Abstract
AIM: A single-chain antibody fragment, ND-1scFv, against human colorectal carcinoma was constructed and expressed in E.coli, and its biodistribution and pharmacokinetic properties were studied in mice bearing tumor.
METHODS: VH and VL genes were amplified from hybridoma cell IC-2, secreting monoclonal antibody ND-1, by RT-PCR, and connected by linker (Gly4Ser)3 to form scFv gene, which was cloned into expression vector pET 28a(+) and finally expressed in E.coli. The expressed product ND-1scFv was purified by metal affinity chromatography using Ni-NTA, its purity and biological activity were determined using SDS-PAGE and ELISA. ND-1scFv was labeled with 99mTc, and then injected into mice bearing colorectal carcinoma xenograft for phamacokinetic study in vivo.
RESULTS: SDS-PAGE analysis showed that the relative molecular weight of recombinant protein was 30 kDa with purity of 94%. ELIAS assay revealed that ND-1scFv retained the immunoactivity of parent mAb, being capable of binding specifically to human colorectal carcinoma cell line expressing associated antigen. Radiolabeled ND-1scFv exhibited rapid tumor targeting, with specific distribution in mice bearing colorectal carcinoma xenograft observed as early as 1 h following injection. In vivo pharmacokinetic studies also demonstrated that ND-1scFv had very rapid plasma clearance (T1/2α of 5.7 min, T1/2β of 2.6 h).
CONCLUSION: ND-1scFv shows significant immunoactivity, and better pharmacokinetic and biodistribution characteristics compared with intact mAbs, demonstrating the possibility as a carrier for tumor-imaging.
INTRODUCTION
Colorectal carcinoma is one of the common malignant tumors with relatively high incidence, occupying the fourth rate of mortality in China. Therefore, efficient diagnosis and therapeutic approaches are important for colorectal carcinoma research. Although in recent years some progress has been made in respect to application of monoclonal antibodies for the therapy and diagnosis of colorectal carcinoma, most mAbs are of murine origin, so that repeated administration can induce human anti-mouse antibodies (HAMA), moreover, intact mAbs are generally too large (Mt 150000) to penetrate tumor masses, which can severely limit the efficacy of antibody in clinical utilization[1]. To overcome such deficiencies, gene engineering antibody, including human origin antibodies, single chain Fv (scFv), human-murine chimed antibodies are developed to improve murine origin mAbs[2-9]. ScFv, which is comprised of immunoglobulin heavy- and light-chain variable regions that are connected by a short peptide linker, is the gene engineered antibody employed most widely at present. The main advantages of scFv over intact mAbs and Fab fragment are their small size (Mr 30000, amounting to one sixth of intact mAbs), making them penetrate a solid tumor mass rapidly and evenly. In addition, the lack of Fc domains makes them less immunogenic responsive and less capable of binding to Fc receptors distributed on normal cells. These characteristics make scFv potentially useful in tumor diagnosis and therapy as a carrier[10,11].
ND-1 is a murine monoclonal antibody against tumor-associated antigen LEA , mainly expressed in human colorectal carcinoma, developed by Jindan Song in 1986, which was obtained by immunizing Balb/c mouse with CCL-187 human colorectal carcinoma cell line. The histochemical determination of one thousand pathologic samples showed that ND-1 binded specifically to well differentiated and moderately differentiated colorectal carcinoma tissues and its specificity is superior to mAbs against CEA. 131I labeled ND-1 also exhibited excellent imaging of tumor tissue in mice bearing colorectal carcinoma xenograft. We constructed a scFv by gene engineering technology from the VL and VH of ND-1, a monoclonal antibody against human colorectal carcinoma, and determined the biological properties of ND-1 scFv in vivo and in vitro.
MATERIALS AND METHODS
Materials
IC-2 is murine hybridoma cell that secrets monoclonal antibody ND-1against human colorectal carcinoma. Both IC-2 and HeLa human cervical carcinoma cell line were from our group. pET28a(+) expression vector and E.coli BL21 were kindly provided by Dr. YH. Chen. CCL-187 human colorectal carcinoma cell line was kindly provided by Tumor Research Institution of Medical College of Harvard University. pMD18-T vector, E.coli JM109 component cell, DNA polymerase, restriction enzyme, and DNA recovery kit were purchased from TarkaRa Biotechnology (Dalian, China). mRNA purification kit and T4 DNA ligase were bought from Pharmacia Biotech. Anti-His6 tag antibody was from Invitrogen. Ni-NTA resin was provided by Qaigen company. MDP and 99mTc were kindly provided by Department of Nuclear Medicine at China Medical University. Heavy chain primer 1 and 2, light chain primer mix, linker primer mix, and RS primer mix was purchased from Pharmacia Biotech.
