Abstract
AIM: To explore the seropositive rate of antibodies against H. pylori (anti-HP) in Taipei City and to compare the relationship of ABO blood groups and H. pylori infection.
METHODS: In 1993, high school students in Shih-Lin District were randomly selected for blood samplings by their registration number at school. In addition, similar procedures were performed on the well-children clinics of Taipei Veterans General Hospital. Besides, randomly selected sera from the adults who took the physical examination were recruited for evaluation. Informed consents were obtained from all the subjects before blood samplings and parents were simultaneously informed for those who were younger than 18-year-old. Blood tests for anti-HP and ABO blood groupings were performed by enzyme-linked immunosorbent assay. Chi square tests were used for the comparisons between seroprevalence of H. pylori and ABO blood groups.
RESULTS: Totally, 685 subjects were recruited (260 children aged 1-14 years, 425 high school students aged 15-18 years) were evaluated, and another 88 adult healthy volunteers were studied as well for comparison. The age-specific seropositive rate of anti-HP was 1.3% at age 1-5 years, 7.7% at age 6-10 years, and 11.5% at age 11-14 years. The seroprevalence of H. pylori infection was abruptly increased in young adolescence: 18.6% at age 15 years, 28.1% at age 16 years, 32.4% at age 17 years and 41.0% at age 18 years, respectively. In the 425 high school students, ABO blood groupings were performed, which disclosed 48.5% (206/425) of blood group O, 24% (102/425) of blood group A, 21.8% (93/425) of blood group B and 5.6% (24/425) of blood group AB. In comparison of the subjects with blood group O and the other blood groups, no statistical significance could be identified in the seroprevalence of H. pylori (P = 0.99).
CONCLUSION: The seroprevalence of H. pylori infection in Taipei City in adults is similar to the developed countries, and the abrupt increase of H. pylori during high school may be resulted from marked increase of interpersonal social activities. Although blood group O was reported to be related to H. pylori infection in previous literature, we found no association between H. pylori infection and ABO blood groups.
INTRODUCTION
Helicobacter pylori (H. pylori) infection is the most common chronic bacterial infection in the world. Previous seroepidemiologic studies indicated that about 50% of adults in the developed countries and nearly 90% of adults in the developing countries were positive of serum antibodies against H. pylori[1]. Chronic H. pylori infection may be related to several conditions, including chronic gastritis[2,3], peptic ulcer disease[4,5], primary gastric lymphoma (mostly mucosa-associated lymphoid tissue, MALToma)[6,7], and gastric adenocarcinoma[8,9].
The seroprevalence of H. pylori infection of adults in Taiwan varied from 54.4% to 59% in previous reports, which was similar to developed countries[10-12]. However, the seroprevalence of H. pylori infection was low in the preschool children in Taiwan. The seropositive rate of antibodies against H. pylori was 8.1% in 2 551 healthy preschool children aged 3-6 years[13], and it was significantly increased to 21.1% in young adolescence[14]. Therefore, marked increase of interpersonal social activities during the school age was proposed to be the most likely source of H. pylori infection.
People with blood group O have been noted to be more susceptible to peptic ulcer disease for decades without appropriate explanations[15,16]. In 1993, Boren et al[17] reported that people with blood group O had more H. pylori receptors, and Lewisb antigens mediated the attachment of H. pylori to the gastric mucosa. Furthermore, higher density of colonization of H. pylori was noted in the gastric mucosa of people with blood group O[18]. However, absence of correlation between H. pylori infection and ABO blood groups was reported in some following studies[19,20].
In Taiwan, blood group O was reported to correlate with the prevalence of H. pylori infection in patients with gastroduodenal diseases[21], but it remained unknown for those asymptomatic individuals who were infected by H. pylori. Therefore, we conducted a study to evaluate the relationship between H. pylori and ABO blood groups in those healthy volunteers in Taipei City to clarify the possible association.
