Abstract
AIM: Bioartificial liver is a hope of supporting liver functions in acute liver failure patients. Using polysulfon fibers, a new bioartificial liver was developed. The aim of this study was to show whether this bioartificial liver could support liver functions or not.
METHODS: Hepatocytes were procured from swine using Seglen’s methods. The bioartificial liver was constructed by polysulfon bioreactor and more than 1010 hepatocytes. It was applied 14 times in 12 patients, who were divided into 7 cases of simultaneous HBAL and 5 cases of non-simultaneous HBAL. Each BAL treatment lasted 6 hours. The general condition of the patients and the biochemical indexes were studied.
RESULTS: After treatment with bioartificial liver, blood ammonia, prothrombin time and total bilirubin showed significant decrease. 2 d later, blood ammonia still showed improvment. within one month period, 1 case (1/7) in simultaneous group died while in non-simultaneous group 2 cases (2/5) died. The difference was significant. Mortality rate was 25%.
CONCLUSION: The constructed bioartificial liver can support liver functions in acute liver failure. The simultaneous HBAL is better than non-simultaneous HBAL.
INTRODUCTION
Acute liver failure (ALF) is commonly seen in the mainland of China. The patients are always characterized by infection of hepatitis B. Liver cell damage is the main reason of ALF. When the amount of normal cells decrease below its limit, liver function will deteriorate and a vicious cycle will be formed.
Liver transplantation (LT) has already been a wise choice for these patients[1-3]. The 1 year survival rate could be improved to more than 70% when LT is applied[4,5]. But the donor is scarce, so it limits the wide practice of LT. Many patients exacerbated and died during the period of waiting donor liver.
Bioartificial liver (BAL) is designed to take the responsibility of supporting liver functions temporarily in acute liver failure[6-9]. It consists of a semi-permeable membrane and living allogeneic or syngeneic liver cells. The flow of blood or plasma from the patient can exchange the substances with those cells through this membrane. The ammonia and other toxins in blood then are detoxified, as well as some useful factors are secreted into blood. Many scholars reported that BAL was effective and could be used as a bridge to LT[8-10]. Also this technique gave the chance of spontaneous recovery of the native function because of liver cell regeneration in some cases[8,11].
It is estimated that 1 × 109 hepatocytes are the lowest limit that are needed in BAL[10]. Such large number of hepatocytes are very difficult to be cultured in a small bioreactor. Enlarging the volume of the bioreactor and adding a hepatocytes cell pool are both good methods to solve such problems.
Using a bioreactor made of polysulfon, we developed a bioartificial liver recently and applied it in 12 patients. The results were exciting.
MATERIALS AND METHODS
Animals
Healthy Chinese experimental miniature swine were purchased from the Animal Center of Beijing Agriculture University. On receipt, swine were kept in a temperature and humidity controlled environment (20-25 °C, humidity 50%-70%) in a 12/12 hour light/dark cycle and fed with a cereal based diet with free access to water. More than a week later, they were used to get the hepatocytes. 12 hours before procedure only free water ad libitum was allowed. The animals were treated in accordance with the guidelines established by Affiliated Drum Tower Hospital of Medical College of Nanjing University.
Hepatocytes preparation
Hepatocytes were isolated from the swine by in situ liver perfusion and enzymatic collagenase digestion according to the process described by Seglen[12]. Briefly, under katamine (50 mg/kg) anesthesia, a median laparotomy and cannulation of the portal vein were performed. The inferior vena cava was ligated just above the renal vein and then was cannulated close to the heart. The liver was perfused at 4 °C and pH7.6 with 3000 ml Hanks solution through the portal vein. Then the liver was circularly perfused with 500 ml 0.5‰ collagenase IV solution (Gibco, New York, USA) at a constant flow of 20 ml/minute. The softened liver was then excised and hepatocytes were separated from the connective liver tissue by gentle agitation. The resulting cell suspension was filtered though 50 µm sterile metal mesh. The cells were washed three times, suspended in non serum RPMI 1640 culture medium (Sigma, Louis, USA) with 200 µg/L hydrocortisone, 1 mg/L HGF, 10 µg/L EGF, 20 µg/L NGF, 100 µg/Linsulin, 4 µg/L glucagon, 6.25 mg/L transferrin, 10 mg/L linoleic acid, 2 mmol glutamine, 0.5 g/L bovine serum albumin, 3 nmol sodium selenate, 0.1 µg/L CuSO4·5H2O, 50 pM ZnSO4·7H2O, 15 mmol HEPES, 200 µg/L cefaperazone, 1 × 105 U/Lpenicillin and 100 mg/L streptomycycin. Cell viability was determined by the trypanum exclusion test. Only suspentions with cell viability of ≥ 95% were used. Cells suspension was then stirring incubated overnight in that non serum RPMI 1640 culture medium at 37 °C.