Genetic construction of ND-1scFv
ND-1scFv gene was constructed as previously described. Briefly, mRNA was extracted from 5 × 106 hybridoma cells IC-2 and cDNA was synthesized by reverse transcription using random primer. VH and VL gene were separately amplified from the cDNA by PCR using heavy chain primer and light primer mix. The VH and VL gene fragments were recovered and mixed in equimolar ratios for two PCR reactions, the first one using linker primer mix for 7 cycles, followed by the second one using RS primer mix for 30 cycles. As a result, VH and VL gene fragments were connected to form scFv gene by extension overlap splicing PCR, and then, obtained ND-1 scFv gene was cloned into pMD18-T, and transformed into E.coli JM109, positive clones were identified by colony PCR and DNA sequencing.
Oligonucleotide primers S1 and S2 were designed to add EcoR I site at the 5’ end of ND-1scFv, and Hind III site, Sal I site at the 3’end. S1: 5’ACTGAATTCATGGCCCAGGTGCAGCTGCAGC3’, S2: 5’CGCAAGCTTCTAGTCGACTTTCCAGCTTGGTC3’. pMD18-T-ND-1scFv was used as template for a PCR by primer S1 and S2, and the product was cloned into the vector pET28a(+) after digestion with EcoR I and Hind III, and then transformed into competent E.coli BL21cells for protein expression.
DNA sequencing
ND-1scFv genes cloned into pMD18T and pET28a(+) were sequenced by the dideoxy chain termination method with M13 primer, T7 promoter primer and T7 terminator primer.
Expression and purification of ND-1scFv
E.coli BL21 cells containing pET28a(+)-ND-1scFv plasmid were grown in 100 ml LB broth with 50 µg/mL kanamycin at 37 °C, when O.D600 of the culture attained about 0.6, IPTG was added in a final concentration of 1 mmol, and cells were shaken at 37 °C, after 3.5 h, the culture was centrifuged at 5000 rpm for 10 min, the cell pellet was treated with lyses solution. After sonication and centrifugation, inclusion body containing scFv protein was solubilized and denatured in the presence of 6 mol/L Guanidine hydrochloride. Affinity chromatography on Ni-NTA resin was performed to purify scFv, the column was eluted with 8 mol/L urea at pH8.0, pH6.5 and pH4.2, and the component of pH4.2, containing scFv, was collected, following renaturing by dialysis. Purity and concentration of protein were determined with Bradford assay.
ELISA assay for activity of ND-1scFv
CCL-187 cells and HeLa cells (5 × 104) were grown in 96-well microtiter plates at 37 °C for 24 h, then fixed with 2.5% glutaradehyde and blocked with 1% BSA, followed by incubation with ND-1IgG or ND-1scFv at 37 °C for 2 h; after washing 3 times with PBS, anti-His6 antibody was added into wells with ND-1scFv and incubated as above, the plate was washed and HRP-labeled goat anti-mouse IgG was added into both ND-IgG and ND-1scFv wells, incubating at 37 °C for 2 h, substrate TMB was added, incubated in darkness for 30 min, the reaction was terminated with1N H2SO4; PBS was used as a negative control.
Tumor model
Human colorectal carcinoma cells (1 × 106 ) were injected s.c. into the back of athymic mice (nu/nu) (4-6 weeks old). When a tumor developed at 0.5-1.5 cm in diameter, biodistribution and pharmacokinetics were studied.
Biodistribution and pharmacokinetics studies
ND-1scFv and ND-1IgG were labeled with 99mTc using MDP. Excess β-mercaptoethanol was added to the solution containing ND-1scFv and ND-1IgG, reduced product (1 mg) was mixed with 40 µl MDP (2.5 mg/mL) and 370MBq 99mTc. Biodistribution study was performed using tumor-bearing mice injected i.p. with 0.2 ml 99mTc-ND-1scFv, the mice were killed at different periods. Blood, tumor and all the main organs were collected and weighed. The radioactivity was counted in a gamma scintillation counter. The T/NT value for each organ was calculated.
