Abstract
AIM: To evaluate the value of right trisectionectomy, previously named right trisegmentectomy, in the treatment of primary liver cancer by summarizing our 13-year experience for this procedure.
METHODS: Thirty three primary liver cancer patients undergoing right trisectionectomy from Apr. 1987 to Dec. 1999 were investigated retrospectively. The impacts in survival of patients by cancerous biological behavior, such as tumor thrombi and satellite nodules, were discussed respectively. All right trisectionectomies were performed under normothermic interruption of porta hepatis at single time. Ultrasonic dissector (CUSA system 200) was used in dissection of hepatic parenchyma from Nov. 1992, instead of finger fracture.
RESULTS: 1-, 3- and 5-year survival rates were 71.9%, 40.6% and 34.4%, respectively. The longest survival term with free cancer was 150 months (alive). There were no significant differences in survival curves between cases with and without tumor thrombi (right branch of portal vein) and satellite nodules. Operative mortality was 3.0% (1/33). Main surgical complications occurred in 5 cases.
CONCLUSION: Right trisectionectomy should be regarded as an effective and safe procedure for huge primary liver cancers and is worth using more widely.
INTRODUCTION
It has been documented that primary liver cancer has been the more common cancer killer worldwide, especially in the areas with high incidence, such as China[1]. Several methods has been developed for the therapy of the malignancy[2-11]. The outcomes have taken marked progress, but recurrence and metastasis rates remain high[12,13]. Up to now, the difficult point is still existing in treatment of large liver cancers, due to worse results and higher risk[14,15]. Since the middle of last century[16], right trisectionectomy (previous trisegmentectomy) has been used for huge hepatic neoplasms covering right and left medial section. In 1975, Starzl described and clearly defined in detail a safe technique of right trisectionectomy[17]. Then he reported on 30 cases of the operation in 1980, including malignant and benign hepatic lesion[18]. In the past two decades, some papers described the procedure in treatment of hepatic malignant neoplasm, such as liver infiltration of gallbladder cancer and metastatic and primary liver cancer[19-23]. Most reports demonstrated that right trisectionectomy was effective in extensive hepatic malignancy, based on some individuals with long-term survival[19,20]. But the risk (morbidity and mortality) of liver resection remains high according to some authors[20,22,23], especially for primary liver cancer with cirrhosis. It is related to the fact that most primary liver cancer patients have a history of hepatitis and suffer a higher incidence of hepatic failure after major resection. Another reason perhaps, is occurrence of bleeding during the operation. Currently, we lack data about comprehensive evaluation of right trisectionectomy in treatment of primary liver cancer. In this study, we investigate 33 cases of right trisectionectomies retrospectively to explore the value of the procedure to deal with primary liver cancer patients.
MATERIALS AND METHODS
Patients
From April 1987 to December 1999, total of 459 primary liver cancer patients were hepatomized. Of them, 33 cases of right trisectionectomies were performed. There were 24 (72.7%) males and 9 (27.3%) females. Ages ranged from 15 to 69 years (mean ± SD, 45.9 ± 16.7 years). Hepatitis B surface antigen (HBsAg): 28 (84.8%) were positive and 5 (15.2%) were negative. There were 8 (24.2%) with slight cirrhosis and 25 (75.8%) without. Child-Pugh’s classification: 22 (66.7%) were A grade and 11 (33.3%) were B grade when the patients were hospitalized, but they were all A grade before surgical procedures through positive hepatic protective therapy. α-fetoprotein (AFP): 27 (81.8%) were elevated and 6 (18.2%) were normal. And the highest value of AFP was 20000 ng/mL. Sizes of tumor ranged from 8 to 20 cm (mean ± SD, 13.9 ± 3.4 cm). Staging (TNM[24]) of Cancer: All tumors were stage IVA (T4N0M0). Pathology: 27 (81.8%) were hepatocellular carcinoma, 2 (6.1%) were cholangiocarcinoma and 4 (12.1%) were mixed hepatocellular-cholangiocarcinoma. There were 17 cases (51.5%) with tumor thrombi in the right branch of the portal vein. 19 macroscopic satellite nodules were found in 15 cases (45.5%), and they didn’t presented in left lateral section of the liver.
