Abstract
AIM: To investigate the changes of microvascular architecture, ultrastructure and permeability of rat jejunal villi at different ages.
METHODS: Microvascular corrosion casting, scanning electron microscopy, transmission electron microscopy and Evans blue infiltration technique were used in this study.
RESULTS: The intestinal villous plexus of adult rats consisted of arterioles, capillary network and venules. The marginal capillary extended to the base part of the villi and connected to the capillary networks of adjacent villi. In newborn rats, the villous plexus was rather simple, and capillary network was not formed. The villous plexus became cone-shaped and was closely arrayed in ablactation rats. In adult rats, the villous plexus became tongue-shaped and was enlarged both in height and width. In aged rats, the villous plexus shrank in volume and became shorter and narrower. The diametral ratio of villous arteriole to villous venule increased as animals became older. The number of endothelial holes, the thickness of basal membrane and the permeability of microvasculature were increased over the entire course of development from newborn period to aged period.
CONCLUSION: The digestive and absorptive functions of the rat jejunum at different ages are highly dependent upon the state of villous microvascular architecture and permeability, and blood circulation is enhanced by collateral branches such as marginal capillary, through which blood is drained to the capillary networks of adjacent villi.
INTRODUCTION
The microvasculature of intestinal villi play an important role in the process of digestion, absorption and mucosal barrier protection[1,2]. Intestinal diseases, such as diarrhea and enteritis, are reported to be related to the change of microvasculature[3,4]. More recent studies on the intestinal microvasculature demonstrated that the damage to surface microvasculature is an early event inducing mucosal injury in the intestine[5-7]. However, these studies were only limited in collecting pathological or clinical data, and tissue materials were obtained from adult subjects[8-10]. In addition, little information is available regarding the changes of microvasculature throughout different age periods. Therefore, the current work was meant to elucidate the morphological and permeability changes of jejunal microvasculature at different ages. Microvascular corrosion casting technique, scanning electron microscopy (SEM), transmission electron microscopy (TEM), Evans blue infiltration methods were used and morphometry was conducted in this study.
MATERIALS AND METHODS
Animals
Fifty-four male Sprague-Dawley rats were used. They were divided into 4 groups according to their ages after birth: (1) newborn rats (1 d, n = 9); (2) ablactation rats (3 weeks, n = 15); (3) adult rats (3 months, n = 15) and (4) aged rats (24 months, n = 15). For the groups with 15 animals, six were used for corrosion casting, three were observed under TEM, the other six were used to determine the permeability with Evans blue infiltration technique. The newborn rats were too small to be used in determining the permeability. All rats were fasting for 12 hours prior to each experiment.
Microvascular corrosion casting and SEM
Methyl-methacrylate and methacrylate were mixed at a ratio of 9:1. Polymerization was allowed by adding benzoyl-methacrylate at 80 °C. The viscosity of this solution was maintained to a level corresponding to that of 30% glycerine. The solution was cooled for later use. The microvascular architecture was then performed in the following procedure. Animals were deeply anesthetized with ether and a catheter was inserted into the thoracic aorta. One percent of saline solution (500 ml/kg i.m.) was perfused to flush the blood out of cardiovascular system, and this step was immediately followed by a perfusion of 5 ml methyl-methacrylate. Meanwhile, the same volume of the pre-prepared solution mixed with N, N-dimethylaniline (1% of the total volume) was added, serving as a polymerization accelerator. twelve hours later when polymerization was completed, the small intestine was sampled and stored in a 10% NaOH solution for 1-2 weeks. The tissue was rinsed with tap water until the corroded tissue was washed out, and then trimmed under the dissection microscope and dried at 40 °C for 48 hours. The dried tissue was then coated using an IB-3 ionic splashing and shooting device. Photomicrographs were taken under a Hitachi S-570 scanning electron microscope.
TEM
Small pieces of the jejunum were immersed in phosphate-buffered 3% glutaraldehyde for 2 hours, and then postfixed in phosphate-buffered 1% OsO4 for 2 hours. After postfixation, the tissue was dehydrated with ethanol and embedded in Epon 812. Ultra-thin sections stained with uranyl acetate and lead citrate were observed and photomicrographs were taken under a Hitachi TEM S-600.
