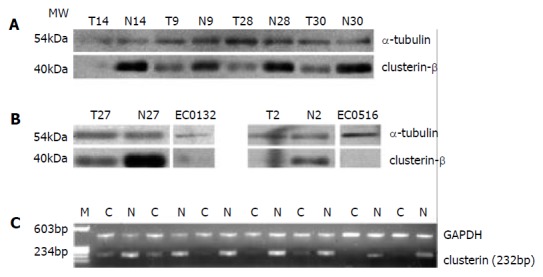
(A) Western blot analysis of clusterin proteins expressed in patient-matched normal and tumor epithelium; (B) Western blot analysis of clusterin proteins expressed in two kinds of esophageal squamous cell carcinoma, EC0156 and EC0132. Alpha-tubulin was used as a loading control. (C) Expression analysis of clusterin in patient-matched esophageal cancer tissues by semi-quantitative RT-PCR. GAPDH was used as an internal control.
DISCUSSION
To identify the differentially secreted proteins or polypeptides associated with tumorigenesis of esophageal squamous cell carcinoma, we carried out differentially proteomic analysis of human serum in two groups. First we compared the pre- and post-surgery sera of ESCC patient to identify the differentiated proteins from the same individuals. Second, the sera from the disease (pre-surgery) and age- and gender-matched healthy populations (normal control) had been analyzed through 2DE-MS strategy. There were 3 protein spots quantitatively changed between the two groups and were identified as clusterin, which was completely loss or dramatically down-regulated in pre-surgery serum of esophageal cancer compared with the post-surgery and the healthy controls. In addition, clusterin was also lost or decreased in tumor cell lines and tissues. Clusterin has never been known to be associated with esophageal cancer. This study reports identified clusterin as a candidate protein of a tumor-associated serum marker in esophageal squamous cell carcinoma via proteomic approaches for the first time.
Clusterin, so-called the testosterone-repressed prostate message-2[13], sulfated glycoprotein 2, complement-associated protein SP-40, complement cytolysis inhibitor, a 80 kDa heterodimeric highly conserved secreted glycoprotein expressed in a wide variety of tissues and was found in all human fluids. It responses to a number of diverse stimuli, including hormone ablation and has been attributed functions in several diverse physiological processes such as sperm maturation, lipid transportation, complement inhibition, tissue remodeling, membrane recycling, cell adhesion and cell-substratum interactions, stabilization of stressed proteins in a folding-competent state and promotion or inhibition of apoptosis[14-16].
Clusterin gene is differentially regulated by cytokines, growth factors and stress- inducing agents, while another defining prominent and intriguing clusterin feature is its upregulation in many severe physiological disturbances states and in several neurodegenerative conditions mostly related to advanced aging[17-22]. Active cell death (ACD) in hormone-dependent tissues such as the prostate and mammary gland is readily induced by hormone ablation and by treatment with anti-androgens or anti-estrogens, calcium channel agonists and TGF beta[23]. Clusterin has been found up-regulated in several cases of in vivo cancer progression and tumor formation such as human prostate carcinomas[18,24], renal cell carcinoma (RCC)[25,26], breast carcinoma[17], ovarian cancer[22], glioblastoma, testicular tumor cells, the normal and cancerous endometrium, hemangioma[21], anaplastic large-cell lymphomas[20], transitional cell carcinoma (TCC) of the bladder[27], as well as hepatoma cells. Clusterin, on the other hand, is a membrane-stabilizing protein that appears to be involved in limiting the autophagic lysis of epithelial cells during apoptosis. Recent studies have shown the clusterin expression mediates antiapoptotic activity against a wide variety of stimuli[28] and using antisense oligonucleotides of clusterin, also could enhance androgen sensitivity and chemosensitivity in prostate cancer therapy[29]. On the other hand, it has recently been shown that decreased synthesis and delayed processing of clusterin in testicular germ cell tumors, colorectal cancer[16,30] and human polycystic kidneys cells[31,32]. However, there is no definitive biochemical evidence to support a specific function for clusterin except for its role in the modulation of the immune system and the functional roles of clusterin are still enigmatic.
A robust antigen capture assay for the measurement of serum clusterin concentrations has been developed and validated for increased clusterin expression, and alterations in serum clusterin levels associated with a number of disease states[33]. Whether the decrease of clusterin in pre-surgery serum is a predictor of ESCC progression and prognosis, still need more efforts to address and the molecular mechanisms of clusterin implicated in tumorigenesis needs to be elucidated.