Evaluation for feasibility of surgery
The feasibility of right trisectionectomy for each patient was considered carefully according to the following standards: (1) Tumors (including satellite nodules no more than 2) were limited in right and left medial section of the liver. There weren’t any evidence about cancer invasion in left lateral section. (2) Tumor masses had clear borders or psuedocapsule, and without tumor thrombus in trunk of portal vein and hepatic vein. (3) No evidence for distant metastasis. (4) Compensative enlargement of left lateral section. (5) Child-Pugh’s classification of liver function was A and indocyanine green retention rate at 15 minutes (ICGR15)[25] was lower than 15% before surgery. (6) Serum bilirubin smaller than 34 mmol·L-1, serum albumin higher than 30 g·L-1 and serum prothrombin time larger than 60% before surgery.
The situations of tumor were detected chiefly by image examinations, including B-type ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI) and angiography. To assess liver function reserve of these patients before operation, we adopted classical Child-Pugh’s classification, ICGR test and some concrete parameters, as described in standards. 146 cases of huge primary liver cancer were eliminated from the indication according to above standards. Of them, 69 cases were due to advanced tumor and the rest 77 due to bad liver function reserve.
Surgical procedures
All of the right trisectionectomies were on standard style, i.e. the resection edges were along the falciform ligaments of the liver and removed blocks were Couinaud segments 4 to 8. From November 1992, the ultrasonic dissector (CUSA system 200) was adopted for dissecting hepatic parenchyma, instead of previous finger fracture technique, introduced by Lin et al[26]. The procedures were all performed under normothermic interruption of porta hepatis at single time. Interruption lasted 15 to 40 minutes (mean ± SD, 25.3 ± 6.8 minutes). The total surgical time ranged from 165 to 312 minutes (mean ± SD, 236 ± 63 minutes). The amount of bleeding ranged from 300 to 3000 ml (mean ± SD, 1240 ± 560 ml). The quantities of transfused blood ranged from 0 to 2200 ml (mean ± SD, 1020 ± 550 ml). There were 2 cases that did not require blood transfusion during right trisectionectomy. For dissection of the liver parenchyma, we used finger fracture in 9 cases (27.3%) before November 1992 and ultrasonic dissector (CUSA System 200) in 24 cases (72.7%) after the time. Net weight of specimens ranged from 1500 to 3100 g (mean ± SD, 2 330 ± 520 g).
Adjuvant therapy and follow-up
All patients were covered in our strict follow-up after the procedure. AFP, B-type ultrosonography, computed tomography (CT) magnetic resonance imaging (MRI) and angiography were used as monitors of recurrence and metastasis. Moreover, follow-ups by mail, E-mail and telephone were taken for patients without reexaminations every year. Follow-up terms were 3 to 150 months. The latest follow-up was in November 2000 and survival terms of patients alive were calculated to October 2000. When the recurrences were found, they were treated by transcatheter arterial chemoembolization (TACE), percutaneous ethanol injection (PEI) etc. 11 patients underwent TACE, 5 patients underwent PEI and 7 patients underwent both.
Statistical analysis
Survival curves were analyzed by Kaplan-Meier and Log rank test. The χ2 and Student t test were used to determine comparability of groups. Statistically significant P value was defined as < 0.05.
RESULTS
Survival rates, recurrence and metastasis after right trisectionectomy
The postoperative survival rates at 1-, 2-, 3-, 4- and 5-years were 71.9%, 50%, 40.6%, 37.5% and 34.4%, respectively (Kaplan-Meier method). Ten cases have survived from 13 to 150 months according to the November 2000 follow-up. Survival curve of all patients is shown in Figure 1. The longest tumor-free survival term was 150 months (alive). The clinicopathological features in cases with and without satellite nodules and tumor thrombi in the right branch of the portal vein are shown in Table 1, and their survival curves were presented in Figure 2 and Figure 3, there were no significant differences (P > 0.05, Log rank test). During follow-up term, recurrence and metastasis of cancer was found in 27 patients (81.8%).
Figure 1.