Pharmacokinetic study was performed by the tumor- bearing mice injected via the tail vein with 0.1 ml 99mTc-ND-1scFv and 99mTc-ND-1 IgG. Blood samples were obtained via tail bleeds at 0, 5, 10, 15, 30, 60, 120, 180 min and 24 h after injection, the radioactivity was counted in a gamma scintillation counter, and pharmacokinetic parameters were calculated.
RESULTS
Clone of ND-1scFv gene
VH and VL gene were amplified from hybridoma cell IC-2 that secreted monoclonal antibody against human colorectal carcinoma, and then were connected by a linker (Gly3Ser)4 using extension overlap splicing PCR to construct scFv gene, which had EcoR I site at 5’ end and Hind III site at 3’ end. scFv gene was cloned into the vector pET28a(+) and expressed in E.coli BL21. Restriction enzyme digestion analysis showed scFv gene had been accurately inserted into vector pET28a (+). Sequence analysis revealed that scFv gene consisted of 732 bp, encoding 243 amino acids. Of which, 354 bp for heavy chain gene, was located upstream of scFv gene, and 330 bp for the light chain gene, was located downstream. They were connected by a 45 bp linker sequence. The deduced protein sequence of ND-1scFv was showed in Figure 1.
Figure 1.
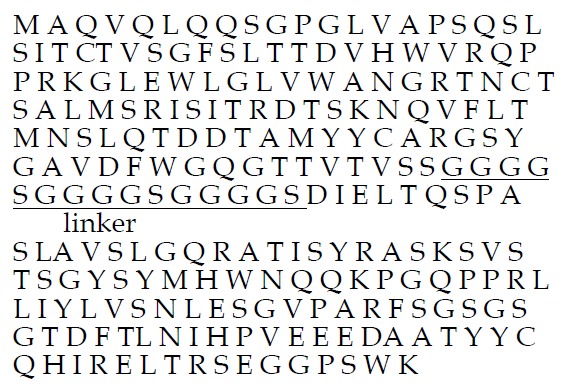
Amino acid sequences of ND-1scFv deduced from nucleotide sequences.
Expression and purification of ND-1scFv
Plasmid ND-1scFv-pET28a(+) was transformed into E.coli BL21, the protein was expressed with induction of IPTG. SDS-PAGE analysis showed that the lysates of BL21 cell expressing scFv protein exhibited a new protein band with molecular weight at 30 kDa (Figure 2). Because a sequence encoding a short peptide His-tag exists at the upstream of multi-clone site (MCS) of vector pET28a(+), ND-1scFv was expressed as a recombinant fusion protein with His tag, consisting of 26 kDa for scFv and 4 kDa for His-tag and its upstream sequence, which was consistent with the theoretically predicted value. SDS-PAGE analysis also showed that no new protein component was found in the supernatant of cell lysate of E.coli BL21induced by IPTG, which indicated scFv protein was expressed in the form of inclusion body. Inclusion body protein was purified by metal affinity chromatography using Ni-NTA resin which could bind to the His-tag protein marker located on the N terminal end of scFv specifically, purity of purified scFv was 94% purity.
Figure 2.
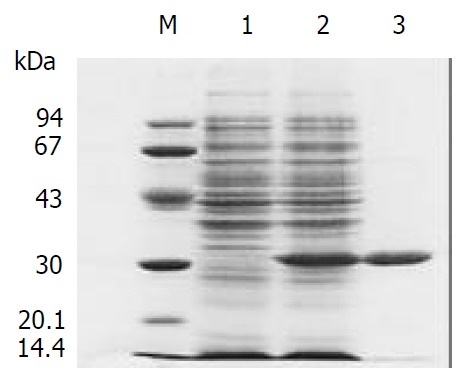
Expressed ND-1scFv. M: Protein marker; 1: Expres-sion of pET28a(+)-ND-1scFv without induction; 2: Expression of pET28a(+)-ND-1scFv with induction of IPTG; 3: Purified ND-1scFv protein.
