Abstract
AIM: To investigate and compare frequencies of serum positive cagA in patients from two separate regions of Turkey who were grouped according to the presence of peptic ulcer disease or non-ulcer dyspepsia.
METHODS: One hundred and eighty H. pylori-positive patients with peptic ulcer disease or non-ulcer dyspepsia were included in the study. One hundred and fourteen patients had non-ulcer dyspepsia and 66 had peptic ulcer disease (32 with gastric ulcers and/or erosions and 34 with duodenal ulcers). Each patient was tested for serum antibody to H. pylori cagA protein by enzyme immunoassay.
RESULTS: The total frequency of serum positive cagA in the study group was 97.2%. The rates in the patients with peptic ulcers and in those with non-ulcer dyspepsia were 100% and 95.6%, respectively. These results were similar to those reported in Asian studies, but higher than those that have been noted in other studies from Turkey and Western countries.
CONCLUSION: The high rates of serum positive cagA in these patients with peptic ulcer disease and non-ulcer dyspepsia were similar to results reported in Asia. The fact that there was high seroum prevalence regardless of ulcer status suggests that factors other than cagA might be responsible for ulceration or other types of severe pathology in H. pylori-positive individuals.
INTRODUCTION
Helicobacter pylori (H. pylori) infection is very common, especially in developing countries; however, patients with this infection rarely develop clinically significant conditions, such as peptic ulcer disease. This situation has prompted researchers to investigate the possible roles of host and environmental factors, and factors related to the bacterium itself in cases that show severe pathologies[1-3]. Earlier works identified associations between H. pylori strains that harbor cytotoxin-associated gene A (cagA) and significant gastroduodenal pathology; however, the results of more recent studies are conflicting. In Europe, investigators have reported a significantly higher seroprevalence of cagA antigen in gastroduodenal ulcer cases than that in non-ulcer dyspepsia cases[4,5]. In contrast, most studies from Asian countries have noted that there was no significant difference between these patient groups with respect to anti-cagA antibody positivity[5-7]. Interpretation of these findings has been further complicated by reports from Japan and China. Some of these results differ from those of other Asian studies, and are in line with findings in Western countries[8,9].
Turkey is geographically situated between two continents that are reported to have different cagA seroprevalence rates. Our aim in this study was to compare the frequencies of serum positive cagA in Turkish patients with peptic ulcer disease and those with non-ulcer dyspepsia.
MATERIALS AND METHODS
General data of patients
The study included 180 patients (79 males and 101 females; mean age 43.4 ± 11.2 years old) with dyspepsia who were confirmed H. pylori-positive by rapid urease testing. The patients came from two Baskent University medical centers in two different Turkish cities. Ninety-nine were from the southern city of Adana, and 81 were from Ankara in central Turkey. Individuals who met at least one of the following criterias were excluded from the study: history of H. pylori eradication treatment; anti-secretory and/or non-steroidal anti-inflammatory drug therapy in the 4 weeks prior to the study; chronic organ failure (chronic renal, pulmonary, or liver disease); chronic alcohol intake and cigarette smoking.
Methods
Gastric specimens from the antrum and corpus of each patient from the Adana Hospital were examined with hematoxylin/eosin and Giemsa stains. For each specimen, chronic inflammation, neutrophil activity, and H. pylori density were scored separately, according to the updated Sydney system: 0 = normal, 1 = mild, 2 = moderate, and 3 = severe[10]. We modified the four-point scale for histological scoring slightly in order to facilitate statistical analysis. Scores of 0-1 were categorized together as “low score” and scores of 2-3 were categorized together as “high score.” The same pathologist examined all the histological sections.
Three groups were divided according to the patients’ endoscopic findings: a non-ulcer dyspepsia (NUD) group (n = 114); a duodenal ulcer (DU) group (n = 34); and a gastric ulcer and/or erosion (GU/E) group (n = 32).
Enzyme immunoassay (Equipar Diagnostici, Rome, Italy) was used to test for the presence of serum IgG and IgA antibodies to H. pylori cagA protein. Since there is no international standard for IgG levels, this was quantitated by means of a standard curve calibrated in arbitrary units per milliliter (Uarb/mL). Serum levels above 5 Uarb/mL were considered to indicate positivity.