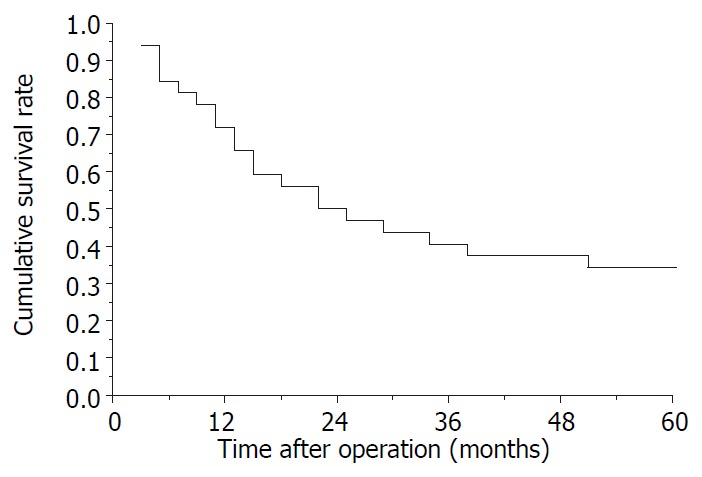
Survival curve of 32 patients underwent right trisectionectomy except 1 hospital death.
Table 1.
Clinicopathological features of patients
| Variables | TT present (17) | TT absent (16) | P | SN present (15) | SN absent (18) | P |
| Age | 43.4 (18.2) | 47.6 (16.5) | > 0.05b | 46.8 (17.6) | 45.1 (15.7) | > 0.05b |
| Sex | > 0.05a | > 0.05a | ||||
| Male | 14 (82.4) | 10 (62.5) | 13 (86.7) | 11 (61.1) | ||
| Female | 3 (17.6) | 6 (37.5) | 2 (13.3) | 7 (38.9) | ||
| LC | > 0.05a | > 0.05a | ||||
| Present | 4 (23.5) | 4 (25.0) | 5 (33.3) | 3 (16.7) | ||
| Absent | 13 (76.5) | 12 (75.0) | 10 (66.7) | 15 (83.3) | ||
| AFP | > 0.05a | > 0.05a | ||||
| Elevated | 15 (88.2) | 12 (75.0) | 13 (86.7) | 14 (77.8) | ||
| Normal | 2 (11.8) | 4 (25.0) | 2 (13.3) | 4 (22.2) | ||
| TS (cm) | 12.5 (2.6) | 16.7 (5.8) | > 0.05b | 14.4 (3.8) | 13.2 (3.1) | > 0.05b |
| BL (ml) | 1290 (640) | 1210 (450) | > 0.05b | 1300 (680) | 1220 (390) | > 0.05b |
| BT (ml) | 1100 (720) | 980 (370) | > 0.05b | 1050 (510) | 990 (570) | > 0.05b |
| WS (g) | 2280 (490) | 2350 (530) | > 0.05b | 2380 (570) | 2300 (460) | > 0.05b |
| Pathology | > 0.05a | > 0.05a | ||||
| HCC | 13 (76.5) | 14 (87.6) | 12 (80.0) | 15 (83.3) | ||
| CC | 1 (5.9) | 1 (6.2) | 1 (6.7) | 1 (5.6) | ||
| MHCC | 3 (17.6) | 1 (6.2) | 2 (13.3) | 2 (11.1) |
Values in parentheses are percentages or standard errors. TT, tumor thrombus; SN, satellite nodule; LC, liver cirrhosis; AFP, α-fetoprotein; TS, tumor size; BL, blood loss; BT, blood transfusion; WS, weight of specimen; HCC, hepatocellular carcinoma; CC, cholangiocarcinoma; MHCC, mixed hepatocellular-cholangiocarcinoma.
: χ2 test,
: Student t test.
Figure 2.
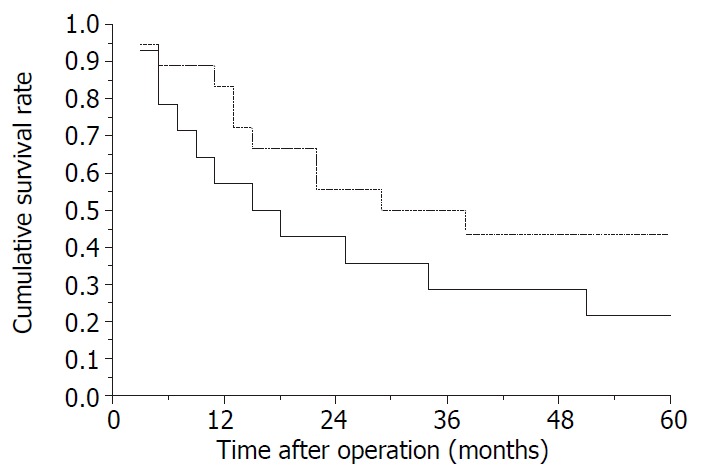
Survival curves of patients. —, with satellite nodules (SN); —, without satellite nodules. P > 0.05, Log rank test.
