Abstract
AIM: To investigate the effect of gastrin on differentiation of IEC-6 cell line in vitro.
METHODS: IEC-6 cells were incubated with gastrin. On day 7 after treatment, cell morphology was examined by light microscope, and on day 20, the cellular ultrastructures were examined by electron microscope. After exposure to gastrin for 6 hours, villin mRNA was analyzed by reverse transcription-polymerase chain reaction, and on day 7, the expression of villin was examined by immunocytochemical analysis with laser confocal microscope.
RESULTS: After exposure to gastrin, IEC-6 cells showed differentiated phenotypes as villas enterocytes and contained an abundance of plasma, small nuclei with nucleoli, and were arranged regularly. There were numerous microvilli around edge of the cells, and several cells showed columnar structures. Villin mRNA expression in cytoplasm was increased in comparison with control.
CONCLUSION: Differentiated characteristics of villus enterocytes and phenotypic changes of rat intestinal epithelial cells (IEC-6) are induced by gastrin, and the effects of gastrin are correlated to increased villin expression.
INTRODUCTION
Gastrin stimulates cell proliferation in gastric mucosa under physiological conditions[1]. Studies have demonstrated that gastrin many increase ornithine decarboxylase activity of IEC-6 cells, cause intracellular polyamine synthesis, and therefore promote cell proliferation[2,3] and migration[4]. Polyamine has been demonstrated to be closely correlated to cytoskeleton reconstitution, an important process of cellular differentiation[5-9], but it is not clear yet whether gastrin plays roles in differentiation of IEC-6 cells. This study was to investigate gastrin-induced morphological changes of intestinal epithelial cell (IEC-6) and the intracellular expression of villin.
MATERIALS AND METHODS
Cell culture
IEC-6 cells (ATCC, Rockville, MD) were grown at 37 °C in a 900 mL·L-1 air-100 mL·L-1 CO2 atmosphere in Dulbecco’s modified Eagle’s medium (pH7.2) containing 50 g·L-1 dFBS, 10 mg·L-1 insulin, 50 mg·L-1 gentamycin sulfate, and subcultured once a week. When cultured cells became confluence, they were dissociated with 0.5 g·L-1 trypsin and 0.2 g·L-1 EDTA, and seeded into 6-well cell culture plates. Pentagastrin (Sigma. Louis, MO) was dissolved in two or three drops of 300 g·L-1 ammonium hydroxide (sterile), the solution was adjusted to pH 7.5, and then diluted with medium to 62.5 mg·L-1 before use.
Morphology
Light microscopy Monolayer of IEC-6 cells was prepared on glass coverslips, which were placed in 6-well cell culture plates (Corning Glass Works). The cells were seeded at a concentration of 1.0 × 105 per well, and incubated at 37 °C in a 900 mL·L-1 air-100 mL·L-1 CO2 atmosphere for 24 h. The media containing cDMEM 2000 μL, PBS 490 μL and 10 μL pentagastrin solution were replaced to make a final concentration of pentagastrin 250 μg·L-1 in culture. The medium in control group was the same as that in the treatment group except gastrin. Cells were harvested on day 7 from the initial treatment. The coverslips were removed and fixed for 15 min at room temperature in 3.5% paraformaldehyde in PBS, washed with distilled water, followed by HE staining and examined under light microscope.
Electron microscopy The cells were seeded in 6-well cell culture plates with a concentration of 1.0 × 105 per well under the same culture condition as above. Cultured cells were harvested on day 20 from the initial treatment of gastrin, washed with PBS, fixed in 2% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated, and embedded in Epon, and examined under electron microscope.