Determination of immunoactivity of ND-1scFv
The immunoreactivity of purified ND-1scFv was determined by ELISA, the result revealed that scFv exhibited an immunoreactivity similar to the parent ND-1 antibody, and showed strong binding to CCL-187 cells expressing colorectal carcinoma associated antigen LEA, and weak binding to LEA-negative HeLa cells. This suggested that scFv had excellent specificity and still retained higher activity after undergoing refolding and purifying procedures(Table 1).
Table 1.
Immune activity of ND-1scFv determined by ELISA
| Sample |
OD450nm (-x ± s) |
|
| CCL-187 | HeLa | |
| ND-1IgG | 1.92 ± 0.28 | 0.20 ± 0.06 |
| ND-1scFv | 0.87 ± 0.17 | 0.19 ± 0.03 |
| PBS | 0.14 ± 0.03 | 0.13 ± 0.01 |
In vivo distribution studies
ND-1scFv was labeled with 99mTc. 99mTc-ND-1scFv was injected into mice bearing the CCL-187 xenograft for biodistribution studies. Radioactivity in blood, tumor and normal tissue was determined at 1 and 3 h following injection, and the ratios of radioactivity between tumor tissue and normal tissue (T/NT) were evaluated. The result showed that labeled scFv displayed rapid localization in tumors, accumulation was found in tumors in high concentrations 1 hour after injection, and scFv uptake in tumor was significantly higher than that in other normal tissues (Table 2).
Table 2.
Distribution of 99mTc- labeled ND-1scFv in mice-bear-ing tumor (-x ± s)
| Tissue |
T/NT value |
|
| 1 h | 3 h | |
| Blood | 2.61 ± 0.97 | 2.16 ± 1.05 |
| Liver | 1.20 ± 0.40 | 1.75 ± 1.10 |
| Spleen | 2.72 ± 0.10 | 1.23 ± 0.65 |
| Kidney | 0.07 ± 0.05 | 0.26 ± 0.01 |
| Heart | 1.75 ± 0.51 | 1.90 ± 0.60 |
| Lung | 0.83 ± 0.31 | 0.62 ± 0.16 |
Pharmocokinetic studies
Studies were conducted to define the pharmocokinetic properties of plasma clearance of 99mTc labeled ND-1scFv in mice bearing tumor (Table 3). Compared with intact ND-1 IgG, 99mTc-ND-1scFv exhibited an extremely rapid clearance from the plasma, 80% of the scFv was cleared out of the plasma pool at 15 min following injection, T1/2α phase for the scFv was 5.7 min, T1/2β phase was 2.6 h, while T1/2α and T1/2β for the ND-1 were 60 min and 18 h, respectively.
Table 3.
Pharmacokinetic parameter of 99mTc-labeled ND-1scFv in mice-bearing tumor
| ND-1IgG | ND-1scFv | |
| Alpha half-life (min) | 5.7 | 60 |
| Beta half-life (h) | 2.6 | 18 |
DISCUSSION
The critical issue in application of mAbs is its high specificity and good in vivo biological features. In this study, single chain Fv ND-1scFv against human colorectal carcinoma was constructed by fusing gene of variable region of heavy chain with gene of variable region of light chain, and the ND-1scFv protein was functionally expressed in E.coli. ELISA analysis showed that ND-1scFv had an immunoactivity similar to the parent ND-1 mAbs, and binded specifically to the CCL-187 human colorectal carcinoma cell that expressed associated antigen LEA. This suggests that ND-1 mAb with only a Fv segment still retained its immunoactivity of binding to corresponding antigens, which is consistent to the previous reports. In addition, ND-1scFv also exhibited excellent specific distribution and pharmacokinetic characteristic in tumor-bearing mice.
A linker sequence was required to connect VH and VL for the construction of scFv, the linker widely used at present was a 15-amino acid sequence consisting of repetition of four Gly and one Ser (GGGGS)[12-15]. In this study, the (Gly4Ser)3 sequence was used, and the fusion molecule was constructed in VH-linker-VL order, the expressed ND-1scFv protein retained favorable stability during the renaturing and dialyzing, and retained biological activity similar to the parent antibody. In addition, a Sal I site was provided at scFv 3’ end except for adding a Hind III site for ligasing the vector. In another experiment, we have already constructed a fusion protein of ND-1scFv and yeast cytosine deaminase using the same restriction site.