Quantification of microvascular permeabilityof the jejunal tissue
Evans blue was used to measure the permeability of microvasculature. Rats were anesthetized with ether, and Evans blue (75 mg/kg i.m.) in 0.9% saline solution was injected into a femoral vein. Two hours later, rats were perfused transcardially with 0.9% saline solution (500 ml/kg i.m.). Evans blue was extracted from the jejunum by incubation in 5 ml of formamide at 54 °C for 24 hours. Evans blue was then quantified by measuring its absorption at wavelength of 620 nm using spectrometer. Results were expressed as µg Evans blue/gram fresh tissue.
Data processing
According to the methods of stereology[11], the measurement and calculation of the thickness of basal membrane (nm), the number of endothelial hole (per mm capillary perimeter) and plasmalemmal vesicles (per mm2 endothelium) were conducted on the photographs of TEM. Results were expressed as mean ± SEM, and statistics was performed using Student’s t test.
RESULTS
Microvascular architecture of adult rats villi
The villous plexi stood on the surface of the ileum. Between the adjacent villous plexi there were numerous different cryptal plexi. The villous plexus consisted of villous arteriole, villous capillary network and the villous venule. The villous capillaries connected to each other in a form of “net-basket” (Figure 1). The villous arteriole coming from the arterial plexus in the submucosa reached the base of the villi through the cryptal plexus gap, extending to the tip along the axis of the villi. The villous arterioles did not bifurcate within the villi, but formed the villous capillary network at the tips of the villi in the pattern of a “netted bag”. This villous capillary wrapped the villous arteriole. The villous venules were formed in the middle and upper part of the villi, through which the blood from capillary venules converged into the venous plexus in the submucosa (Figure 1).
Figure 1.
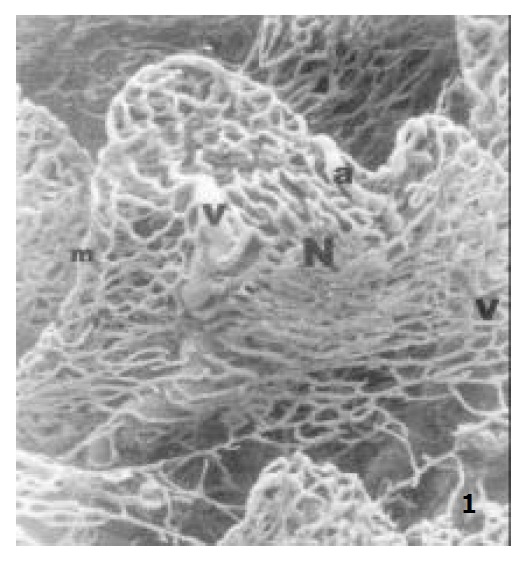
The microvascular architecture of adult rat jejunal villi, showing the villous arteriole (a), the villous capillary network (N), the villous venule (v), the marginal capillary (m) × 150.
In addition, there was a straight capillary along the margin of the villous capillary network, and it is called marginal capillary. Its diameter was twice that of its adjacent capillaries (Figure 1 and Figure 2). This capillary ran along the villous margin and did not merge into the villous venules of itself. It reached the base of the villi and connected to the basal part of its adjacent villous capillary networks (Figure 2).
Figure 2.
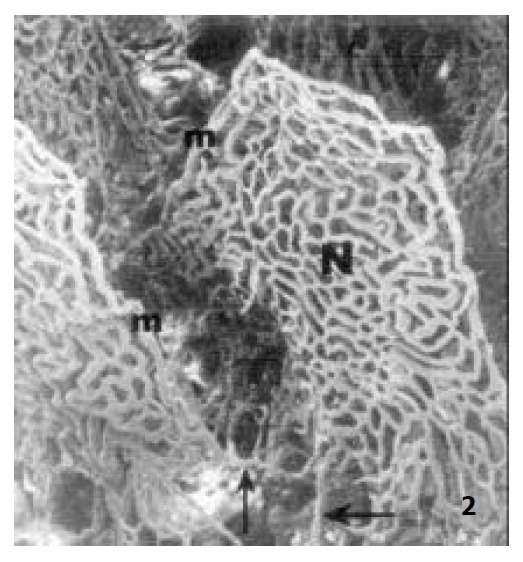
The microvascular architecture of adult rat jejunal villi, showing the marginal capillary (m) connecting to the basal part of its adjacent villous plexus (↑) × 130.