Villin expression
mRNA level analysis After incubated with gastrin for 6 hours, cultured cells were harvested for extraction of total RNA with RNA TRIzol reagent (Gibco, Gaithersburg, MD). Isolation was performed according to the manufacturer’s protocols. The concentration of extracted RNA was determined. RT-PCR kit (Gibco, Gaithersburg, MD) was used for RT-PCR reaction following the attached protocol of the product. The primers (Seagon, Shanghai, China) were synthesized according to sequences of rat villin gene (GenBankTM accession number M98454) as follows: coding strand primer: 5’-ATGCCCAAGTCAAAGGCTCTCTCAACATCAC-3’, noncoding strand primer: 5’-TGCAACAGTCGCTGGACATCACAGG-3’[10]. The reference primers (Seagon, Shanghai, China) were according to sequences of rat β-actin gene (GenBankTM accession number AB028846) as follows: coding strand primer: 5’-TTCCAGCCTTCCTTCCTGG-3’, noncoding strand primer: 5’-TTGCGCTCAGGAGGAGCAAT-3’. 2 µL of RT products was added to the PCR master mix. After incubation at 94 °C for 2 min, reaction was done for 35 cycles at 94 °C for 60 s, at 55 °C for 60 s, and at 72 °C for 30 s. The expected cDNA amplification products were 408 bp for villin and 238 bp for β-actin. After electrophoresis on agarose gel and staining with ethidium bromide, DNA bands were visualized with an ultraviolet transilluminator.
Protein level analysis On day 7 from the initial treatment, cultured cells were fixed for 15 min at room temperature in 3.5% paraformaldehyde in PBS, and washed three times. For study of villin expression, the cells were permeabilized by incubation with 0.2% Triton X-100 in PBS for 4 min, washed three times with PBS, and then treated with goat serum for 10 min. The permeabilized cells were incubated with goat anti-rat antibody with dilution of 1:100 in PBS (Santa Cruz) for 2 hours at room temperature, washed, and then incubated with FITC-conjugated rabbit anti-goat IgG with dilution of 1:50 in PBS (Sigma. Louis, MO). The treated cells were visualized under TCS SP confocal laser scanning microscope (Leica, Heidelberg, Germany).
RESULTS
Effect of gastrin on morphology of IEC-6 cells
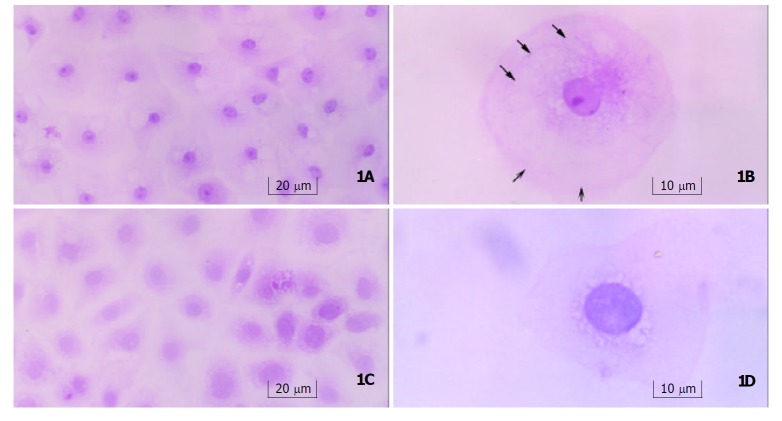
Light microscopy Seven-days after treatment with gastrin, cells were arranged regularly with an abundance of plasma, and small nuclei with nucleoli. Typically differentiated cells showed a tendency to form microvilli on the edge, and remarkable cytoskeleton-like structure, which was similar to cytoskeleton distribution in well-differentiated enterocytes. Cells in control group contained sparse plasma, large nuclei without nucleoli, and were arranged irregularly (Figure 1).
Figure 1.
Morphology of IEC-6 cells. a: Gastrin-treated cells(250×) contained an abundance of plasma, small nuclei with nucleoli, and were arranged regularly. b: One of gastrin-treated cells (400×) showed the tendency to form microvilli on the edge(open arrows), and cytoskeleton-like staining in plasma (solid arrows). c: Control cells (250×) contained sparse plasma, large nuclei without nucleoli, and were in irregular arrangement and immature shape. d: One of control cells (400×) showed no tendency to form microvilli on the edge, and nucleus was relatively larger and had no nucleolus.