Configuration of bioartificial liver
Polysulfon bioreactor was purchased from TECA Corp. (Hongkong, China). The molecular cutoff of the membrane was 100 kD. Total fiber internal surface area was 1620 cm2, external surface area was 2060 cm2. Before use, the bioreactor was sterilized and rinsed by 3000 ml normal saline. A hepatocytes reservoir, a rolling pump and a circulation cycle were designed to connect to the extra-fiber compartment of bioreactor. The aim was to ensure more than 1010 hepatocytes being used and enough nutrition could be provided. Then the cultured suspended cells were filled into the extra-fiber compartment of bioreactor. The rolling rate of the pump was 80 ml/minute.
Blood was removed from the patient through a double lumen catheter in superficial femoral vein at a rate of 100 ml/minute and run through a plasma separator. The separated plasma passed through a charcoal column or bilirubin absorption column and then run into the intra-fiber compartment of bioreactor in simultaneous HBAL, while in non-simultaneous HBAL the plasma run directly into the intra-fiber compartment of bioreactor. The reacted plasma were then reconstituted with red blood cells and returned to the patient via the venous cannula. (Figure 1).
Figure 1.
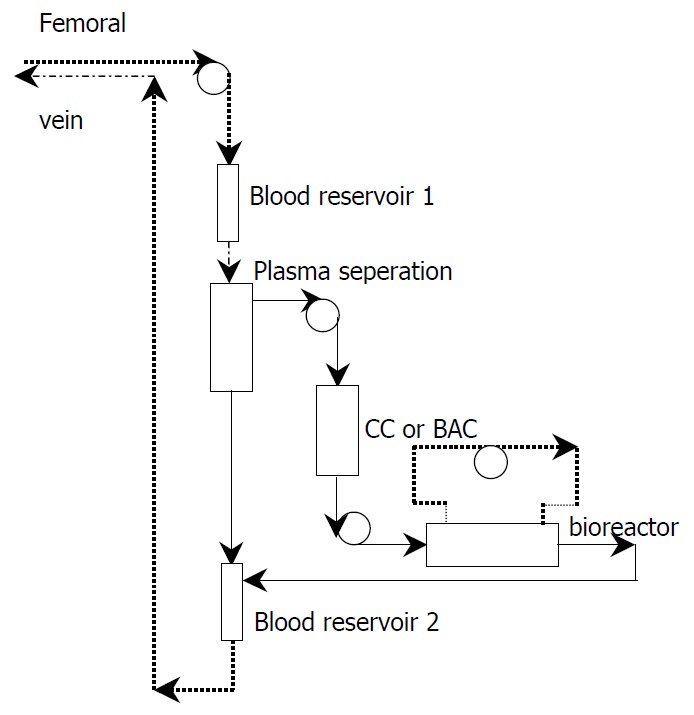
The constructed bioartificial liver. CC: charcoal column. BAC: bilirubin absorption column.
Clinical use
12 patients, which include 9 male and 3 female, suffering from acute liver failure were adopted to this study. The age ranged from 13 to 56. All the patients were found having hepatitis B infection (Table 1). Before the BAL supporting treatment, an evaluation of the patient’s psychic state was conducted by a psychologist, and an agreement of BAL application was signed by the patient and/or his direct relatives.
Table 1.