Changes of microvascular architecture of jejunal villi at different ages
In newborn rats, the villous plexus was rather simple and was composed of only one or two loops of capillary vessels. A capillary network was not formed at this age and villous arteriole and venule could not be differentiated (Figure 3). For the ablactation rats, the villous plexus became cone-shaped and was closely arrayed. The marginal capillary was as wide as villous arteriole. Each villous plexus had only one villous venule and was formed in the basal part of the villi. However, the diametral ratio of villous arteriole to it was very small (Figure 4). In adult rats, the villous plexus became tongue-shaped and was enlarged both in height and width. Each villous plexus usually had one villous venule which was formed in the middle and upper part of the villi, but the wide villi had two venules that were zygomorphous (Figure 1). In the aged rats, the villous plexus shrank in volume and became shorter and narrower. The capillaries were irregularly arrayed (Figure 5). The villous arteriole became wider and lower than villous venule (Figure 6). The diametral ratio of villous arteriole to venule was increased as animals became older (Table 1).
Figure 3.
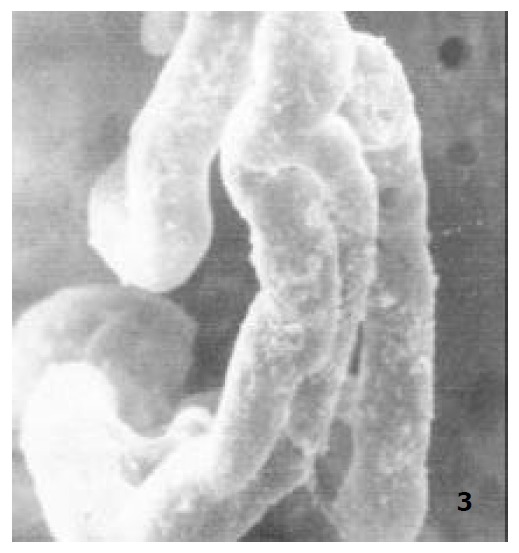
The microvascular architecture of newborn rat jejunal villi, × 400.
Figure 4.
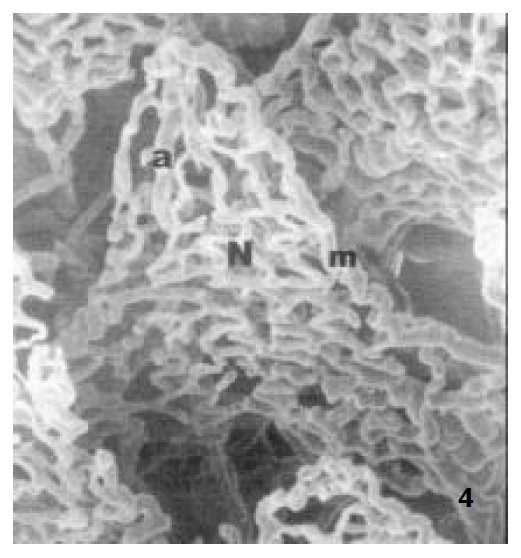
The microvascular architecture of ablactation rat jejunal villi, showing the villous arteriole (a), The villous capillary network (N), the marginal capillary (m) × 300.
Figure 5.
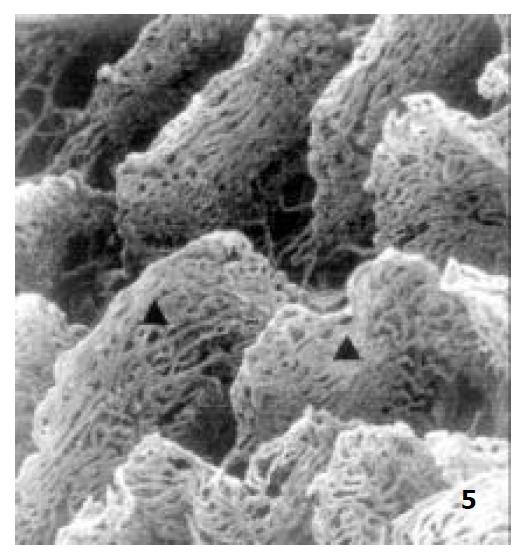
The microvascular architecture of aged rat jejunal villi × 100.
Figure 6.