Electron microscopy Twenty days after 20-day treatment with gastrin, numerous microvilli appeared on the edge of IEC-6 cells, many endocytic vesicles occurred under the apical membrane, and columnar structures were seen in some cells. Control cells were thin and flat, the nuclei were relatively large with scanty of cytoplasm. Only sparse microvilli were observed on the edge of control cells, and few endocytic vesicles were noticed (Figure 2).
Figure 2.
Ultrastructural changes of IEC-6 cells. a: Gastrin-treated cells (5000×, bar = 1 μm) showed columnar structures(the nuclei were shown by open arrows) with numerous microvilli on the edge (solid arrows). b: Gastrin-treated cells (12000×, bar = 500 nm)developed numerous microvilli (open arrows) and lots of endocytic vesicles appeared under the apical membrane (solid arrows). c: Control cells (5000×, bar = 1 μm) were thin and flat. Relatively large nuclei (open arrows) and scanty plasma were observed. d: Only sparse microvilli (open arrow) and endocytic vesicles (solid arrow) were seen in control cells (12000×, bar = 500 nm).
Effect of gastrin on villin expression in IEC-6 cells
mRNA level After exposure to gastrin for 6 hours, villin mRNA expression in gastrin-treated cells was stronger than that in control cells (Figure 3).
Figure 3.
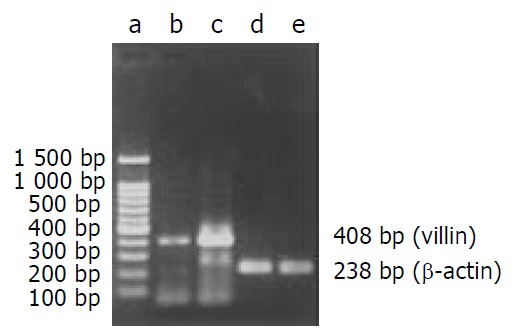
RT-PCR products from IEC-6 cells on agarose electrophoresis. a: Marker (brighter band: 500 bp), b: Control, c: Gastrin treated cells, d: β-actin(Control), e: β-actin (Gastrin).
Protein level On day 7 after treatment, plenty of cytoplasmic villins were observed obviously in gastrin-treated cells and few in control cells (Figure 4).
Figure 4.
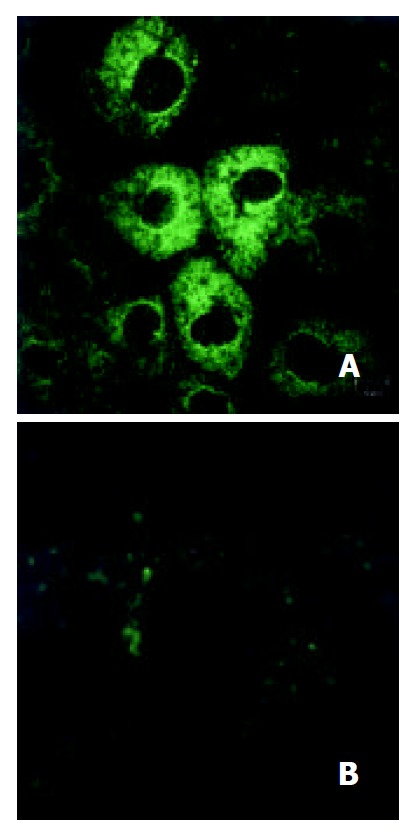
Villin expression in IEC-6 cells. a: Gastrin-treated cells (800×), b: Control cells (800×).
DISCUSSION
The barrier function of intestine is based on the physiological renewal or pathological repair of intestinal epithelia. The process includes proliferation, migration and terminal differentiation of the crypt cells. Development and differentiation of intestinal epithelia proceed in at least two distinct steps: the conversion of a nonepithelial cell to a protoepithelium, followed by a process of terminal differentiation. Terminal differentiation continues to occur in adult animals in the intestine[11], and is the last process. It not only indicates the completion of renewal or repair and the degree of differentiation, but also determines whether the new epithelia have physiological functions. There are two morphological characteristics in differentiated intestinal epithelia. One is the columnar shape cells with microvilli at the apical membrane, the other is organization of intestinal epithelial cells on a basement membrane into multicellular structures.