MATERIALS AND METHODS
We conducted a cross-sectional survey among senior high schools in the Shih-Lin District, Taipei City in 1993. All the recruited subjects were randomly selected in each age group (from 15 to 18) according to the registration number in schools. Blood samplings were performed after the informed consents were signed by themselves or their parents (if the subjects were younger than 18-year-old). In addition, children at age 1 to 14 years from the well-children clinics of Taipei Veterans General Hospital were recruited if their parents agreed with the study and signed the informed consents. Moreover, we randomly collected the sera of adults who underwent physical examinations from the Department of Physical Examination to evaluate the seroprevalence of H. pylori. This study was evaluated and approved by the Ethical Committee of Taipei Veterans General Hospital.
The blood samples were centrifuged and the sera were stored in aliquot at -80 °C until analysis. The serum antibodies against H. pylori were tested by the commercial enzyme-linked immunosorbent assay (ELISA) kit (HEL-P test, AMRAD, Sydney, Australia). In addition, ABO blood groupings were also done by the ELISA test (Gamma Biologicals, Houston, TX, USA). ABO blood groupings were not performed in children from the well-children clinics because we reserved the sera from the blood samplings in the beginning, which were not possible for ABO blood groupings by the commercial kit.
Data of the recruited individuals were expressed in categories. The comparisons of seroprevalence of H. pylori between each ABO blood group were evaluated by chi-square test or Fisher’s exact test if appropriate. A P value of less than 0.05 would be considered statistically significant. All the available data were analyzed by a computer program (SPSS, Chicago, IL, USA).
RESULTS
Totally, 685 subjects were recruited, including 260 children aged 1-14 years from the Well-children Clinic, and 425 young adolescents aged 15-18 years from high school students in Shih-Lin District, Taipei City. In addition, sera of 88 randomly selected subjects from the Department of Physical Examination were evaluated for the referential seroprevalence of H. pylori in adults (Table 1).
Table 1.
Age and sex distribution of seroprevalence of anti-bodies against Helicobacter pylori (anti-HP), and comparisons between sex and age groups
| Age |
Male |
Female |
P | ||
| Anti-HP (+)/n | (%) | Anti-HP (+)/n | (%) | ||
| 1-5 | 0/26 | 0 | 1/42 | 2.4 | 0.32 |
| 6-10 | 4/39 | 10.3 | 2/39 | 5.1 | 0.40 |
| 11-14 | 9/53 | 17.0 | 3/51 | 5.9 | 0.08 |
| Subtotal | 13/118 | 11.0 | 6/132 | 4.5 | 0.06 |
| 15 | 7/34 | 20.6 | 11/63 | 17.5 | 0.72 |
| 16 | 9/38 | 23.7 | 27/90 | 30 | 0.46 |
| 17 | 21/65 | 32.3 | 24/74 | 32.4 | 0.99 |
| 18 | 14/41 | 34.1 | 11/20 | 55.0 | 0.14 |
| Subtotal | 51/179 | 28.5 | 73/246 | 29.7 | 0.79 |
| Adults | 27/49 | 55.1 | 19/39 | 48.7 | 0.56 |
| Total | 91/346 | 26.3 | 98/417 | 23.5 | 0.37 |
The age-specific seropositive rate of antibodies against H. pylori was 1.3% at age 1-5 years, 7.7% at age 6-10 years, and 11.5% at age 11-14 years. The seroprevalence of H. pylori infection abruptly increased in young adolescence: 18.6% at age 15 years, 28.1% at age 16 years, 32.4% at age 17 years and 41.0% at age 18 years, respectively. In randomly selected adults, the seropositive rate of anti-HP reached 52.3% (Figure 1).
Figure 1.
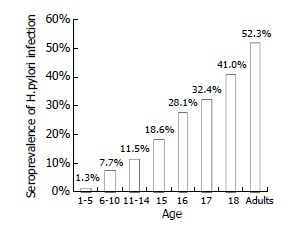
Age-specific seroprevalence of H. pylori infection in 773 healthy subjects in Taipei.