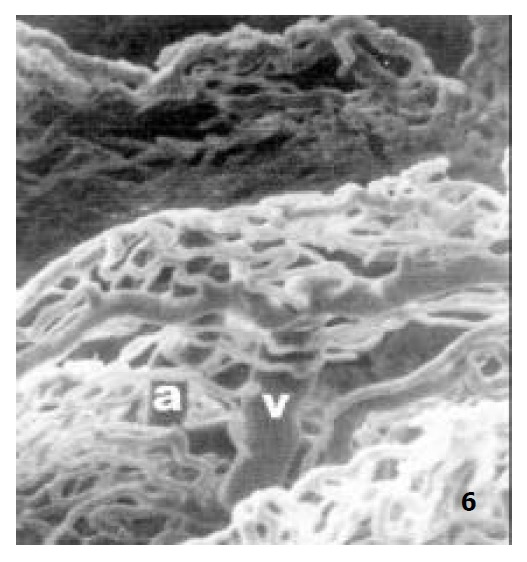
The microvascular architecture of aged rat jejunal villi, showing the villous arteriole (a), the villous venule (v) × 250.
Table 1.
Comparison of the microvascular architecture of villi at different ages (mean ± SEM)
| Ablactation rats | Adult rats | Aged rats | |
| Diameter of arteriole (µm) | 6.17 ± 1.51 | 10.47 ± 2.25a | 16.73 ± 3.28c |
| Diameter of venule (µm) | 20.43 ± 3.17 | 25.45 ± 3.07b | 25.50 ± 3.34 |
| Ratio: arteriole/venule | 0.30 ± 0.04 | 0.41 ± 0.04a | 0.65 ± 0.06c |
P < 0.01,
P < 0.05 vs ablactation rats;
P < 0.01 vs adult rats.
Changes of endothelial ultrastructure of villous capillary at different ages
Under TEM, the endothelium of capillary in the vicinity of epithelium of villi was so polarized that the nucleus was far away from epithelium and the side near the epithelium was thin. In the endothelium of newborn rats, bigger nucleus, more cytoplast as well as several plasmalemmal vesicles were seen, but no endothelial hole and basal membrane were observed. However, endothelial hole and basal membrane were found in ablactation animals (Figure 7). For the adult rats, a plenty of plasmalemmal vesicles were noticed in the endothelium (Figure 8). The endothelium in the aged rats, however, became thinner whereas its basal membrane became thicker. Endothelial holes were found to be increased whereas plasmalemmal vesicles were decreased (Figure 9). The number of endothlial holes, the thickness of basal membrane and the permeability of microvasculature were increased over the entire course of development from newborn period to aged period (Table 2).
Figure 7.
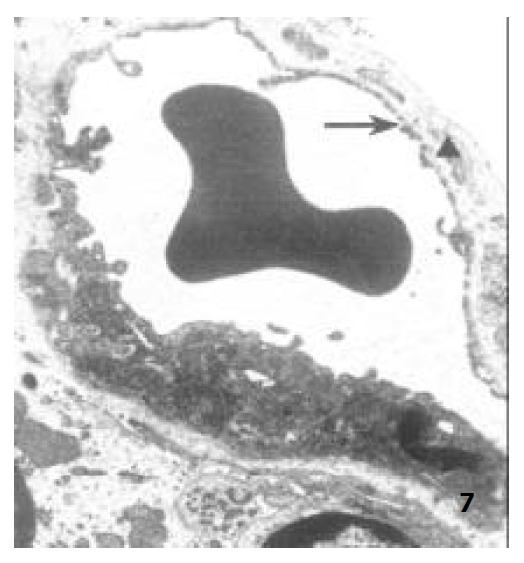
The capillary endothelium of ablactation rats, showing the endothelial hole (↑) and the basal membrane (▲) × 8000.
Figure 8.