Clinical data of the patients
| Patient No. | Sex | Age | Hepatitis B infection | Regimen of treatment | Number of treatment | Result |
| 1 | M | 13 | + | HBAL (CC) | 1 | Improved |
| 2 | M | 38 | + | 1. HBAL (BAC) | 1 | Improved |
| 2. BAL | 1 | |||||
| 3 | F | 34 | + | HBAL (BAC) | 1 | Improved |
| 4 | M | 55 | + | HBAL (BAC) | 1 | Death |
| 5 | M | 52 | + | HBAL (BAC) | 1 | Improved |
| 6 | M | 46 | + | HBAL (BAC) | 1 | Improved |
| 7 | M | 58 | + | HBAL (BAC) | 1 | Improved |
| 8 | M | 33 | + | PE and BAL | 1 | Improved |
| 9 | F | 50 | + | PE and BAL | 2 | Death |
| 10 | F | 52 | + | HF and BAL | 1 | Death |
| 11 | M | 40 | + | HF and BAL | 1 | Improved |
| 12 | M | 30 | + | BAC and BAL | 1 | Improved |
M: Male, F: Female, +: Positive, HBAL: Hybrid bioartificial liver, BAL: Bioartificial liver, (CC): With charcoal column, (BAC): With bilirubin absorption column, PE and BAL: Plasma exchange and 24 hours later BAL only, HF and BAL: Hemofiltration and 24 hours later BAL only, BAC and BAL: Bilirubin absorption and 24 hours later BAL only.
The treatment regimen included simultaneous HBAL and non-simultaneous HBAL. The only difference was bilirubin absorption treatment or plasma exchange treatment being used 1day before the bioreactor was applied in non-simultaneous HBAL while in simultaneous HBAL they were applied simultaneously. Other traditional treatments were all the same. Some liver function indexes and the one month mortality rate were used to evaluate the function of BAL.
Statistical analysis
Mortality rate was expressed as percentage. Others were expressed as mean ± SD. Paired T test was used (SPSS software, SPSS Inc. USA). Probability of less than 0.05 was accepted as significant.
RESULTS
In 12 patients, 14 times BAL treatments were conducted. The period of one BAL treatment lasted 6 hours. All patients experienced the procedure successfully.
3 patients died soon after the procedure and 9 were improved. The mortality rate was 25%. The criteria of improvement included improvement of general condition of the patient, persistent hepatic function improvement, improved psychic state, and recovery.
In biochemical test, ALT showed slight decrease in post-treatment period and restored to pre-treatment level 2 d later. No change in blood albumin. Blood ammonia, prothrombin time and total bilirubin indexes showed significant decrease after treatment. 2 d later, only blood ammonia still maintained significant low level (Figure 2).
Figure 2.
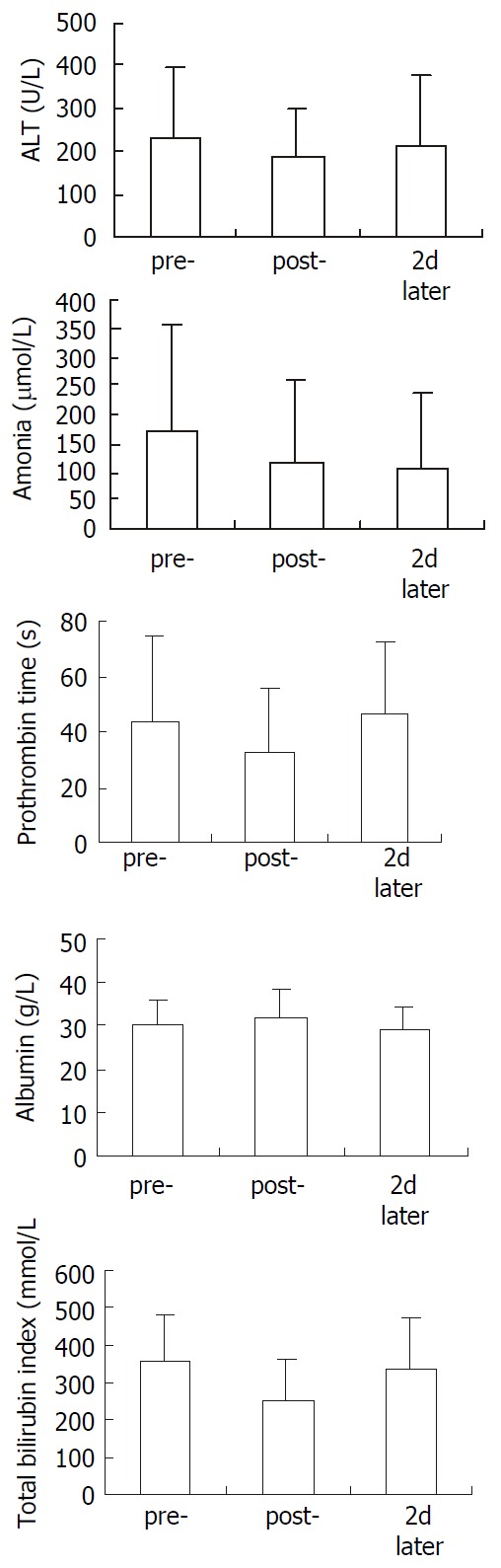