Intestinal epithelial cells (IEC-6) have features of undifferentiated small intestinal crypt cells[12], and are often used as a model of intestinal mucosal repair and cell differentiation[13,14]. The differentiation of intestinal crypt cell is a complex process, which is controlled by multiple factors. It has been known that several genes, such as p38 mitogen-activated protein kinases (p38MAPK)[15], Cdx gene family[16-20], pancreatic-duodenal homeobox (Pdxs) gene[21-23], sucrase-isomaltase (SI) gene[18,24,25], villin[26], activin[27], provoke cells towards the phenotype of differentiated villus enterocytes. Some cytokines such as epidermal growth factor (EGF)[6,7], insulin, insulin-like growth factor (IGF)-I and II[28,29], transforming growth factor (TGF)-β1[29], glucagon-like peptide-2 (GLP-2)[30] also have effects on the process. Astragalus injection could promote IEC-6 cell differentiation by inducing ODC activity and polyamine biosynthesis[31].
Moreover, the interaction between cells or between cells and extracellular matrix (ECM) also plays an important role in differentiation of IEC-6 cells[32]. Both humoral and matrix factors from intestinal mesenchyme are involved in intestinal epithelial differentiation and these factors appear to be organ specific[33,34]. And in conjunction with cell-cell contact and/ or ECM, many regulatory cytokines such as enteroglucagon, interleukin-2 (IL-2), fibroblast growth factor (FGF), and EGF family members lead to specific differentiation signals[35]. Cdx2 gene provokes pleiotropic effects triggering cells towards the phenotype of differentiated villus enterocytes, but its expression is also modulated by basement membrane components[18].
Previous studies on differentiation of IEC-6 cells have found that laminin can lead the organization of IEC-6 cells on a basement membrane into multicellular structures[36], and the down-regulation of c-jun expression mediated by laminin might result in the event[37]. IEC-6 cell culture on Englebreth-Holm-Swarm (EHS) extracellular matrix proteins also displays morphological changes, correlated with loss of nuclear localization of c-myc protein and development of cell surface alkaline phosphatase (ALP) enzymatic activity[14]. And it has been documented that striking morphological and functional alterations can be induced by glucocorticoid in IEC-6 cells. These effects are consistent with the activation or modulation of multiple genes important in physiological functions of absorptive villous cells[38]. Other data showed differentiation of IEC-6 cells was associated with upregulation of 11β-hydroxysteroid dehydrogenase (11β-HSD2) activity[39]. Members of the Cdx gene family play a fundamental role in both the establishment of the intestinal phenotype during development and maintenance of this phenotype via transcriptional activation of differentiated intestinal genes[40-43].
Our results showed that significantly morphological changes were observed in IEC-6 cells treated with gastrin in comparison with control group. The cells were in regular arrangement. Typically differentiated cell had an abundance of cytoplasm and a small nucleus containing nucleolus. There was a tendency to form microvilli and cytoskeleton-like structures were observed in the cytoplasm. Twenty-days after treatment of gastrin, a great number of microvilli appeared on the edges of the cells, and several cells displayed a simple columnar structure, and were fund amentally different from adenocarcinoma-like differentiation induced by Cdx1 transfection, which exhibited stratified columnar structure[16]. The absence of a multilayer structure indicated that these cells did not lose their contact inhibition characteristics, and they were not tumor cells. The existence of lots of endocytic vesicles as found under the apical membrane was also a typical feature of terminally differentiated enterocytes[11,44]. These results indicated that the cells might have the function of endocytosis as well as enterocytes. In control cells, only few microvilli were observed on the edge of cells with few endocytic vesicles.