E.coli gene expression system is known as the earliest developed and most widely applied system for gene engineering. Although expressed proteins are usually lack of the effective modification such as glycosylation, there are some evidences suggesting that a variety of antibody fragments expressed in E.coli were able to fold and assemble correctly into bioactive products without the processing[16-20], which was also confirmed in own studies. pET vector which belongs to the T7 expression system propagating in E.coli was used to express of ND-1scFv. This vector contains T7 promoter, which can achieve high level controlled gene transcription in the presence of T7 RNA polymerase[19,21-24]. ND-1scFv protein was intensively expressed to 17% of the total bacterial protein. In addition, a 6×His tag sequence exists at the upstream of clone site in the pET vector, which was expressed in the form of fusion protein with the downstream scFv. Since it did not influence the bioactivity of expressed products, no enzyme hydrolysis process was required to remove it from the final products. This simplified the whole expression procedure. It was even more worth noticing that this sequence could be used as a protein marker for the determination and purification of expressed proteins[25-27]. ND-1scFv protein was purified by metal affinity chromatography using Ni-NTA resin which can bind to His tag specifically located on -NH3 of scFv. SDS-PAGE analysis showed that the purity of ND-1scFv was as high as 94%, and the concentration was 1.5 mg/mL, demonstrating its potential usefulness in clinical application.
After being reconstructed into small molecules, the molecular weight of mAbs usually reduced to as 1/3 or 1/6 of intact mAbs, which significantly increased their penetrability to tumor tissue. Related experimental observation revealed that intact mAbs mainly concentrated nearby the blood vessel, while scFv seemed to be distributed uniformly within the tumor tissue and performing targeting function with high efficiency[28,29]. Furthermore, scFv exhibited two-phase pharmacokinetic characteristic in vivo, its T1/2α (equilibrium phase) is much shorter than that of intact mAbs, implying that the in vivo equilibrated distribution of scFv may be reached rapidly, and its penetration into the interior of solid tumor could be achieved in a short time. In our experiment, 99mTc labeled ND-1scFv accumulated in tumor tissue in high concentration rapidly only 1 h after being injected into mice bearing xenograft. The radioactivity was significantly higher than that in most of normal tissues, while intact ND-1 required 20-24 h to obtain similar accumulation. Plasma pharmacokinetic studies in mice bearing tumor also showed a rapid plasma clearance of ND-1scFv superior to intact ND-1. Strong penetrability, rapid localization and elimination are the main biological behavior of scFv in vivo, making it an ideal localizing diagnostic agent for clinical applications. These were further validated by the immunoimaging experiments in mice bearing tumor using a various of scFvs against different tumor antigen[30-32]. Hitherto, the superiority of ND-1 developed by our group over the commercial product, mAb vs CEA, both in specificity and affinity, has been demonstrated in a number in vivo and in vitro experiments. Thus ND-1scFv, constructed from VH and VL of ND-1, may provide a new approach for clinical diagnosis and treatment of human colorectal carcinoma.
In this study, we observed that labeled scFv simultaneously accumulated intensively in kidney and in tumors of mice bearing xenograft, which also has been reported by other researchers[30]. On one hand, relative small size of scFv promotes its rapid uptake by kidney, so that the accumulation in kidney occurs shortly after injection, on the other hand, the half life of 99mTc is shorter, which, although beneficial for in vivo fast imaging, also increases the uptake of labeled scFv by kidney[28]. Recently, Goel et al[33,34] constructed divalent [sc(Fv)2] and tetravalent {[sc(Fv)2]2} by covalent interaction, which increased the valence of scFvs and improved their affinity. Compared to the monovalent scFv, the divalent scFvs showed approximately 20-fold higher affinities. Furthermore, the molecular weight of multivalent scFvs was larger than scFv, but still smaller than intact IgG, so the in vivo pharmacokinetic behavior would be more promising[35-40]. Some researchers suggested that this uptake also may be related to the IP of the scFv, thus, there exists the possibility of directly modifying the isoelectric point of the scFv by introducing mutation in framework regions. A lower IP may reduce non-specific uptake into tissues such as the kidney[31]. The ND-1scFv constructed in this study retained the immunoactivity of parent mAbs and the clinical application are demonstrated preliminarily in radiolabling experiment with mice bearing tumor. With further development, it may become a promising targeting carrier for clinical diagnosis.