In the 425 randomly selected high school students, ABO blood groupings were performed, which disclosed 48.5% (206/425) blood group O, 24% (102/425) blood group A, 21.8% (93/425) blood group B and 5.6% (24/425) blood group AB. Further analysis on the ABO blood groupings and seroprevalence of H. pylori demonstrated that seropositive rate of anti-HP was 27.7% (57/206) in blood group O, 31.4% (32/102) in blood group A, 30.1% (28/93) in blood group B, and 29.2% (7/24) in blood group AB (Table 2). In comparison of the subjects with blood group O with the other blood groups, no statistical difference could be identified in the seroprevalence of H. pylori (P = 0.98). Neither was the difference significant among the groups as for being vulnerable to H. pylori infection.
Table 2.
Relationship of ABO blood groups and seropositivity of antibodies against Helicobacter pylori (anti-HP) infection
| Blood group | Serum anti-HP (+) | Serum anti-HP (-) | P |
| O | 57 (27.7%) | 149 (72.3%) | - |
| A | 32 (31.4%) | 70 (68.6%) | 0.99 |
| B | 28 (30.1%) | 65 (69.9%) | 0.99 |
| AB | 7 (29.2%) | 17 (60.8%) | 0.99 |
| Total | 124 (100%) | 301 (100%) |
DISCUSSION
H. pylori infection is the most prevalent chronic bacterial infection in the world. Despite of the worldwide infection, the transmission pattern remains uncertain. H. pylori infection is rare before first two decades of life in developed countries, ranging from 6% to 16%[22-24]. However, children in Gambia and Nigeria are almost all infected by H. pylori at age of 5 years[25,26]. According to the previous reports, the seroprevalence of H. pylori infection in Taiwanese preschool children (3-6 years old) was 8.1%, with the age-dependent progression (4.5% in 3-year-old children, 4.4% in 4-year-old children, 9.4% in 5-year-old children, and 11.7% in 6-year-old children)[13]. Furthermore, the seropositivity of antibodies against H. pylori was reported to be 21.1% in adolescents[14], and 54.4% in adults over 30-year-old in Taiwan[10]. Moreover, age-specific prevalence of H . pylori infection in patients with gastroduodenal diseases was 11.1% in those aged 1 to 20, 73.1% at age 21-30, and 79.8% at age 51-60 in Central Taiwan[27]. In this seroepidemiological study, we also found a similar pattern of age-dependent progression. However, the seroprevalence of H. pylori in this study (1.3% at age 1-5 years, 7.7% at age 6-10 years, and 11.5% at age 11-14 years) was significantly lower than that in the Taiwan islandwide survey. The better socioeconomic status in Taipei City may account for the differences of seroprevalence of H. pylori infection. However, the seroprevalence of H. pylori infection among adults in Taipei City was about the average of the Taiwan islandwide survey.
The transmission pattern of H. pylori currently remains uncertain, but the role of fecal-oral route seems to be minor[28,29]. The genotypic study did not support oral-oral transmission pattern of H. pylori infection, either[30]. Intrafamilial and person-to-person transmission has been shown being more important in H. pylori infection[31,32]. Risk factors analysis of H. pylori infection has been extensively performed including gender, race, family income, type of housing, location of housing, water supply, health status, and keeping pets, but only the socioeconomic status was better confirmed[33-36]. Broutet et al[37] proposed that male gender deserve more attention in epidemiological studies of H. pylori infection. In this study, the male predominance of H. pylori infection was observed. However, similar findings were not supported in previous epidemiological reports despite of different areas, ethnicity, and age[38-41]. The sex difference of H. pylori infection at age 11-14 years was unclear, which deserves further investigations.
The seropositive rate of anti-HP increased abruptly in subjects at age 15 years to 18 years (18.6% to 41.0%) in this study. The estimated annual incidence was 7.5% in this cross-sectional survey, which might be resulted from the extensive social activities at this stage. On the other hand, smoking is an important factor in H. pylori infection, particularly in young adults[42,43]. Most smokers start smoking at their young adolescence. Therefore, smoking may be another explanation of such an abrupt increase of H. pylori infection among people aged 15 to 18.