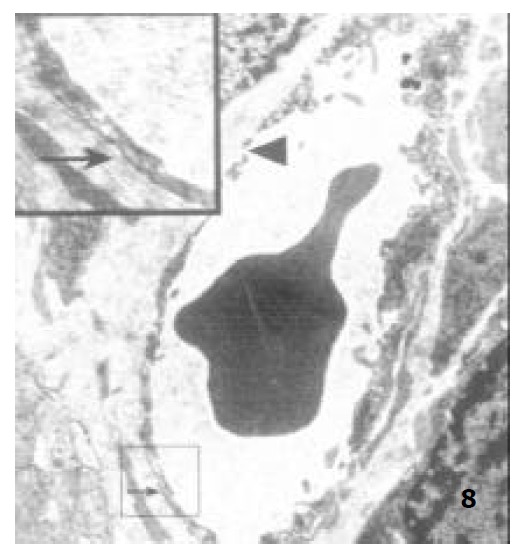
The capillary endothelium of adult rats, showing the basal membrane (↑) and the endothelial hole (▲) × 8000.
Figure 9.
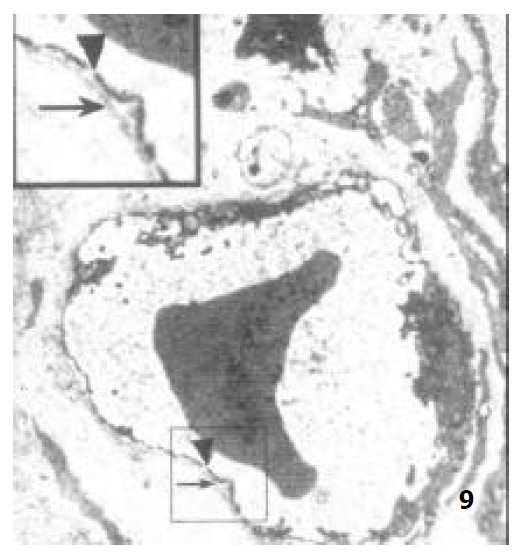
The capillary endothelium of aged rats, showing the basal membrane (↑) and the endothelial hole (▲) × 8000.
Table 2.
Comparison of the endothelial ultrastructure of villi at different ages (mean ± SEM)
| Newborn | Ablactation | Adult | Aged rats | |
| Endothelial holes (number/µm) | 3.01 ± 0.38 | 3.51 ± 0.35b | 5.19 ± 0.48d | |
| Plasmalemmal vesicles (number/0.1 µm2) | 17.7 ± 4.25 | 39.1 ± 10.36a | 75.3 ± 12.13c | 38.0 ± 11.14d |
| Basal membrane (nm) | 25.2 ± 5.26 | 43.5 ± 5.86c | 60.5 ± 6.89d | |
| Vascular permeability (µg/g) | 28.1 ± 4.64 | 64.4 ± 9.34c | 116.4 ± 15.63d |
P < 0.01 vs newborn rats;
P < 0.05,
P < 0.01 vs ablactation rats;
P < 0.01 vs adult rats.
Changes of microvascular permeability of villi at different ages
The microvascular permeability at different ages was determined with Evans Blue infiltration technique. The amount of Evans blue that penetrated into intestinal tissue was 28.1 µg/g tissue in ablactation rats and 64.4 µg/g tissue in adult rats. The microvascular permeability of the aged rats was twice as high as that of the adult rats (Table 2).
DISCUSSION
Injection of certain materials into blood vessels enables three dimensional visualization of vascular architecture and has provided abundant information which is not available by reconstruction of serial sections[12-14]. A line of evidence demonstrated in rats, horses and human embryos, that the “fountain type” microvascular architecture of mammalian small intestine is common[15-17]. Among these studies, Ohashi et al[16] found that two or more villous venules originating in the base of villi. The results of our study appear to suggest that the villous venules of the rat small intestine are formed in the middle and upper part of the villi, not in the base and at the tip. The villous venules are shorter than villous arterioles, and do not form arterio-venous anastamoses. There is big difference in diameter between villous venules and villous arteries. The former is about one third of that of the latter. All of these properties are important in creating a low blood pressure and speedy circulatory system in the villous capillary network, which is important to the process of nutritional absorption and transportation[18].