Footnotes
Supported by the Major State Basic Research Development Program of China, No.G19980512 and No.2001CB510201; the National Hi-Tech R & D Program of China, No.2001AA227091 and No. 2001AA233061; National Natural Science Foundation of China, No. 39990570, No.30171049, 30225045 and No.39990600
Edited by Ren SY
References
- 1.Zhang W, Bailey-Wilson JE, Li W, Wang X, Zhang C, Mao X, Liu Z, Zhou C, Wu M. Segregation analysis of esophageal cancer in a moderately high-incidence area of northern China. Am J Hum Genet. 2000;67:110–119. doi: 10.1086/302970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang DX, Li W. Advances on pathogenesis of esophageal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:1029–1030. [Google Scholar]
- 3.Hu SP, Yang HS, Shen ZY. Study on etiology of esophageal carcinoma: retrospect and prospect. Zhongguo Aizheng Zazhi. 2001;11:171–174. [Google Scholar]
- 4.Oka M, Yamamoto K, Takahashi M, Hakozaki M, Abe T, Iizuka N, Hazama S, Hirazawa K, Hayashi H, Tangoku A, et al. Relationship between serum levels of interleukin 6, various disease parameters and malnutrition in patients with esophageal squamous cell carcinoma. Cancer Res. 1996;56:2776–2780. [PubMed] [Google Scholar]
- 5.Lu J, Liu Z, Xiong M, Wang Q, Wang X, Yang G, Zhao L, Qiu Z, Zhou C, Wu M. Gene expression profile changes in initiation and progression of squamous cell carcinoma of esophagus. Int J Cancer. 2001;91:288–294. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1063>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Paweletz CP, Ornstein DK, Roth MJ, Bichsel VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH, Herring J, et al. Loss of annexin 1 correlates with early onset of tumorigenesis in esophageal and prostate carcinoma. Cancer Res. 2000;60:6293–6297. [PubMed] [Google Scholar]
- 7.Zhou G, Li H, DeCamp D, Chen S, Shu H, Gong Y, Flaig M, Gillespie JW, Hu N, Taylor PR, et al. 2D differential in-gel electrophoresis for the identification of esophageal scans cell cancer-specific protein markers. Mol Cell Proteomics. 2002;1:117–124. doi: 10.1074/mcp.m100015-mcp200. [DOI] [PubMed] [Google Scholar]
- 8.Xiong XD, Xu LY, Shen ZY, Cai WJ, Luo JM, Han YL, Li EM. Identification of differentially expressed proteins between human esophageal immortalized and carcinomatous cell lines by two-dimensional electrophoresis and MALDI-TOF-mass spectrometry. World J Gastroenterol. 2002;8:777–781. doi: 10.3748/wjg.v8.i5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F, Guan YJ, Chen ZC. Proteomics in cancer research. Shengwu Huaxue Yu Shengwu Wuli Jinzhan. 2001;28:164–167. [Google Scholar]
- 10.Wang H, Hanash SM. Contributions of proteome profiling to the molecular analysis of cancer. Technol Cancer Res Treat. 2002;1:237–246. doi: 10.1177/153303460200100404. [DOI] [PubMed] [Google Scholar]
- 11.Vercoutter-Edouart AS, Lemoine J, Le Bourhis X, Louis H, Boilly B, Nurcombe V, Révillion F, Peyrat JP, Hondermarck H. Proteomic analysis reveals that 14-3-3sigma is down-regulated in human breast cancer cells. Cancer Res. 2001;61:76–80. [PubMed] [Google Scholar]
- 12.Zhan XQ, Chen ZC, Guan YJ, Li C, He CM, Liang SP, Xie JY, Chen P. Analysis of human lung squamous carcinoma cell line NCI-H520 proteome by two-dimensional electrophoresis and MALDI-TOF-mass spectrometry. Aizheng. 2001;20:575–582. [Google Scholar]
- 13.Parczyk K, Pilarsky C, Rachel U, Koch-Brandt C. Gp80 (clusterin; TRPM-2) mRNA level is enhanced in human renal clear cell carcinomas. J Cancer Res Clin Oncol. 1994;120:186–188. doi: 10.1007/BF01202200. [DOI] [PubMed] [Google Scholar]
- 14.Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol. 2002;34:1430–1448. doi: 10.1016/s1357-2725(02)00041-9. [DOI] [PubMed] [Google Scholar]