Change of ALT, ammonia, prothrombin time, albu-min and total bilirubin index in 12 patients. Compared with pre-, ammonia, prothrombin time and total bilirubin index showed significant decreace in post-. 2 d later, only ammo-nia showed significant lowing. pre-: pre-treatment, post-: post-treatment, 2 d later: 2 d after the treatment. Paired T test was used to test the difference.
In non-simultaneous HBAL, the mortality rate was 40%. While in simultaneous HBAL, the mortality rate was 14.3%. Significant difference was found comparing them (Figure 3).
Figure 3.
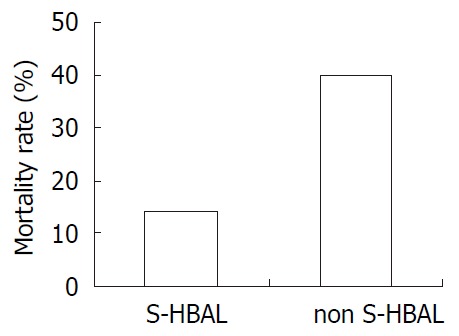
The mortality rate of simultaneous HBAL and non-simultaneous HBAL. Significant difference was found between this two groups (P < 0.05).
Typical case report: A male, aged 38 years old, was admitted for “fatigue and yellow urine for a week”. Physical examination showed yellowish face, moderate jaundice in skin and sclera, palpable liver that was 2 cm below the costal arch in right mid-clavicular line, and tenderness in right upper abdomen. Laboratory examination showed positive HBs antibody, Hbe antibody and HBc antibody, abnormal liver function. B type ultrasound examination showed image of liver injury. The diagnosis was severe acute hepatitis with hepatitis B virus infection. After admission, the patient was given conventional treatment without any improvement and ran downhill. One week later, He received a simultaneous HBAL (bilirubin absorption) treatment. Total bilirubin and prothrombin time decreased immediately. 6 d later he received another HBAL treatment because of worsening some biochemical indexes. 2 weeks after that the patient was recovered and was waiting for liver transplantation. (Table 2).
Table 2.
Change of some biochemical index after the HBAL treatment
| Time | ALT (U/L) | GOT (U/L) | TBI (µmol/L) | DBI (µmol/L) | TP (g/L) | ALB (g/L) | PT (s) | |
| Admission | 77.5 | 58.8 | 341.8 | 265.4 | 60.1 | 32.8 | 68.8 | |
| 2 hours later | 58.7 | 52.5 | 343.0 | 255.2 | 52.4 | 31.9 | 32.2 | |
| HBAL | 4 hours later | 66.3 | 69.5 | 345.7 | 280.4 | 52.9 | 31.4 | 40.9 |
| (BA) | 6 hours later | 68.7 | 79.9 | 260.8 | 291.6 | 54.5 | 31.4 | 26.7 |
| 2 days later | 80.6 | 68.0 | 423.2 | 336.0 | 70.4 | 37.1 | 20.2 | |
| Before HBAL | 421.2 | 371.9 | 375.4 | 109.0 | 44.6 | 27.4 | 21.9 | |
| 2 hours later | 427.9 | 412.0 | 389.0 | 102.1 | 42.7 | 26.5 | 28.5 | |
| HBAL | 4 hours later | 431.8 | 411.4 | 373.9 | 99.5 | 43.0 | 26.7 | 28.7 |
| 6 hours later | 371.0 | 218.0 | 339.8 | 122.1 | 45.6 | 28.8 | 17.4 | |
| 2 days later | 301.2 | 159.6 | 444.6 | 125.7 | 43.6 | 26.5 | 28.7 |
HBAL: Hybrid bioartificial liver; BA: Bilirubin absorption.