People with blood group O were found more susceptible to peptic ulcer disease for decades without known cause until the relationship between Lewisb antigens and the attachment of H. pylori to gastric mucosa was observed[15-18]. However, the correlation between H. pylori infection and ABO blood types was not supported in some reports[19,20]. Nevertheless, Lin et al[21] demonstrated the close relationship between H. pylori infection and blood group O patients with gastroduodenal diseases in Central Taiwan. In our study, healthy individuals rather than symptomatic patients with blood group O were not particularly vulnerable to H. pylori infection.
In conclusion, the abrupt increase of H. pylori infection in high school students was noted with the estimated yearly incidence to be 7.5%. Subjects with blood group O do not increase clinical susceptibility to H. pylori infection than those with other blood groups.
Footnotes
Edited by Xu XQ
References
- 1.Mégraud F. Epidemiology of Helicobacter pylori infection. Gastroenterol Clin North Am. 1993;22:73–88. [PubMed] [Google Scholar]
- 2.Czinn SJ, Dahms BB, Jacobs GH, Kaplan B, Rothstein FC. Campylobacter-like organisms in association with symptomatic gastritis in children. J Pediatr. 1986;109:80–83. doi: 10.1016/s0022-3476(86)80579-0. [DOI] [PubMed] [Google Scholar]
- 3.Ashorn M. What are the specific features of Helicobacter pylori gastritis in children. Ann Med. 1995;27:617–620. doi: 10.3109/07853899509002480. [DOI] [PubMed] [Google Scholar]
- 4.Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 5.Macarthur C, Saunders N, Feldman W. Helicobacter pylori, gastroduodenal disease, and recurrent abdominal pain in children. JAMA. 1995;273:729–734. [PubMed] [Google Scholar]
- 6.Wotherspoon AC. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu Rev Med. 1998;49:289–299. doi: 10.1146/annurev.med.49.1.289. [DOI] [PubMed] [Google Scholar]
- 7.Wu TC, Chen LK, Lai CR. Primary gastric lymphoma associated with Helicobacter pylori in a child. J Pediatr Gastroenterol Nutr. 2001;32:608–610. doi: 10.1097/00005176-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 9.Nomura A, Stemmermann GN. Helicobacter pylori and gastric cancer. J Gastroenterol Hepatol. 1993;8:294–303. doi: 10.1111/j.1440-1746.1993.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin JT, Wang JT, Wang TH, Wu MS, Lee TK, Chen CJ. Helicobacter pylori infection in a randomly selected population, healthy volunteers, and patients with gastric ulcer and gastric adenocarcinoma. A seroprevalence study in Taiwan. Scand J Gastroenterol. 1993;28:1067–1072. doi: 10.3109/00365529309098311. [DOI] [PubMed] [Google Scholar]
- 11.Lin JT, Wang JT, Wu MS, Wang TH, Lee TK, Chen CJ. Seroprevalence study of Helicobacter pylori infection in patients with gastroduodenal diseases. J Formos Med Assoc. 1994;93:122–127. [PubMed] [Google Scholar]
- 12.Lin JT, Wang LY, Wang JT, Wang TH, Yang CS, Chen CJ. A nested case-control study on the association between Helicobacter pylori infection and gastric cancer risk in a cohort of 9775 men in Taiwan. Anticancer Res. 1995;15:603–606. [PubMed] [Google Scholar]
- 13.Lin DB, Nieh WT, Wang HM, Hsiao MW, Ling UP, Changlai SP, Ho MS, You SL, Chen CJ. Seroepidemiology of Helicobacter pylori infection among preschool children in Taiwan. Am J Trop Med Hyg. 1999;61:554–558. doi: 10.4269/ajtmh.1999.61.554. [DOI] [PubMed] [Google Scholar]