Figure 3.
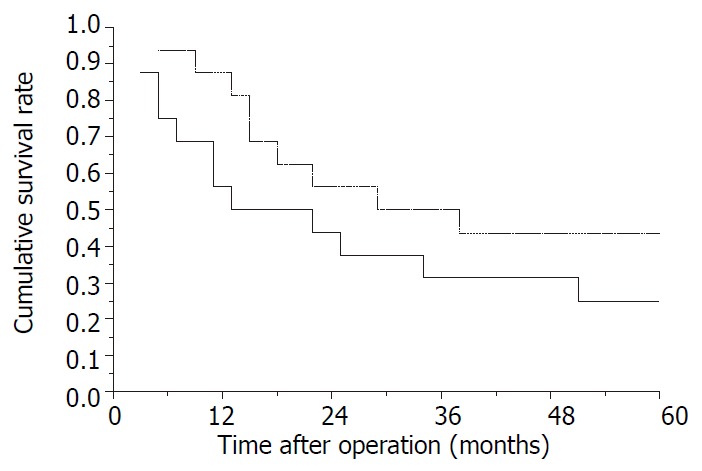
Survival curves of patients. —, with tumor thrombus (TT); —, without tumor thrombus. P > 0.05, Log rank test.
Operative mortality after right trisectionectomy
There was one patient who died of hepatic failure within one month after the operation. The operative mortality was 3.0% (1/33).
Surgical complications after right trisectionectomy
There were 5 cases (15.2%) that developed main surgical complications after right trisectionectomies, including 2 cases of hepatic failure, 2 cases of bile leakage and 1 case of secondary bleeding. Four patients recovered by positive reoperation excluding 1 case of hepatic failure.
DISCUSSION
In the therapy of primary liver malignancy, curative hepatic resection has been regarded as a primary method for radical treatment. But for hepatoma mainly in the right lobe and which invades the medial section of the left lobe, resection has always been difficult. Despite the fact that there has been research about right trisectionectomy, previous trisegmentectomy, mortality and morbidity remains high. There are few studies with large sample groups of primary liver cancer patients treated with right trisectionectomy. Starzl[18] reported on 19 cases of primary hepatic malignant tumors (14 primary liver cancers and 5 other types of tumors) treated by the procedure, The 1-year survival rate was more than 50%. Holbrook et al[27] reported on 13-years of experience in resection of malignant primary liver tumors, including 9 right trisectionectomies. Overall 1-, 2-, 3-, and 5-year survival rates were 57%, 52%, 40% and 33%, respectively. Mortality was 7% and complication incidence was 26%. Kumada et al[28] reported on 11 cases of tri- and bi-sectionectomy for HCC, mean survival time was 16 months, mortality was 15.4%. We had reported 4 right trisectionectomies to treat extensive primary liver cancers in 1991[29]. Three cases survived more than 1 year. From April 1987 to December 1999, we performed 33 right trisectionectomies for primary liver cancer patients, 1-, 2-, 3-, 4- and 5-year survival rates were 71.9%, 50%, 40.6%, 37.5% and 34.4%, respectively. The longest term of survival with free cancer was 150 months. Mortality was 3.0% and the surgical complication rate was 15.2%. These results are related to new advances in liver surgery. To control severe intraoperative bleeding, we used normothermic interruption of the porta hepatis at single time. Previously, we reported on 20 cases of hemihepatectomy using this interruption method. Manipulation appeared simple and convenient. Mortality was 0%[30]. These data suggested that normothermic interruption of the porta hepatis at single time should be regarded as an effective and safe method to limit bleeding in liver surgery. There were two patients in this study that were trisectionectomized without blood transfusion. Besides, the use of ultrasonic dissector in later period made the operative fields more clear. Thus, manipulations became more convenient and accurate. Meanwhile, the low incidence of complications and mortality were related to the accurate estimation of liver functional reserve prior to operation. We adopted the Child-Pugh’s classification, some detailed parameters and ICGR test. Child-Pugh’s classification was a classical method for estimating liver function. It could become more accurate if helped by other concrete markers, such as serum bilirubin, prothrombin time and albumin. Besides, ICGR test, a proven sensitive indicator of liver function reserve[25], also provided important information. Our experience is that the combination of these parameters could accurately predict liver function reserve.