Villin is one of the actin-binding proteins which have been reported to play a major role in the formation of the microvillus core bundle[45]. These proteins are known to modulate the dynamics of the actin cytoskeleton by mediating the state of actin polymerization and the spatial arrangement of actin protofilaments[46-49]. Villin may also respond to the apical calcium gradient, fragmenting actin microfilaments (MFs), and thus locally facilitate actin remodeling[50], and has a very important role in the alteration of cell morphology. The villin mRNA was expressed at high levels in the small intestine, to a lesser degree in the colon, and was not detected in the brain or liver[51]. The results indicate that villin is a kind of intestine-specific structure protein. In HT-29 cells, increase of villin mRNA levels was consistent with the process of enterocyte differentiation. Similarly, villin gene expression was induced in Caco-2 cells during postconfluence differentiation[51]. Immunolocalization studies on the distribution of the brush border-specific microvillar protein, villin, in human colonic mucosa indicated that localization of this protein was disrupted in certain dysplastic and neoplastic states. Thus, the expression and/or distribution of brush border-specific proteins such as villin may be useful markers for defects in the differentiation state of enterocytes[52].
Changes of cellular morphology and expression of mRNA and protein of villin in IEC-6 were investigated in order to observe the effects of gastrin on the differentiation of IEC-6 cells. The results showed that gastrin could obviously up-regulate villin expression at both mRNA and protein levels. These results were in consistent with the morphological alterations of these cells, and indicated that there was causality between the two events, i.e., gastrin induced characteristic features of differentiated enterocytes may account for its up-regulation to villin expression in IEC-6 cells. All these results indicate that gastrin can promote differentiation of IEC-6 cells, which is correlated to the up-regulation of villin expression.
ACKNOWLEDGEMENTS
We are grateful to Professor Peixun Wang, Associate Professors Weiwei Lei and Qin Xu, and Dr. Haibin Wang for their technical advice and excellent assistance.
Footnotes
Supported by the Major State Basic Research Development Program of China (973 Program) No.G19990544 and the National Natural Science Foundation of China, No.39970906
Edited by Ren SY and Wang XL
References
- 1.Jønson L, Bundgaard JR, Johnsen AH, Rourke IJ. Identification and expression of gastrin and cholecystokinin mRNAs from the turtle, Pseudemys scripta: evidence of tissue-specific tyrosyl sulfation(1) Biochim Biophys Acta. 1999;1435:84–93. doi: 10.1016/s0167-4838(99)00197-1. [DOI] [PubMed] [Google Scholar]
- 2.Wang JY, McCormack SA, Viar MJ, Johnson LR. Secretin inhibits induction of ornithine decarboxylase activity by gastrin in duodenal mucosa and IEC-6 cells. Am J Physiol. 1994;267:G276–G284. doi: 10.1152/ajpgi.1994.267.2.G276. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZL, Chen WW. Proliferation of intestinal crypt cells by gastrin-induced ornithine decarboxylase. World J Gastroenterol. 2002;8:183–187. doi: 10.3748/wjg.v8.i1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack SA, Wang JY, Viar MJ, Tague L, Davies PJ, Johnson LR. Polyamines influence transglutaminase activity and cell migration in two cell lines. Am J Physiol. 1994;267:C706–C714. doi: 10.1152/ajpcell.1994.267.3.C706. [DOI] [PubMed] [Google Scholar]
- 5.McCormack SA, Wang JY, Johnson LR. Polyamine deficiency causes reorganization of F-actin and tropomyosin in IEC-6 cells. Am J Physiol. 1994;267:C715–C722. doi: 10.1152/ajpcell.1994.267.3.C715. [DOI] [PubMed] [Google Scholar]