- 14.Wang LY, Lin JT, Cheng YW, Chou SJ, Chen CJ. Seroepidemiology of Helicobacter pylori among adolescents in Taiwan. Zhonghua Minguo Weisheng Wujimian Yixue Zazhi. 1996;29:10–17. [PubMed] [Google Scholar]
- 15.CLARKE CA, EDWARDS JW, HADDOCK DR, HOWEL-EVANS AW, MCCONNELL RB, SHEPPARD PM. ABO blood groups and secretor character in duodenal ulcer; population and sibship studies. Br Med J. 1956;2:725–731. doi: 10.1136/bmj.2.4995.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mentis A, Blackwell CC, Weir DM, Spiliadis C, Dailianas A, Skandalis N. ABO blood group, secretor status and detection of Helicobacter pylori among patients with gastric or duodenal ulcers. Epidemiol Infect. 1991;106:221–229. doi: 10.1017/s0950268800048366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 18.Atherton JC, Tham KT, Peek RM, Cover TL, Blaser MJ. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996;174:552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 19.Loffeld RJ, Stobberingh E. Helicobacter pylori and ABO blood groups. J Clin Pathol. 1991;44:516–517. doi: 10.1136/jcp.44.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niv Y, Fraser G, Delpre G, Neeman A, Leiser A, Samra Z, Scapa E, Gilon E, Bar-Shany S. Helicobacter pylori infection and blood groups. Am J Gastroenterol. 1996;91:101–104. [PubMed] [Google Scholar]
- 21.Lin CW, Chang YS, Wu SC, Cheng KS. Helicobacter pylori in gastric biopsies of Taiwanese patients with gastroduodenal diseases. Jpn J Med Sci Biol. 1998;51:13–23. doi: 10.7883/yoken1952.51.13. [DOI] [PubMed] [Google Scholar]
- 22.De Giacomo C, Lisato L, Negrini R, Licardi G, Maggiore G. Serum immune response to Helicobacter pylori in children: epidemiologic and clinical applications. J Pediatr. 1991;119:205–210. doi: 10.1016/s0022-3476(05)80728-0. [DOI] [PubMed] [Google Scholar]
- 23.Thomas JE, Whatmore AM, Barer MR, Eastham EJ, Kehoe MA. Serodiagnosis of Helicobacter pylori infection in childhood. J Clin Microbiol. 1990;28:2641–2646. doi: 10.1128/jcm.28.12.2641-2646.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oderda G, Vaira D, Holton J. Age-related increase of Helicobacter pylori frequency in symptom-free and in dyspeptic children. Lancet. 1992;340:671–672. doi: 10.1016/0140-6736(92)92204-s. [DOI] [PubMed] [Google Scholar]
- 25.Holcombe C, Tsimiri S, Eldridge J, Jones DM. Prevalence of antibody to Helicobacter pylori in children in northern Nigeria. Trans R Soc Trop Med Hyg. 1993;87:19–21. doi: 10.1016/0035-9203(93)90403-d. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan PB, Thomas JE, Wight DG, Neale G, Eastham EJ, Corrah T, Lloyd-Evans N, Greenwood BM. Helicobacter pylori in Gambian children with chronic diarrhoea and malnutrition. Arch Dis Child. 1990;65:189–191. doi: 10.1136/adc.65.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CW, Chang YS, Lai PY, Cheng KS. Prevalence and heterogeneity of Helicobacter pylori in gastric biopsies of patients with gastroduodenal diseases. Zhonghua Minguo Weisheng Wujimian Yixue Zazhi. 1997;30:61–71. [PubMed] [Google Scholar]
- 28.Webb PM, Knight T, Newell DG, Elder JB, Forman D. Helicobacter pylori transmission: evidence from a comparison with hepatitis A virus. Eur J Gastroenterol Hepatol. 1996;8:439–441. [PubMed] [Google Scholar]
- 29.Lin DB, Tsai TP, Yang CC, Wang HM, Nieh WT, Ling UP, Changlai SP, You SL, Ho MS, Chen CJ. Association between seropositivity of antibodies against hepatitis a virus and Helicobacter pylori. Am J Trop Med Hyg. 2000;63:189–191. doi: 10.4269/ajtmh.2000.63.189. [DOI] [PubMed] [Google Scholar]