We found that branching did not take place when the villous arterioles climbed towards the tip of the villous and the capillary networks were not formed until the villous arteria reached the tip of the villous. After entering the villi, blood was supplied to the tip portion of villi and partially to its surrounding area. The cryptal plexus mainly supplied the blood to the basal part of the villi, which lacked artery terminals and branches. Therefore, this collateral circulation can provide blood supplies to the villi. Although there was supplementary blood supply in the base of the villi, blood supply was inefficient due to a long distance of transportation from the branching point at the tips of villi which were full of arterial blood[19]. Therefore, the villi at the tip received much more arterial blood supply than in the base. From the base to the tip of the villi, the absorption rate of epithelium increased proportionately with the volume of its blood supply. However, there were still aging cells at the tip of villous that were less dynamic physiologically than the younger cells at the basal part. The cells of villi at the tip might not be able to adapt themselves very well to any abnormal blood circulation, and therefore would easily lose their normal physiology under certain harsh conditions[20]. Injuries due to hypoxia usually emerged later in both intestinal crypts and basal part of villi than in the apical area of villi[21]. This may indicate that the crypts, as compared with villi, are relatively more resistant to hypoxic injuries. Such resistance is important in maintaining the optimal endocrine function and the regenerative capability of mucosa even after shock resulting from excessive blood loss.
Our results also showed that there were marginal capillaries on both sides of the small intestinal villi. These capillaries descended from the apical part of the villi to the basal part along its margin. It crossed the cryptal plexus and connected to the capillary network. So far, there have been no report regarding this connection. Before this study, it had been considered that there was only one venous return path from the small intestinal capillary. In other words, blood from capillary venules converged into the venous plexus in the submucosa[16,17,22]. Studies on marginal capillary have been developed since Mohiuddin[19] described the marginal capillary. It was reported recently that marginal capillary was more manifest in jejunum than in ileum[23,24], but its relationship with its adjacent villous plexus was not found in both jejunum and ileum. It is thus considered that the marginal capillary transports blood to the villous margin. Therefore, the findings from the this study regarding the marginal capillary and its relation with its adjacent villi suggest that the collateral circulation may exist before the venous return from the villi. That is, the marginal capillary delivers blood partially into the adjacent villous capillary networks. This architecture of the villous plexus may have been adapted to the movement of intestines as well as to the digestive and absorptive activities. After eating, the small intestine speeds up its motility. Villi not only have tight contacts with the chyme but also are subjected to mechanical pressure from the passing chyme. If the villous venules are depressed, blood return to the villi will be blocked. Therefore, blood can be collected into the adjacent villous plexi through the marginal capillary. This helps maintain a normal blood circulation in the villi, and their digestive and absorptive functions are carried out normally.
In the newborn rat villi, capillary network was not formed although only simple microvascualr architecture was formed. The later developed capillary network is probably due to the formation of slender transcapillary tissue pillars, which give rise to new vascular meshes[25,26]. During villous formation, the developing vascular nets played an important role and the apical capillary loop in a villous was anchored to epithelial basal membrane or to the adjoining matrixes[27]. After 3 weeks, the villous microvascular architecture tended to grow in full scale. During ablactation period, the villous capillary network was formed, and the diametral ratio of villous arteriole to villous venule was one-third, which consolidated the normal digestion and absorption. The villous plexus in adult rats became higher and wider, providing more nutrition for the body. In aged rats, the tip of the villous plexus caved in, the position of villous venule was relatively high. The increases in diametral ratio of villous arteriole to villous venule caused high blood pressure, which degraded the transportation and absorption of nutrition. All of these demonstrate the important role of the vascular architecture in maintaining an efficient absorption of nutrition for the malfunctional intestines in the aged animals.
The primary penetrating vessels in the submucosa formed an extensive submucosal plexus that supplied the tunica serosa, tunica muscularis, and tunica mucosa[28]. Fewer branches of this plexus supply the muscle layers, and most of branches run towards the mucosa and to supply blood to the villous and the cryptal plexus[29]. Therefore the vascular permeability mainly depends on the villous plexus, namely vascular architecture and endothelial structure. Under normal physiological condition, small molecules can easily enter or come out of blood vessels, but the large size substances must be transported through endothelial hole or plasmalemmal vesicle[30]. Since there were no endothelial holes and very few plasmalemmal vesicles in newborn rats, these large size substances must be selected to pass through the endothelium, which may be a protection for the immature immune system. The emergence of a few of endothelial holes and plasmalemmal vesicles during ablactation period indicate that the big molecular substances can be transported. The endothelium of adult rats have a plenty of plasmalemmal vesicles, and microvascular permeability was twice as high as that of the ablactation rats. In the aged rats, the endothelium became thinner, and the number of plasmalemmal vesicles became smaller while the number of endothelial holes became bigger. The diametral ratio of villous arteriole to villous venule increased as animals become older. The blood pressure was higher in old rat villi. The larger number of endothelial holes and higher blood pressure resulted in double increase in the microvascular permeability of the aged rats as compared with the adult rats. The thickened basal membrane may be due to a compensatory response, which may prevent any injurants from entering the blood vessels in the aged rats. Based upon the above information, one can say that aging or degradation of blood vessels may underlie the mechanisms of systematic aging.