Footnotes
Supported by the Natural Science Foundation of China, No.85-722-18-02
Edited by Ren SY
References
- 1.Borsi L, Balza E, Bestagno M, Castellani P, Carnemolla B, Biro A, Leprini A, Sepulveda J, Burrone O, Neri D, et al. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer. 2002;102:75–85. doi: 10.1002/ijc.10662. [DOI] [PubMed] [Google Scholar]
- 2.He FT, Nie YZ, Chen BJ, Qiao TD, Fan DM, Li RF, Kang YS, Zhang Y. Expression and identification of recombinant soluble single-chain variable fragment of monoclonal antibody MC3. World J Gastroenterol. 2002;8:258–262. doi: 10.3748/wjg.v8.i2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu ZC, Ding J, Nie YZ, Fan DM, Zhang XY. Preparation of single chain variable fragment of MG(7) mAb by phage display technology. World J Gastroenterol. 2001;7:510–514. doi: 10.3748/wjg.v7.i4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SH, Chun JH, Park SY. Characterization of monoclonal antibodies against carcinoembryonic antigen (CEA) and expression in E. coli. Hybridoma. 2001;20:265–272. doi: 10.1089/027245701753179857. [DOI] [PubMed] [Google Scholar]
- 5.Pavlinkova G, Colcher D, Booth BJ, Goel A, Batra SK. Pharma-cokinetics and biodistribution of a light-chain-shuffled CC49 single-chain Fv antibody construct. Cancer Immunol Immunother. 2000;49:267–275. doi: 10.1007/s002620000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arakawa F, Yamamoto T, Kanda H, Watanabe T, Kuroki M. cDNA sequence analysis of monoclonal antibody FU-MK-1 specific for a transmembrane carcinoma-associated antigen, and construction of a mouse/human chimeric antibody. Hybridoma. 1999;18:131–138. doi: 10.1089/hyb.1999.18.131. [DOI] [PubMed] [Google Scholar]
- 7.de Kleijn EM, Punt CJ. Biological therapy of colorectal cancer. Eur J Cancer. 2002;38:1016–1022. doi: 10.1016/s0959-8049(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 8.Allison DE, Gourlay SG, Koren E, Miller RM, Fox JA. Pharmaco-kinetics of rhuMAb CD18, a recombinant humanised monoclonal antibody fragment to CD18, in normal healthy human volunteers. Bio Drugs. 2002;16:63–70. doi: 10.2165/00063030-200216010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Al-Yasi AR, Carroll MJ, Ellison D, Granowska M, Mather SJ, Wells CA, Carpenter R, Britton KE. Axillary node status in breast cancer patients prior to surgery by imaging with Tc-99m humanised anti-PEM monoclonal antibody, hHMFG1. Br J Cancer. 2002;86:870–878. doi: 10.1038/sj.bjc.6600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlinkova G, Beresford GW, Booth BJ, Batra SK, Colcher D. Pharmacokinetics and biodistribution of engineered single-chain antibody constructs of MAb CC49 in colon carcinoma xenografts. J Nucl Med. 1999;40:1536–1546. [PubMed] [Google Scholar]
- 11.Kim DJ, Chung JH, Ryu YS, Rhim JH, Kim CW, Suh Y, Chung HK. Production and characterisation of a recombinant scFv reactive with human gastrointestinal carcinomas. Br J Cancer. 2002;87:405–413. doi: 10.1038/sj.bjc.6600365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi N, Shibahara K, Ikegashira K, Shibusawa K, Goto J. Single-chain Fv fragments derived from an anti-11-deoxycortisol antibody. Affinity, specificity, and idiotype analysis. Steroids. 2002;67:733–742. doi: 10.1016/s0039-128x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 13.Ren X, Gao H, Su K, Chen W, Yang A, Yu Z. Construction and nucleotide sequence assay of human anti-HBs variable region single-chain antibody gene. Shengwu Yixue Gongchengxue Zazhi. 2000;17:484–486. [PubMed] [Google Scholar]