- 6.McCormack SA, Blanner PM, Zimmerman BJ, Ray R, Poppleton HM, Patel TB, Johnson LR. Polyamine deficiency alters EGF receptor distribution and signaling effectiveness in IEC-6 cells. Am J Physiol. 1998;274:C192–C205. doi: 10.1152/ajpcell.1998.274.1.C192. [DOI] [PubMed] [Google Scholar]
- 7.McCormack SA, Ray RM, Blanner PM, Johnson LR. Polyamine depletion alters the relationship of F-actin, G-actin, and thymosin beta4 in migrating IEC-6 cells. Am J Physiol. 1999;276:C459–C468. doi: 10.1152/ajpcell.1999.276.2.C459. [DOI] [PubMed] [Google Scholar]
- 8.Teti D, Visalli M, McNair H. Analysis of polyamines as markers of (patho)physiological conditions. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:107–149. doi: 10.1016/s1570-0232(02)00669-4. [DOI] [PubMed] [Google Scholar]
- 9.Weiss TS, Bernhardt G, Buschauer A, Thasler WE, Dolgner D, Zirngibl H, Jauch KW. Polyamine levels of human colorectal adenocarcinomas are correlated with tumor stage and grade. Int J Colorectal Dis. 2002;17:381–387. doi: 10.1007/s00384-002-0394-7. [DOI] [PubMed] [Google Scholar]
- 10.Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274:6476–6482. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- 11.Hikita C, Vijayakumar S, Takito J, Erdjument-Bromage H, Tempst P, Al-Awqati Q. Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J Cell Biol. 2000;151:1235–1246. doi: 10.1083/jcb.151.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wróblewski R, Jalnäs M, Van Decker G, Björk J, Wroblewski J, Roomans GM. Effects of irradiation on intestinal cells in vivo and in vitro. Histol Histopathol. 2002;17:165–177. doi: 10.14670/HH-17.165. [DOI] [PubMed] [Google Scholar]
- 14.Wood SR, Zhao Q, Smith LH, Daniels CK. Altered morphology in cultured rat intestinal epithelial IEC-6 cells is associated with alkaline phosphatase expression. Tissue Cell. 2003;35:47–58. doi: 10.1016/s0040-8166(02)00103-9. [DOI] [PubMed] [Google Scholar]
- 15.Houde M, Laprise P, Jean D, Blais M, Asselin C, Rivard N. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J Biol Chem. 2001;276:21885–21894. doi: 10.1074/jbc.M100236200. [DOI] [PubMed] [Google Scholar]
- 16.Soubeyran P, André F, Lissitzky JC, Mallo GV, Moucadel V, Roccabianca M, Rechreche H, Marvaldi J, Dikic I, Dagorn JC, et al. Cdx1 promotes differentiation in a rat intestinal epithelial cell line. Gastroenterology. 1999;117:1326–1338. doi: 10.1016/s0016-5085(99)70283-0. [DOI] [PubMed] [Google Scholar]
- 17.Freund JN, Domon-Dell C, Kedinger M, Duluc I. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem Cell Biol. 1998;76:957–969. doi: 10.1139/o99-001. [DOI] [PubMed] [Google Scholar]
- 18.Lorentz O, Duluc I, Arcangelis AD, Simon-Assmann P, Kedinger M, Freund JN. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J Cell Biol. 1997;139:1553–1565. doi: 10.1083/jcb.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor JK, Levy T, Suh ER, Traber PG. Activation of enhancer elements by the homeobox gene Cdx2 is cell line specific. Nucleic Acids Res. 1997;25:2293–2300. doi: 10.1093/nar/25.12.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moucadel V, Soubeyran P, Vasseur S, Dusetti NJ, Dagorn JC, Iovanna JL. Cdx1 promotes cellular growth of epithelial intestinal cells through induction of the secretory protein PAP I. Eur J Cell Biol. 2001;80:156–163. doi: 10.1078/0171-9335-00148. [DOI] [PubMed] [Google Scholar]
- 21.Yamada S, Kojima H, Fujimiya M, Nakamura T, Kashiwagi A, Kikkawa R. Differentiation of immature enterocytes into enteroendocrine cells by Pdx1 overexpression. Am J Physiol Gastrointest Liver Physiol. 2001;281:G229–G236. doi: 10.1152/ajpgi.2001.281.1.G229. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida S, Kajimoto Y, Yasuda T, Watada H, Fujitani Y, Kosaka H, Gotow T, Miyatsuka T, Umayahara Y, Yamasaki Y, et al. PDX-1 induces differentiation of intestinal epithelioid IEC-6 into insulin-producing cells. Diabetes. 2002;51:2505–2513. doi: 10.2337/diabetes.51.8.2505. [DOI] [PubMed] [Google Scholar]