In the present study, we analyzed the influence of some pathological features on outcome of right trisectionectomy for huge primary liver cancers. The clinicopathological features showed in Table 1 suggested the comparability between patients with and without tumor thrombi or satellite nodules (all P > 0.05). And no significant differences could be found in their survival curves (P > 0.05), in spite of some differences in these curves. These findings suggest that surgeons should use curative resection in therapy of huge tumors, even in those with a few satellite nodules and tumor thrombi, if the tumor thrombi are only in the right branch of the portal vein, a satisfactory effect could be expected.
In conclusion, right trisectionectomy is an effective and safe procedure and should become one of strategies, and surgical arts in the treatment of huge tumor of primary liver cancers.
Footnotes
Edited by Yuan HT
References
- 1.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the world-wide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Makuuchi M, Takayama T, Kubota K, Kimura W, Midorikawa Y, Miyagawa S, Kawasaki S. Hepatic resection for hepatocellular carcinoma -- Japanese experience. Hepatogastroenterology. 1998;45 Suppl 3:1267–1274. [PubMed] [Google Scholar]
- 3.Yamamoto J, Iwatsuki S, Kosuge T, Dvorchik I, Shimada K, Marsh JW, Yamasaki S, Starzl TE. Should hepatomas be treated with hepatic resection or transplantation? Cancer. 1999;86:1151–1158. doi: 10.1002/(sici)1097-0142(19991001)86:7<1151::aid-cncr8>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen P, Hoffman A, Howerton R, Loggie BW. Cryosurgery of close or positive margins after hepatic resection for primary and metastatic hepatobiliary malignancies. Am Surg. 2002;68:695–703; discussion 703. [PubMed] [Google Scholar]
- 5.Pelletier G, Ducreux M, Gay F, Luboinski M, Hagege H, Dao T, Van Steenbergen W, Buffet C, Rougier P, Adler M, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129–134. doi: 10.1016/s0168-8278(98)80187-6. [DOI] [PubMed] [Google Scholar]
- 6.Livraghi T, Benedini V, Lazzaroni S, Meloni F, Torzilli G, Vettori C. Long term results of single session percutaneous ethanol injection in patients with large hepatocellular carcinoma. Cancer. 1998;83:48–57. [PubMed] [Google Scholar]
- 7.Ohmoto K, Tsuduki M, Shibata N, Takesue M, Kunieda T, Yamamoto S. Percutaneous microwave coagulation therapy for hepatocellular carcinoma located on the surface of the liver. AJR Am J Roentgenol. 1999;173:1231–1233. doi: 10.2214/ajr.173.5.10541094. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 9.Cheng SH, Lin YM, Chuang VP, Yang PS, Cheng JC, Huang AT, Sung JL. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025–1033. doi: 10.1046/j.1440-1746.1999.01994.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu CL, Fan ST, Ng IO, Lo CM, Poon RT, Wong J. Treatment of advanced hepatocellular carcinoma with tamoxifen and the correlation with expression of hormone receptors: a prospective randomized study. Am J Gastroenterol. 2000;95:218–222. doi: 10.1111/j.1572-0241.2000.01688.x. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, Bianchi L, Ayuso C, Vargas V, Rodés J, Bruix J. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000;31:54–58. doi: 10.1002/hep.510310111. [DOI] [PubMed] [Google Scholar]
- 12.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuto T, Hirohashi K, Kubo S, Tanaka H, Yamamoto T, Ikebe T, Kinoshita H. Efficacy of major hepatic resection for large hepatocellular carcinoma. Hepatogastroenterology. 1999;46:413–416. [PubMed] [Google Scholar]