- 14.Nakayashiki N, Yoshikawa K, Nakamura K, Hanai N, Okamoto K, Okamoto S, Mizuno M, Wakabayashi T, Saga S, Yoshida J, et al. Production of a single-chain variable fragment antibody recognizing type III mutant epidermal growth factor receptor. Jpn J Cancer Res. 2000;91:1035–1043. doi: 10.1111/j.1349-7006.2000.tb00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto Y, Ikenaga T, Tanigawa K, Ueda T, Ezak I, Imoto T. Expression and characterization of human rheumatoid factor single-chain Fv. Biol Pharm Bull. 2000;23:941–945. doi: 10.1248/bpb.23.941. [DOI] [PubMed] [Google Scholar]
- 16.Luo YM, Mu Y, Wei JY, Yan GL, Luo GM. [Studies on the optimal expression condition, purification and its characterization of ScFv-2F3] Shengwu Gongcheng Xuebao. 2002;18:74–78. [PubMed] [Google Scholar]
- 17.Norton EJ, Diekman AB, Westbrook VA, Flickinger CJ, Herr JC. RASA, a recombinant single-chain variable fragment (scFv) an-tibody directed against the human sperm surface: implications for novel contraceptives. Hum Reprod. 2001;16:1854–1860. doi: 10.1093/humrep/16.9.1854. [DOI] [PubMed] [Google Scholar]
- 18.Zeng JZ, Zhou ZY, Wu YQ, Liu ZP, Wang WX, Huang HL, Cai ZN, Yu JL. [Expression of single-chain Fv antibody for anti-beet necrotic yellow vein virus in Escherichia coli] Yichuan Xuebao. 2000;27:1006–1011. [PubMed] [Google Scholar]
- 19.He HJ, Yang WD, Chang YN, Shi HJ, Yang GZ, Wu XF, Yuan QS. [Fusion and expression of the gene encoding human Mn-SOD to anti-CEA single-chain antibody in Escherichia coli] Shengwu Gongcheng Xuebao. 2000;16:566–569. [PubMed] [Google Scholar]
- 20.Fernández LA, Sola I, Enjuanes L, de Lorenzo V. Specific secretion of active single-chain Fv antibodies into the supernatants of Escherichia coli cultures by use of the hemolysin system. Appl Environ Microbiol. 2000;66:5024–5029. doi: 10.1128/aem.66.11.5024-5029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MH, Park TI, Park YB, Kwak JW. Bacterial expression and in vitro refolding of a single-chain fv antibody specific for human plasma apolipoprotein B-100. Protein Expr Purif. 2002;25:166–173. doi: 10.1006/prep.2002.1623. [DOI] [PubMed] [Google Scholar]
- 22.Song LX, Yu WY. [Construction and expression of anticolonic cancer scFv fragment] Shengwu Gongcheng Xuebao. 2000;16:82–85. [PubMed] [Google Scholar]
- 23.Cho WK, Sohn U, Kwak JW. Production and in vitro refolding of a single-chain antibody specific for human plasma apolipoprotein A-I. J Biotechnol. 2000;77:169–178. doi: 10.1016/s0168-1656(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 24.Guo L, Yan X, Qian S, Meng G. Selecting and expressing protective single-chain Fv fragment to stabilize L-asparaginase against inactivation by trypsin. Biotechnol Appl Biochem. 2000;31(Pt 1):21–27. doi: 10.1042/ba19990062. [DOI] [PubMed] [Google Scholar]
- 25.Sandee D, Tungpradabkul S, Tsukio M, Imanaka T, Takagi M. Construction and high cytoplasmic expression of a tumoricidal single-chain antibody against hepatocellular carcinoma. BMC Biotechnol. 2002;2:16. doi: 10.1186/1472-6750-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel A, Beresford GW, Colcher D, Pavlinkova G, Booth BJ, Baranowska-Kortylewicz J, Batra SK. Divalent forms of CC49 single-chain antibody constructs in Pichia pastoris: expression, purification, and characterization. J Biochem (Tokyo) 2000;127:829–836. doi: 10.1093/oxfordjournals.jbchem.a022676. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto Y, Tanigawa K, Nakashima M, Sonoda K, Ueda T, Watanabe T, Imoto T. Construction of the single-chain Fv from 196-14 antibody toward ovarian cancer-associated antigen CA125. Biol Pharm Bull. 1999;22:1068–1072. doi: 10.1248/bpb.22.1068. [DOI] [PubMed] [Google Scholar]