Statistical analysis
The unpaired Student’s T-test and the χ2 test were used to analyze the data, as appropriate. It was considered to be statistical significant when P < 0.05.
RESULTS
Of the 180 patients, 175 (97.2%) were cagA (+). The rate serum positive cagA in the NUD group was 95.6%, and they were 100% in both DU and GU/E groups. The overall rate in each hospital and the group rates in each center were shown in Table 1. The NUD, DU, and GU/E groups had similar mean serum levels of IgG-type cagA antibodies (45.2 ± 40.3 Uarb/mL, 54.3 ± 42.4 Uarb/mL, and 51.2 ± 41.3 Uarb/mL, respectively; P > 0.05).
Table 1.
The rates of serum positive cagA overall and accord-ing to endoscopic diagnosis in the patients from the two dif-ferent centers
|
cagA+ |
P | ||
| Adana hospital n (%) | Ankara hospital n (%) | ||
| Total patients | 98/99 (98.9) | 77/81 (95.1) | NS |
| NUD patients | 73/74 (98.6) | 36/40 (90.0) | NS |
| GU/E patients | 12/12 (100) | 20/20 (100) | NS |
| DU patients | 13/13 (100) | 21/21 (100) | NS |
Denotes: NUD: Non-ulcer dyspepsia; DU: Duodenal ulcer; GU/erosion: Gastric ulcer and/or erosions.
In each group, the percentages of patients with high scores for each histologic parameter were calculated. Separate calculations were made for the antrum and the corpus specimens. The results were then compared to demonstrate whether there was any difference among gastroduodenal pathologies (NUD, DU, and GU/E) with respect to severity of gastritis and H. pylori density in each stomach region (Figure 1). In the antrum, there were no significant differences in the group rates for chronic inflammation, neutrophil activity, and H. pylori density (P > 0.05), and all three groups had very high frequencies of high scores for chronic inflammation and H. pylori density. Analysis of the corpus findings showed that the DU group had a lower percentage of patients with high inflammation scores than those in the other two groups (P < 0.05). Also, the GU/E group had a higher percentage of patients with high H. pylori density scores than those in the other two groups (P < 0.05).
Figure 1.
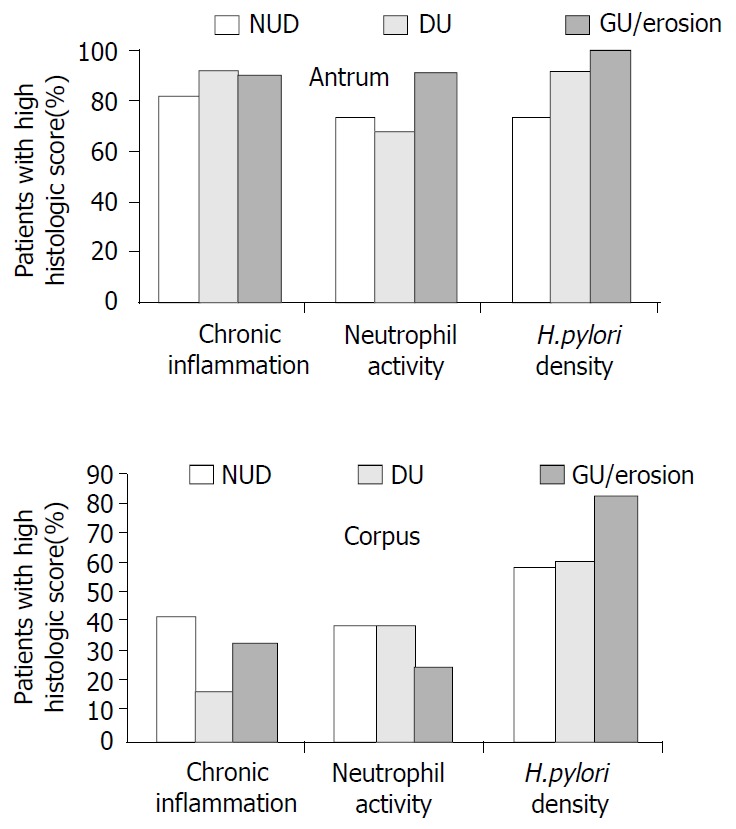
The percentages of patients with high scores (moderate or severe findings) for inflammation, neutrophil activity, and H. pylori density in the antrum and corpus. (NUD: Non-ulcer dyspepsia; DU: Duodenal ulcer; GU/erosion: Gas-tric ulcer and/or erosions).