In summary, the microvascular architectures of rat intestinal villi are complex. Each villi has its own blood supply and venous return paths. Furthermore, one villi can supply blood to its adjacent villi through the marginal capillary. These complex connections in the villi help avoid a shortage of blood supply or blockade of venous blood return. Normal digestive and absorptive functions are thus protected. With regard to the small intestine of other species, whether there is a marginal capillary and venous return collateral circulation is a matter of future study.
Footnotes
Supported by Natural Science Foudation of Hebei Province Educational Committee, No.2002136 and Natural Science Foudation of Hebei Province, No.303158
Edited by Ma JY
References
- 1.Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182–196. doi: 10.1006/jsre.2000.5862. [DOI] [PubMed] [Google Scholar]
- 2.Mailman D. Blood flow an intestinal absorption. Fed Proc. 1982;41:2096–2100. [PubMed] [Google Scholar]
- 3.Lun H. Investigative survey of the intestinal blood vessels. Jiepouxue Zazhi. 1997;20:197–199. [Google Scholar]
- 4.Laroux FS, Grisham MB. Immunological basis of inflammatory bowel disease: role of the microcirculation. Microcirculation. 2001;8:283–301. doi: 10.1038/sj/mn/7800095. [DOI] [PubMed] [Google Scholar]
- 5.Abbas B, Boyle FC, Wilson DJ, Nelson AC, Carr KE. Radiation induced changes in the blood capillaries of rat duodenal villi: a corrosion cast,light and transmission electron microscopical study. J Submicrosc Cytol Pathol. 1990;22:63–70. [PubMed] [Google Scholar]
- 6.Kelly DA, Piasecki C, Anthony A, Dhillon AP, Pounder RE, Wakefield AJ. Focal reduction of villous blood flow in early indomethacin enteropathy: a dynamic vascular study in the rat. Gut. 1998;42:366–373. doi: 10.1136/gut.42.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruh J, Vogel F, Schmidt E, Werner M, Klar E, Secchi A, Gebhard MM, Glaser F, Herfarth C. Effects of hydrogen peroxide scavenger Catalase on villous microcirculation in the rat small intestine in a model of inflammatory bowel disease. Microvasc Res. 2000;59:329–337. doi: 10.1006/mvre.1999.2201. [DOI] [PubMed] [Google Scholar]
- 8.Hu S, Sheng ZY. The effects of anisodamine and dobutamine on gut mucosal blood flow during gut ischemia/reperfusion. World J Gastroenterol. 2002;8:555–557. doi: 10.3748/wjg.v8.i3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima Y, Baudry N, Duranteau J, Vicaut E. Microcirculation in intestinal villi: a comparison between hemorrhagic and endotoxin shock. Am J Respir Crit Care Med. 2001;164:1526–1530. doi: 10.1164/ajrccm.164.8.2009065. [DOI] [PubMed] [Google Scholar]
- 10.Dabareiner RM, Snyder JR, Sullins KE, White NA, Gardner IA. Evaluation of the microcirculation of the equine jejunum and ascending colon after ischemia and reperfusion. Am J Vet Res. 1993;54:1683–1692. [PubMed] [Google Scholar]
- 11.Zheng FS. Steric metrology of cell morphology. Beijing: The Former Beijing Medical University and PUMC Press; 1990. [Google Scholar]
- 12.Kondo S. Microinjection methods for visualization of the vascular architecture of the mouse embryo for light and scanning electron microscopy. J Electron Microsc (Tokyo) 1998;47:101–113. doi: 10.1093/oxfordjournals.jmicro.a023566. [DOI] [PubMed] [Google Scholar]