- 23.Kojima H, Nakamura T, Fujita Y, Kishi A, Fujimiya M, Yamada S, Kudo M, Nishio Y, Maegawa H, Haneda M, et al. Combined expression of pancreatic duodenal homeobox 1 and islet factor 1 induces immature enterocytes to produce insulin. Diabetes. 2002;51:1398–1408. doi: 10.2337/diabetes.51.5.1398. [DOI] [PubMed] [Google Scholar]
- 24.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor JK, Boll W, Levy T, Suh E, Siang S, Mantei N, Traber PG. Comparison of intestinal phospholipase A/lysophospholipase and sucrase-isomaltase genes suggest a common structure for enterocyte-specific promoters. DNA Cell Biol. 1997;16:1419–1428. doi: 10.1089/dna.1997.16.1419. [DOI] [PubMed] [Google Scholar]
- 26.Braunstein EM, Qiao XT, Madison B, Pinson K, Dunbar L, Gumucio DL. Villin: A marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90–102. doi: 10.1002/dvdy.10091. [DOI] [PubMed] [Google Scholar]
- 27.Sonoyama K, Rutatip S, Kasai T. Gene expression of activin, activin receptors, and follistatin in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G89–G97. doi: 10.1152/ajpgi.2000.278.1.G89. [DOI] [PubMed] [Google Scholar]
- 28.Jehle PM, Fussgaenger RD, Blum WF, Angelus NK, Hoeflich A, Wolf E, Jungwirth RJ. Differential autocrine regulation of intestine epithelial cell proliferation and differentiation by insulin-like growth factor (IGF) system components. Horm Metab Res. 1999;31:97–102. doi: 10.1055/s-2007-978705. [DOI] [PubMed] [Google Scholar]
- 29.Kojima H, Hidaka H, Matsumura K, Fujita Y, Nishio Y, Maegawa H, Haneda M, Yasuda H, Fujimiya M, Kikkawa R, et al. Concerted regulation of early enterocyte differentiation by insulin-like growth factor I, insulin, and transforming growth factor-beta1. Proc Assoc Am Physicians. 1998;110:197–206. [PubMed] [Google Scholar]
- 30.Kitchen PA, Fitzgerald AJ, Goodlad RA, Barley NF, Ghatei MA, Legon S, Bloom SR, Price A, Walters JR, Forbes A. Glucagon-like peptide-2 increases sucrase-isomaltase but not caudal-related homeobox protein-2 gene expression. Am J Physiol Gastrointest Liver Physiol. 2000;278:G425–G428. doi: 10.1152/ajpgi.2000.278.3.G425. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZL, Chen WW. [Effect of Astragalus injection in promoting IEC-6 cell differentiation through activating ornithine decarboxylase] Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22:439–443. [PubMed] [Google Scholar]
- 32.Wolpert S, Wong ML, Bass BL. Matrix alters the proliferative response of enterocytes to growth factors. Am J Surg. 1996;171:109–112. doi: 10.1016/S0002-9610(99)80083-X. [DOI] [PubMed] [Google Scholar]
- 33.Ferretti E, Li S, Wang J, Post M, Moore A. Mesenchymal regulation of differentiation of intestinal epithelial cells. J Pediatr Gastroenterol Nutr. 1996;23:65–73. doi: 10.1097/00005176-199607000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- 35.Burgess AW. Growth control mechanisms in normal and transformed intestinal cells. Philos Trans R Soc Lond B Biol Sci. 1998;353:903–909. doi: 10.1098/rstb.1998.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson AD, Pysher T, Bienkowski RS. Organization of intestinal epithelial cells into multicellular structures requires laminin and functional actin microfilaments. Exp Cell Res. 1991;192:543–549. doi: 10.1016/0014-4827(91)90074-5. [DOI] [PubMed] [Google Scholar]
- 37.Wolpert SI, Lally KM, Li J, Wang JY, Bass BL. Extracellular matrix modulates enterocyte growth via downregulation of c-jun but is independent of p21 and p27 expression. J Gastrointest Surg. 1999;3:319–324. doi: 10.1016/s1091-255x(99)80074-2. [DOI] [PubMed] [Google Scholar]