DISCUSSION
Many studies have suggested that cagA+ strains of H. pylori are associated with severe gastrointestinal lesions, such as severe gastritis, peptic ulcer disease, and gastric cancer[11-13]. In infected patients, the cagA protein is translocated into epithelial cells and induces structural changes in these cells[14,15]. A number of investigations have shown that infection with these strains leads to increased secretion of interleukin-8, which plays a pivotal role in the inflammatory response[16,17]. Despite these findings and observations, recent studies of the frequency of cagA+ H. pylori strains in patients with NUD have suggested that factors other than cagA may contribute to severe gastrointestinal pathologies[5-7].
Studies of patients with and without peptic ulcer disease in different countries, and in different regions within countries, have revealed wide variations in cagA serum prevalence in these two groups. This variation is evident if we compared the data from our study group overall (180 H. pylori-positive patients from health centers in central and southern Turkey) and a previously published study of patients from western Turkey[18]. The latter report showed that the rate of serum positive cagA was significantly higher in peptic ulcer patients than that in NUD patients, whereas our results showed higher but similar rates when our patients were categorized in these two groups. When we analyzed our data of patients categorized according to hospital/city origin (Table 1), the overall rates of serum positive cagA were similar, and the corresponding rates were similar when the patients were divided into NUD, GU/E, and DU groups. These observations supported the suggestion that cagA should not be considered a universal marker for the prediction of severe gastrointestinal pathology. The reasons for, and the clinical aspects associated with this variation in serum positive cagA are not clear. Two possible reasons for the discrepancy among studies even from same country are the possible differences between commercial kits in the detection of cagA antibody in the sera of patients and variation in the prevalence of serum positive cagA strains even in areas showing geographical proximity.
In our study, we were unable to compare the severity of gastritis and H. pylori density in patients with cagA (+) and cagA (-) strains because almost all of the 180 patients tested positive for cagA antibodies, regardless of the endoscopic diagnosis. We performed an indirect analysis in attempt to determine whether cagA+ H. pylori strains were associated with severe gastritis. For this, we focused only on individuals with high histological scores, and determined the percentage of patients in each group that had high scores for chronic inflammation, neutrophil activity, and H. pylori density, respectively. The NUD, GU/E, and DU groups all had very high frequencies of high inflammation scores in the antrum. However, in the corpus, the DU group had a significantly lower frequency of high inflammation scores than the other two groups. These findings suggested that cagA may have some impact on the severity of gastritis, but not on duodenal ulcer development. Previous work has shown a negative correlation between severe corpus gastritis and the presence of DU, most likely due to changes in the pattern of gastric acid secretion[19,20]. Our findings were in line with this reported relationship.
Figura et al[21] reported that most patients with NUD have both cagA (+) and cagA (-) strains of H. pylori simultaneously, and suggested that a particular pathological finding may be determined by the dominant strain that colonizes a particular gastric area. This may explain the conflicting results of different studies concerning the serum prevalence of cagA in NUD patients. According to this concept, a patient with anti-cagA antibody in his or her serum may clinically show NUD if the majority of the H. pylori organisms in the gastric mucosa are cagA (-).
There is also another possible explanation for why some patients with cagA (+) strains develop milder upper gastrointestinal pathology than those who show more severe pathology but have the same bacterial strain. The reason may be variations in genetic make-up, as a number of different cagPaI genes are required for the cagA protein to be able to enter epithelial cells. Investigation has shown that inactivation of some of these genes abolishes cagA delivery and phosphorylation[14,22].
In conclusion, our findings reveal that the rates of cagA serum prevalence are high and similar in H. pylori-positive patients from two Turkish cities that are approximately half thousand of kilometers apart. These rates indicate that cagA serum prevalence in the Turkish population is close to the rates reported in Asian countries. The fact that we observed similar frequencies of cagA (+) H. pylori strains in all our dyspeptic patients, regardless of ulcer status, suggests that factors other than cagA may contribute to severe gastrointestinal pathology in patients with H. pylori.