- 13.Poonkhum R, Pongmayteegul S, Meeratana W, Pradidarcheep W, Thongpila S, Mingsakul T, Somana R. Cerebral microvascular architecture in the common tree shrew (Tupaia glis) revealed by plastic corrosion casts. Microsc Res Tech. 2000;50:411–418. doi: 10.1002/1097-0029(20000901)50:5<411::AID-JEMT10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Zahner M, Wille KH. Vascular system in the large intestine of the dog. Anat Histol Embryol. 1996;25:101–108. doi: 10.1111/j.1439-0264.1996.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 15.Bellamy JE, Latshaw WK, Nielsen NO. The vascular architecture of the porcine small intestine. Can J Comp Med. 1973;37:56–62. [PMC free article] [PubMed] [Google Scholar]
- 16.Ohashi Y, Kita S, Murakami T. Microcirculation of the rat small intestine as studied by the injection replica scanning electron microscope method. Arch Histol Jpn. 1976;39:271–282. doi: 10.1679/aohc1950.39.271. [DOI] [PubMed] [Google Scholar]
- 17.Dart AJ, Snyder JR, Julian D, Hinds DM. Microvascular circulation of the small intestine in horses. Am J Vet Res. 1992;53:995–1000. [PubMed] [Google Scholar]
- 18.Hummel R, Schnorr B. [The system of blood vessels of the small intestine of ruminants (author's transl)] Anat Anz. 1982;151:260–280. [PubMed] [Google Scholar]
- 19.Mohiuddin A. Blood and lymph vessels in the jejunal villi of the white rat. Anat Rec. 1966;156:83–89. doi: 10.1002/ar.1091560110. [DOI] [PubMed] [Google Scholar]
- 20.Jiang L, Zhao XM, Tian N, Liu YY, Li XH, Jiang CG. Simulation study of hemodynamic of small intestinal villosity microvessels in vitro. Zhongguo Bingli Shengli Zazhi. 1999;15:60–62. [Google Scholar]
- 21.Morini S, Yacoub W, Rastellini C, Gaudio E, Watkins SC, Cicalese L. Intestinal microvascular patterns during hemorrhagic shock. Dig Dis Sci. 2000;45:710–722. doi: 10.1023/a:1005491509832. [DOI] [PubMed] [Google Scholar]
- 22.Métry JM, Neff M, Knoblauch M. The microcirculatory system of the intestinal mucosa of the rat. An injection cast and scanning electron microscopy study. Scand J Gastroenterol Suppl. 1982;71:159–162. [PubMed] [Google Scholar]
- 23.Tahara T, Yamamoto T. Morphological changes of the villous microvascular architecture and intestinal growth in rats with streptozotocin-induced diabetes. Virchows Arch A Pathol Anat Histopathol. 1988;413:151–158. doi: 10.1007/BF00749677. [DOI] [PubMed] [Google Scholar]
- 24.Kamyshova VV, Karelina NR, Mironov AA, Mironov VA. [Morphofunctional features of different divisions of the microcirculatory bed of jejunal villi in the white rat] Arkh Anat Gistol Embriol. 1985;88:44–50. [PubMed] [Google Scholar]
- 25.Patan S, Alvarez MJ, Schittny JC, Burri PH. Intussusceptive microvascular growth: a common alternative to capillary sprouting. Arch Histol Cytol. 1992;55 Suppl:65–75. doi: 10.1679/aohc.55.suppl_65. [DOI] [PubMed] [Google Scholar]
- 26.Karelina NR. [Circulatory bed of the small intestinal villi of newborn infants] Arkh Anat Gistol Embriol. 1978;75:64–71. [PubMed] [Google Scholar]
- 27.Hashimoto H, Ishikawa H, Kusakabe M. Development of vascular networks during the morphogenesis of intestinal villi in the fetal mouse. Kaibogaku Zasshi. 1999;74:567–576. [PubMed] [Google Scholar]
- 28.Yarbrough TB, Snyder JR, Harmon FA. Jejunal microvasculature of the llama and alpaca. Am J Vet Res. 1995;56:1133–1137. [PubMed] [Google Scholar]
- 29.Chen YM, Li J, Zhang J, Duan XL. The intestinal microvascular architecture of rat. Shijie Huaren Xiaohua Zazhi. 2000;8:1291–1293. [Google Scholar]
- 30.Komuro T, Hashimoto Y. Three-dimensional structure of the rat intestinal wall (mucosa and submucosa) Arch Histol Cytol. 1990;53:1–21. doi: 10.1679/aohc.53.1. [DOI] [PubMed] [Google Scholar]