- 38.Quaroni A, Tian JQ, Göke M, Podolsky DK. Glucocorticoids have pleiotropic effects on small intestinal crypt cells. Am J Physiol. 1999;277:G1027–G1040. doi: 10.1152/ajpgi.1999.277.5.G1027. [DOI] [PubMed] [Google Scholar]
- 39.Pácha J, Lisá V, Miksík I. Effect of cellular differentiation on 11beta-hydroxysteroid dehydrogenase activity in the intestine. Steroids. 2002;67:119–126. doi: 10.1016/s0039-128x(01)00143-x. [DOI] [PubMed] [Google Scholar]
- 40.Suh E, Chen L, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol. 1994;14:7340–7351. doi: 10.1128/mcb.14.11.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moucadel V, Totaro MS, Dell CD, Soubeyran P, Dagorn JC, Freund JN, Iovanna JL. The homeobox gene Cdx1 belongs to the p53-p21(WAF)-Bcl-2 network in intestinal epithelial cells. Biochem Biophys Res Commun. 2002;297:607–615. doi: 10.1016/s0006-291x(02)02250-7. [DOI] [PubMed] [Google Scholar]
- 42.Soubeyran P, Haglund K, Garcia S, Barth BU, Iovanna J, Dikic I. Homeobox gene Cdx1 regulates Ras, Rho and PI3 kinase pathways leading to transformation and tumorigenesis of intestinal epithelial cells. Oncogene. 2001;20:4180–4187. doi: 10.1038/sj.onc.1204551. [DOI] [PubMed] [Google Scholar]
- 43.Patterson AP, Chen Z, Rubin DC, Moucadel V, Iovanna JL, Brewer HB, Eggerman TL. Developmental regulation of apolipoprotein B mRNA editing is an autonomous function of small intestine involving homeobox gene Cdx1. J Biol Chem. 2003;278:7600–7606. doi: 10.1074/jbc.M201601200. [DOI] [PubMed] [Google Scholar]
- 44.Vijayakumar S, Takito J, Hikita C, Al-Awqati Q. Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cells: processes that resemble terminal differentiation. J Cell Biol. 1999;144:1057–1067. doi: 10.1083/jcb.144.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Athman R, Louvard D, Robine S. The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G496–G502. doi: 10.1152/ajpgi.00207.2002. [DOI] [PubMed] [Google Scholar]
- 46.Glenney JR, Bretscher A, Weber K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc Natl Acad Sci U S A. 1980;77:6458. doi: 10.1073/pnas.77.11.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mooseker MS, Graves TA, Wharton KA, Falco N, Howe CL. Regulation of microvillus structure: calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J Cell Biol. 1980;87:809–822. doi: 10.1083/jcb.87.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrary E, Cohen-Tannoudji M, Pehau-Arnaudet G, Lapillonne A, Athman R, Ruiz T, Boulouha L, El Marjou F, Doye A, Fontaine JJ, et al. In vivo, villin is required for Ca(2+)-dependent F-actin disruption in intestinal brush borders. J Cell Biol. 1999;146:819–830. doi: 10.1083/jcb.146.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coluccio LM, Bretscher A. Reassociation of microvillar core proteins: making a microvillar core in vitro. J Cell Biol. 1989;108:495–502. doi: 10.1083/jcb.108.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vidali L, Hepler PK. Actin and pollen tube growth. Protoplasma. 2001;215:64–76. doi: 10.1007/BF01280304. [DOI] [PubMed] [Google Scholar]
- 51.Hodin RA, Shei A, Meng S. Transcriptional activation of the human villin gene during enterocyte differentiation. J Gastrointest Surg. 1997;1:433–48; discussion 438. doi: 10.1016/s1091-255x(97)80130-8. [DOI] [PubMed] [Google Scholar]
- 52.Carboni JM, Howe CL, West AB, Barwick KW, Mooseker MS, Morrow JS. Characterization of intestinal brush border cytoskeletal proteins of normal and neoplastic human epithelial cells. A comparison with the avian brush border. Am J Pathol. 1987;129:589–600. [PMC free article] [PubMed] [Google Scholar]