Footnotes
Edited by Xu XQ
References
- 1.Go MF. What are the host factors that place an individual at risk for Helicobacter pylori-associated disease? Gastroenterology. 1997;113:S15–S20. doi: 10.1016/s0016-5085(97)80005-4. [DOI] [PubMed] [Google Scholar]
- 2.Kurata JH, Nogawa AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol. 1997;24:2–17. doi: 10.1097/00004836-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Atherton JC. The clinical relevance of strain types of Helicobacter pylori. Gut. 1997;40:701–703. doi: 10.1136/gut.40.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburton VJ, Everett S, Mapstone NP, Axon AT, Hawkey P, Dixon MF. Clinical and histological associations of cagA and vacA genotypes in Helicobacter pylori gastritis. J Clin Pathol. 1998;51:55–61. doi: 10.1136/jcp.51.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenks PJ, Mégraud F, Labigne A. Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut. 1998;43:752–758. doi: 10.1136/gut.43.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua J, Zheng PY, Yeoh KG, Ho B. The status of the cagA gene does not predict Helicobacter pylori-associated peptic ulcer disease in Singapore. Microbios. 2000;102:113–120. [PubMed] [Google Scholar]
- 7.Yang JC, Wang TH, Wang HJ, Kuo CH, Wang JT, Wang WC. Genetic analysis of the cytotoxin-associated gene and the vacuolating toxin gene in Helicobacter pylori strains isolated from Taiwanese patients. Am J Gastroenterol. 1997;92:1316–1321. [PubMed] [Google Scholar]
- 8.Takata T, Fujimoto S, Anzai K, Shirotani T, Okada M, Sawae Y, Ono J. Analysis of the expression of CagA and VacA and the vacuolating activity in 167 isolates from patients with either peptic ulcers or non-ulcer dyspepsia. Am J Gastroenterol. 1998;93:30–34. doi: 10.1111/j.1572-0241.1998.030_c.x. [DOI] [PubMed] [Google Scholar]
- 9.Ching CK, Wong BC, Kwok E, Ong L, Covacci A, Lam SK. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996;91:949–953. [PubMed] [Google Scholar]
- 10.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, Rathbone BJ. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 12.Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 13.Rudi J, Kolb C, Maiwald M, Zuna I, von Herbay A, Galle PR, Stremmel W. Serum antibodies against Helicobacter pylori proteins VacA and CagA are associated with increased risk for gastric adenocarcinoma. Dig Dis Sci. 1997;42:1652–1659. doi: 10.1023/a:1018849112533. [DOI] [PubMed] [Google Scholar]
- 14.Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M, et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree JE, Covacci A, Farmery SM, Xiang Z, Tompkins DS, Perry S, Lindley IJ, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma SA, Tummuru M, Miller G, Blaser MJ. Interleukin-8 re-sponse of gastric epithelial cell lines to Helicobacter pylori stimu-lation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirtürk L, Ozel AM, Yazgan Y, Solmazgül E, Yildirim S, Gültepe M, Gürbüz AK. CagA status in dyspeptic patients with and without peptic ulcer disease in Turkey: association with histopathologic findings. Helicobacter. 2001;6:163–168. doi: 10.1046/j.1523-5378.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim HY, Kim YB, Park CK, Yoo JY, Graham DY. Co-existing gastric cancer and duodenal ulcer disease: role of Helicobacter pylori infection. Helicobacter. 1997;2:205–209. doi: 10.1111/j.1523-5378.1997.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 20.El-Zimaity HMT O, Kim JG, Akamatsu T, Gürer IE, Simjee AE, Graham DY. Geographic differences in the distribution of intestinal metaplasia in duodenal ulcer patients. Am J Gastroenterol. 2001;96:666–672. doi: 10.1111/j.1572-0241.2001.03601.x. [DOI] [PubMed] [Google Scholar]
- 21.Figura N, Vindigni C, Covacci A, Presenti L, Burroni D, Vernillo R, Banducci T, Roviello F, Marrelli D, Biscontri M, et al. cagA positive and negative Helicobacter pylori strains are simultaneously present in the stomach of most patients with non-ulcer dyspepsia: relevance to histological damage. Gut. 1998;42:772–778. doi: 10.1136/gut.42.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]