- 14.Hanazaki K, Kajikawa S, Shimozawa N, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Hepatic resection for large hepa-tocellular carcinoma. Am J Surg. 2001;181:347–353. doi: 10.1016/s0002-9610(01)00584-0. [DOI] [PubMed] [Google Scholar]
- 15.Régimbeau JM, Farges O, Shen BY, Sauvanet A, Belghiti J. Is surgery for large hepatocellular carcinoma justified? J Hepatol. 1999;31:1062–1068. doi: 10.1016/s0168-8278(99)80319-5. [DOI] [PubMed] [Google Scholar]
- 16.Quattlebaum JK. Massive resection of the liver. Ann Surg. 1953;137:787–796. doi: 10.1097/00000658-195306000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Bell RH, Beart RW, Putnam CW. Hepatic trisegmentectomy and other liver resections. Surg Gynecol Obstet. 1975;141:429–437. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Koep LJ, Weil R, Lilly JR, Putnam CW, Aldrete JA. Right trisegmentectomy for hepatic neoplasms. Surg Gynecol Obstet. 1980;150:208–214. [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto M, Miura K, Yoshioka M, Matsumoto Y. Disease-free survival for 9 years after liver resection for stage IV gallbladder cancer: report of a case. Surg Today. 1995;25:750–753. doi: 10.1007/BF00311494. [DOI] [PubMed] [Google Scholar]
- 20.Sugiura Y, Nakamura S, Iida S, Hosoda Y, Ikeuchi S, Mori S, Sugioka A, Tsuzuki T. Extensive resection of the bile ducts combined with liver resection for cancer of the main hepatic duct junction: a cooperative study of the Keio Bile Duct Cancer Study Group. Surgery. 1994;115:445–451. [PubMed] [Google Scholar]
- 21.Chi DS, Fong Y, Venkatraman ES, Barakat RR. Hepatic resection for metastatic gynecologic carcinomas. Gynecol Oncol. 1997;66:45–51. doi: 10.1006/gyno.1997.4727. [DOI] [PubMed] [Google Scholar]
- 22.Iwatsuki S, Starzl TE. Experience with resection of primary hepatic malignancy. Surg Clin North Am. 1989;69:315–322. doi: 10.1016/s0039-6109(16)44788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakabayashi H, Okada S, Maeba T, Maeta H. Effect of preoperative portal vein embolization on major hepatectomy for advanced-stage hepatocellular carcinomas in injured livers: a preliminary report. Surg Today. 1997;27:403–410. doi: 10.1007/BF02385702. [DOI] [PubMed] [Google Scholar]
- 24.Skeel RT. Carcinomas of the pancreas, liver, gallbladder. In: Skeel RT, eds , editors. Handbook of cancer chemotherapy (Fifth edition) Philadelphia: Lippincott Williams & Wilkins; 1997. p. 249. [Google Scholar]
- 25.Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–1259. [PubMed] [Google Scholar]
- 26.Lin TY, Lee CS, Chen KM, Chen CC. Role of surgery in the treatment of primary carcinoma of the liver: a 31-year experience. Br J Surg. 1987;74:839–842. doi: 10.1002/bjs.1800740931. [DOI] [PubMed] [Google Scholar]
- 27.Holbrook RF, Koo K, Ryan JA. Resection of malignant primary liver tumors. Am J Surg. 1996;171:453–455. doi: 10.1016/S0002-9610(96)00001-3. [DOI] [PubMed] [Google Scholar]
- 28.Kumada K, Ozawa K, Okamoto R, Takayasu T, Yamaguchi M, Yamamoto Y, Higashiyama H, Morikawa S, Sasaki H, Shimahara Y. Hepatic resection for advanced hepatocellular carcinoma with removal of portal vein tumor thrombi. Surgery. 1990;108:821–827. [PubMed] [Google Scholar]
- 29.Rui JA. [Right trisegmentectomy for primary liver cancer--a report of 4 cases with review of literature] Zhonghua Zhongliu Zazhi. 1991;13:37–39. [PubMed] [Google Scholar]
- 30.Rui JG, Qu JY, Su Y, Li ZW, Wang K, Zhu GJ, Wu JX, Chen GJ, Wang CF, Mao XW. [Hemihepatectomy under hepato-portal interruption at normal temperature for liver malignancies--a report of 20 patients] Zhonghua Zhongliu Zazhi. 1987;9:221–223. [PubMed] [Google Scholar]