DISCUSSION
ALF is a severe disease in clinical practice with a mortality rate of 30%-80%[1-5]. OLT is thought to be the most effective method to improve the prognosis. But it is greatly limited due to shortage of donor liver[3,4]. HBAL is designed to take responsibility of improving the poor physical state of the patients and supporting liver function for a short time which may serve as a bridge to OLT[6-11].
The ideal HBAL should support the patients to pass through the worst period of the course by its metabolic, synthetic, toxin and drug degradational functions. When it works, immune reactions to the living cells may be avoided[13,14], while toxins and some useful factors might be capable to pass the membrane freely.
Construction of HBAL
The external BAL is being studied in many medical centers. Living hepatocytes are first needed in this apparatus. Human hepatocytes are the ideal choice. But the only available human livers are all used for liver transplantation. Human cell strains from some liver neoplasmas were also reported. But the danger of this application is unknown. Porcine hepatocytes are the commonest choice of many centers, because pigs are cheap and easy to get, without any ethical contradictions of killing them.
When the liver cells are procured, the membranes might be damaged by the enzymes or the mechanical factors[15]. The damaged membrane needs 1 to 3 d for recovery[16]. Our experience shows 1 d stirring culture of the procured hepatocytes is enough.
Enough viable hepatocytes are also important in BAL. It was estimated that 1 × 109 hepatocytes are the lowest limit[10]. Such a large number of hepatocytes are very difficult to live and maintain its function in a small bioreactor, through which hepatocytes may exchange substance with blood. If the volume of bioreactor is enlarged greatly, hemodynamics of the patient may be disturbed. We designed a two cycles bioreactor. The first cycle was to supply the nutrition and the other one was to connect to a cell reservoir which ensured 1010 cells were used in this system.
The toxins in blood are sometimes harmful to living hepatocytes. To decrease this effect, mechanical filtration or absorption is used in this system. Due to long period of time to construct the system and urgent situation of the patient requiring this treatment, we designed a non-simultaneous HBAL, that used mechanical filtration or carbon absorption or bilirubin absorption or plasma exchange first, then BAL was applied the next day. The results were not satisfactory. The mortality rate was higher than the simultaneous HBAL.
Effects of HBAL
The material of the bioreactor and the characters of it are most important. Polysulfon was chosen because of its good histocompatibility with body and little toxicity. In dialysis medicine the polysulfon is already widely used as dialyzer. The molecular cut-off is another important index for the bioartificial liver. If it is large enough, cells in it may not be protected completely. Otherwise the toxins cannot be detoxified and useful factors secreted by hepatocytes cannot enter the body. The molecular cut-off we choosed was 100 kD because IgG is about 150 kD and albumin or hepatic growth factor is about 70-80 kD.
Our experiments showed that BAL could function well when applied to the patients. The ammonia, prothrombin time and total bilirubin showed significant decrease in post-treatment examination. ALT also showed a slight decrease in post-treatment study, although no significant difference was found.
Also HBAL showed a delayed reaction, 2 d after application as ammonia still showed significant decrease. We considered that this function might be due to the synthetic function of HBAL.