- 28.Kang N, Hamilton S, Odili J, Wilson G, Kupsch J. In vivo target-ing of malignant melanoma by 125Iodine- and 99mTechnetium-labeled single-chain Fv fragments against high molecular weight melanoma-associated antigen. Clin Cancer Res. 2000;6:4921–4931. [PubMed] [Google Scholar]
- 29.Kang NV, Hamilton S, Sanders R, Wilson GD, Kupsch JM. Efficient in vivo targeting of malignant melanoma by single-chain Fv antibody fragments. Melanoma Res. 1999;9:545–556. doi: 10.1097/00008390-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Reilly RM, Maiti PK, Kiarash R, Prashar AK, Fast DG, Entwistle J, Dan T, Narang SA, Foote S, Kaplan HA. Rapid imaging of hu-man melanoma xenografts using an scFv fragment of the human monoclonal antibody H11 labelled with 111In. Nucl Med Commun. 2001;22:587–595. doi: 10.1097/00006231-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Turatti F, Mezzanzanica D, Nardini E, Luison E, Maffioli L, Bambardieri E, de Lalla C, Canevari S, Figini M. Production and validation of the pharmacokinetics of a single-chain Fv fragment of the MGR6 antibody for targeting of tumors expressing HER-2. Cancer Immunol Immunother. 2001;49:679–686. doi: 10.1007/s002620000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer A, Tsiompanou E, O'Malley D, Boxer GM, Bhatia J, Flynn AA, Chester KA, Davidson BR, Lewis AA, Winslet MC, et al. Radioimmunoguided surgery in colorectal cancer using a genetically engineered anti-CEA single-chain Fv antibody. Clin Cancer Res. 2000;6:1711–1719. [PubMed] [Google Scholar]
- 33.Goel A, Augustine S, Baranowska-Kortylewicz J, Colcher D, Booth BJ, Pavlinkova G, Tempero M, Batra SK. Single-Dose ver-sus fractionated radioimmunotherapy of human colon carcinoma xenografts using 131I-labeled multivalent CC49 single-chain fvs. Clin Cancer Res. 2001;7:175–184. [PubMed] [Google Scholar]
- 34.Goel A, Colcher D, Baranowska-Kortylewicz J, Augustine S, Booth BJ, Pavlinkova G, Batra SK. Genetically engineered tet-ravalent single-chain Fv of the pancarcinoma monoclonal anti-body CC49: improved biodistribution and potential for thera-peutic application. Cancer Res. 2000;60:6964–6971. [PubMed] [Google Scholar]
- 35.Power BE, Caine JM, Burns JE, Shapira DR, Hattarki MK, Tahtis K, Lee FT, Smyth FE, Scott AM, Kortt AA, et al. Construction, expression and characterisation of a single-chain diabody derived from a humanised anti-Lewis Y cancer target-ing antibody using a heat-inducible bacterial secretion vector. Cancer Immunol Immunother. 2001;50:241–250. doi: 10.1007/s002620100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Ma QJ. [Cloning and expressing of an anti-CD5 single chain antibody] Shengwu Gongcheng Xuebao. 2001;17:131–134. [PubMed] [Google Scholar]
- 37.Yazaki PJ, Shively L, Clark C, Cheung CW, Le W, Szpikowska B, Shively JE, Raubitschek AA, Wu AM. Mammalian expression and hollow fiber bioreactor production of recombinant anti-CEA diabody and minibody for clinical applications. J Immunol Methods. 2001;253:195–208. doi: 10.1016/s0022-1759(01)00388-x. [DOI] [PubMed] [Google Scholar]
- 38.Yazaki PJ, Wu AM, Tsai SW, Williams LE, Ikler DN, Wong JY, Shively JE, Raubitschek AA. Tumor targeting of radiometal labeled anti-CEA recombinant T84.66 diabody and t84.66 minibody: comparison to radioiodinated fragments. Bioconjug Chem. 2001;12:220–228. doi: 10.1021/bc000092h. [DOI] [PubMed] [Google Scholar]
- 39.Wu AM, Yazaki PJ. Designer genes: recombinant antibody fragments for biological imaging. Q J Nucl Med. 2000;44:268–283. [PubMed] [Google Scholar]
- 40.Power BE, Hudson PJ. Synthesis of high avidity antibody fragments (scFv multimers) for cancer imaging. J Immunol Methods. 2000;242:193–204. doi: 10.1016/s0022-1759(00)00201-5. [DOI] [PubMed] [Google Scholar]