- 30.Luman W, Zhao Y, Ng HS, Ling KL. Helicobacter pylori infection is unlikely to be transmitted between partners: evidence from genotypic study in partners of infected patients. Eur J Gastroenterol Hepatol. 2002;14:521–528. doi: 10.1097/00042737-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Tindberg Y, Bengtsson C, Granath F, Blennow M, Nyrén O, Granström M. Helicobacter pylori infection in Swedish school children: lack of evidence of child-to-child transmission outside the family. Gastroenterology. 2001;121:310–316. doi: 10.1053/gast.2001.26282. [DOI] [PubMed] [Google Scholar]
- 32.Wizla-Derambure N, Michaud L, Ategbo S, Vincent P, Ganga-Zandzou S, Turck D, Gottrand F. Familial and community environmental risk factors for Helicobacter pylori infection in children and adolescents. J Pediatr Gastroenterol Nutr. 2001;33:58–63. doi: 10.1097/00005176-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Graham DY, Malaty HM, Evans DG, Evans DJ, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 34.Fiedorek SC, Malaty HM, Evans DL, Pumphrey CL, Casteel HB, Evans DJ, Graham DY. Factors influencing the epidemiology of Helicobacter pylori infection in children. Pediatrics. 1991;88:578–582. [PubMed] [Google Scholar]
- 35.Stone MA, Taub N, Barnett DB, Mayberry JF. Increased risk of infection with Helicobacter pylori in spouses of infected subjects: observations in a general population sample from the UK. Hepatogastroenterology. 2000;47:433–436. [PubMed] [Google Scholar]
- 36.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 37.Broutet N, Sarasqueta AM, Sakarovitch C, Cantet F, Lethuaire D, Mégraud F. Helicobacter pylori infection in patients consulting gastroenterologists in France: prevalence is linked to gender and region of residence. Eur J Gastroenterol Hepatol. 2001;13:677–684. doi: 10.1097/00042737-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Elitsur Y, Short JP, Neace C. Prevalence of Helicobacter pylori infection in children from urban and rural West Virginia. Dig Dis Sci. 1998;43:773–778. doi: 10.1023/a:1018866030977. [DOI] [PubMed] [Google Scholar]
- 39.Sinha SK, Martin B, Sargent M, McConnell JP, Bernstein CN. Age at acquisition of Helicobacter pylori in a pediatric Canadian First Nations population. Helicobacter. 2002;7:76–85. doi: 10.1046/j.1083-4389.2002.00063.x. [DOI] [PubMed] [Google Scholar]
- 40.Leal-Herrera Y, Torres J, Perez-Perez G, Gomez A, Monath T, Tapia-Conyer R, Muñoz O. Serologic IgG response to urease in Helicobacter pylori-infected persons from Mexico. Am J Trop Med Hyg. 1999;60:587–592. doi: 10.4269/ajtmh.1999.60.587. [DOI] [PubMed] [Google Scholar]
- 41.Lin JT, Wang LY, Wang JT, Wang TH, Chen CJ. Ecological study of association between Helicobacter pylori infection and gastric cancer in Taiwan. Dig Dis Sci. 1995;40:385–388. doi: 10.1007/BF02065425. [DOI] [PubMed] [Google Scholar]
- 42.Brenner H, Rothenbacher D, Bode G, Adler G. Relation of smoking and alcohol and coffee consumption to active Helicobacter pylori infection: cross sectional study. BMJ. 1997;315:1489–1492. doi: 10.1136/bmj.315.7121.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopański Z, Schlegel-Zawadzka M, Golec E, Witkowska B, Micherdziński J, Cienciala A, Kustra Z. The significance of selected epidemiologico-clinical factors in the prevalence of the Helicobacter pylori infection in young males. Eur J Med Res. 1997;2:358–360. [PubMed] [Google Scholar]