Footnotes
Supported by the Public Health Bureau of Jiangsu Province, China, BQ200020 and Social Development Plan of Scientific and Technological Council of Nanjing Municipal, China. SS200002
Edited by Xu JY
References
- 1.Emond JC, Whitington PF, Thistlethwaite JR, Cherqui D, Alonso EA, Woodle IS, Vogelbach P, Busse-Henry SM, Zucker AR, Broelsch CE. Transplantation of two patients with one liver. Analysis of a preliminary experience with 'split-liver' grafting. Ann Surg. 1990;212:14–22. doi: 10.1097/00000658-199007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw BW. More questions than answers. Liver Transpl Surg. 1995;1:404–407. doi: 10.1002/lt.500010613. [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Lombardero M, Detre KM, Zetterman RK, Wiesner RH, Lake JR, Hoofnagle JH. Increased waiting time for liver transplantation results in higher mortality. Transplantation. 1997;64:1300–1306. doi: 10.1097/00007890-199711150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bilsuttil W, Klintmalm B. 1996. Transplantation of the liver Philadelphia. W. B. Saunders Company; p. 861. [Google Scholar]
- 5.Emond JC, Whitington PF, Thistlethwaite JR, Alonso EM, Broelsch CE. Reduced-size orthotopic liver transplantation: use in the management of children with chronic liver disease. Hepatology. 1989;10:867–872. doi: 10.1002/hep.1840100520. [DOI] [PubMed] [Google Scholar]
- 6.Friedman AL. Why bioartificial liver support remains the Holy Grail. ASAIO J. 1998;44:241–243. [PubMed] [Google Scholar]
- 7.Kamihira M, Yamada K, Hamamoto R, Iijima S. Spheroid formation of hepatocytes using synthetic polymer. Ann N Y Acad Sci. 1997;831:398–407. doi: 10.1111/j.1749-6632.1997.tb52213.x. [DOI] [PubMed] [Google Scholar]
- 8.Sussman NL, Gislason GT, Conlin CA, Kelly JH. The Hepatix extracorporeal liver assist device: initial clinical experience. Artif Organs. 1994;18:390–396. doi: 10.1111/j.1525-1594.1994.tb02221.x. [DOI] [PubMed] [Google Scholar]
- 9.Dixit V. Development of a bioartificial liver using isolated hepatocytes. Artif Organs. 1994;18:371–384. doi: 10.1111/j.1525-1594.1994.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 10.Hui T, Rozga J, Demetriou AA. Bioartificial liver support. J Hepatobiliary Pancreat Surg. 2001;8:1–15. doi: 10.1007/s005340170045. [DOI] [PubMed] [Google Scholar]
- 11.Suh KS, Lilja H, Kamohara Y, Eguchi S, Arkadopoulos N, Neuman T, Demetriou AA, Rozga J. Bioartificial liver treatment in rats with fulminant hepatic failure: effect on DNA-binding activity of liver-enriched and growth-associated transcription factors. J Surg Res. 1999;85:243–250. doi: 10.1006/jsre.1999.5669. [DOI] [PubMed] [Google Scholar]
- 12.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 13.Rozga J, Williams F, Ro MS, Neuzil DF, Giorgio TD, Backfisch G, Moscioni AD, Hakim R, Demetriou AA. Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology. 1993;17:258–265. [PubMed] [Google Scholar]
- 14.Cuervas-Mons V, Colás A, Rivera JA, Prados E. In vivo efficacy of a bioartificial liver in improving spontaneous recovery from fulminant hepatic failure: a controlled study in pigs. Transplantation. 2000;69:337–344. doi: 10.1097/00007890-200002150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Flendrig LM, La-Soe JW, Jorning GGA, Steenbeek A, Karlsen OT, Bovee WMM, Ladiges NC, te Velde AA, Chamuleau RA. In vitro evaluation of a novel bioreactor based on an integral oxygenator and spirally wound nonwoven polyester matrix for hepatocyte culture as small aggregates. J Hepatol. 1997;26:1379–1392. doi: 10.1016/s0168-8278(97)80475-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Ding Y, Zhang H. Cryopreservation of suckling pig hepatocytes. Ann Clin Lab Sci. 2001;31:391–398. [PubMed] [Google Scholar]