- 15.Jones SE, Jomary C. Clusterin. Int J Biochem Cell Biol. 2002;34:427–431. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 16.Yang CR, Leskov K, Hosley-Eberlein K, Criswell T, Pink JJ, Kinsella TJ, Boothman DA. Nuclear clusterin/XIP8, an x-ray-induced Ku70-binding protein that signals cell death. Proc Natl Acad Sci USA. 2000;97:5907–5912. doi: 10.1073/pnas.97.11.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redondo M, Villar E, Torres-Muñoz J, Tellez T, Morell M, Petito CK. Overexpression of clusterin in human breast carcinoma. Am J Pathol. 2000;157:393–399. doi: 10.1016/S0002-9440(10)64552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens P, Jeske W, Wernert N, Wellmann A. Downregulation of clusterin expression in testicular germ cell tumours. Pathobiology. 2001;69:19–23. doi: 10.1159/000048753. [DOI] [PubMed] [Google Scholar]
- 19.Tobe T, Minoshima S, Yamase S, Choi NH, Tomita M, Shimizu N. Assignment of a human serum glycoprotein SP-40,40 gene (CLI) to chromosome 8. Cytogenet Cell Genet. 1991;57:193–195. doi: 10.1159/000133144. [DOI] [PubMed] [Google Scholar]
- 20.Wellmann A, Thieblemont C, Pittaluga S, Sakai A, Jaffe ES, Siebert P, Raffeld M. Detection of differentially expressed genes in lymphomas using cDNA arrays: identification of clusterin as a new diagnostic marker for anaplastic large-cell lymphomas. Blood. 2000;96:398–404. [PubMed] [Google Scholar]
- 21.Hasan Q, Rüger BM, Tan ST, Gush J, Davis PF. Clusterin/apoJ expression during the development of hemangioma. Hum Pathol. 2000;31:691–697. doi: 10.1053/hupa.2000.7638. [DOI] [PubMed] [Google Scholar]
- 22.Hough CD, Cho KR, Zonderman AB, Schwartz DR, Morin PJ. Coordinately up-regulated genes in ovarian cancer. Cancer Res. 2001;61:3869–3876. [PubMed] [Google Scholar]
- 23.Tenniswood MP, Guenette RS, Lakins J, Mooibroek M, Wong P, Welsh JE. Active cell death in hormone-dependent tissues. Cancer Metastasis Rev. 1992;11:197–220. doi: 10.1007/BF00048064. [DOI] [PubMed] [Google Scholar]
- 24.Kadomatsu K, Anzano MA, Slayter MV, Winokur TS, Smith JM, Sporn MB. Expression of sulfated glycoprotein 2 is associated with carcinogenesis induced by N-nitroso-N-methylurea in rat prostate and seminal vesicle. Cancer Res. 1993;53:1480–1483. [PubMed] [Google Scholar]
- 25.Miyake H, Gleave ME, Arakawa S, Kamidono S, Hara I. Introducing the clusterin gene into human renal cell carcinoma cells enhances their metastatic potential. J Urol. 2002;167:2203–2208. [PubMed] [Google Scholar]
- 26.Hara I, Miyake H, Gleave ME, Kamidono S. Introduction of clusterin gene into human renal cell carcinoma cells enhances their resistance to cytotoxic chemotherapy through inhibition of apoptosis both in vitro and in vivo. Jpn J Cancer Res. 2001;92:1220–1224. doi: 10.1111/j.1349-7006.2001.tb02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh J. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochem Cell Biol. 1994;72:537–545. doi: 10.1139/o94-072. [DOI] [PubMed] [Google Scholar]
- 28.Viard I, Wehrli P, Jornot L, Bullani R, Vechietti JL, Schifferli JA, Tschopp J, French LE. Clusterin gene expression mediates resistance to apoptotic cell death induced by heat shock and oxidative stress. J Invest Dermatol. 1999;112:290–296. doi: 10.1046/j.1523-1747.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 29.Zellweger T, Miyake H, Cooper S, Chi K, Conklin BS, Monia BP, Gleave ME. Antitumor activity of antisense clusterin oligonucleotides is improved in vitro and in vivo by incorporation of 2'-O-(2-methoxy)ethyl chemistry. J Pharmacol Exp Ther. 2001;298:934–940. [PubMed] [Google Scholar]
- 30.Yamori T, Kimura H, Stewart K, Ota DM, Cleary KR, Irimura T. Differential production of high molecular weight sulfated glycoproteins in normal colonic mucosa, primary colon carcinoma, and metastases. Cancer Res. 1987;47:2741–2747. [PubMed] [Google Scholar]
- 31.Longo VD, Viola KL, Klein WL, Finch CE. Reversible inactivation of superoxide-sensitive aconitase in Abeta1-42-treated neuronal cell lines. J Neurochem. 2000;75:1977–1985. doi: 10.1046/j.1471-4159.2000.0751977.x. [DOI] [PubMed] [Google Scholar]
- 32.Zellweger T, Chi K, Miyake H, Adomat H, Kiyama S, Skov K, Gleave ME. Enhanced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res. 2002;8:3276–3284. [PubMed] [Google Scholar]
- 33.Morrissey C, Lakins J, Moquin A, Hussain M, Tenniswood M. An antigen capture assay for the measurement of serum clusterin concentrations. J Biochem Biophys Methods. 2001;48:13–21. doi: 10.1016/s0165-022x(00)00137-8. [DOI] [PubMed] [Google Scholar]


