Abstract
AIM: To investigate the toxicities, biodistribution and anticancer effect of 5-fluorouracil controlled release implant (5-FUCI) on Walker 256 carcinosarcoma cells in Wistar rats.
METHODS: Experiment 1: Wistar rats were randomly divided into three groups (27 rats per group). Blank implant was implanted in left lobe of the liver, and rats were treated with saline solution (in group A) or 5-fluorouracil (subcutaneous injection, group B). 5-FUCI was inserted in left lobe of the liver (group C). The gastrointestinal and hematological toxicities were observed and contents of element F in group C were assayed. Experiment 2: on day 6 after Walker-256 carcinosarcoma transplantation in left lobe of the liver, 5-FUCI was implanted in right lobe of the liver (group E) or left lobe (group F), and rats in control group (group D) were inserted blank implant. Tumor inhibition rate and survival time were investigated.
RESULTS: 5-FUCI showed no obvious toxic effect, extraction of Evan’s blue from gastrointestinal tissue was normal, the peripheral white blood cells and bone marrow nucleated cells were not reduced, compared with control group (P > 0.05). Histological examination revealed that there were no visible changes in small intestinal mucosa, The concentration of 5-fluorouracil in left lobe of the liver was 9.84, 28, 34 times as much as those of right lobe of the liver, heart and kidney respectively after the implantation in group C. They kept a high level of fluorouracil in left lobe of the liver, ranging from (4.414% ± 0.482%) to (7.800% ± 0.804%), for eight weeks. Survival days were 28.0 ± 2.2, 30.0 ± 3.2 and 38.7 ± 6.7 d in group D, E and F, respectively.
CONCLUSION: 5-FUCI shows no obvious toxicities to gastrointestinal tract and myelotoxicity. After implantation, it kept a high level of 5- fluorouracil in surrounding tissues of the implant for eight weeks. Its antitumor effect on Walker-256 carcinosarcoma is demonstrated.
INTRODUCTION
5-Fluorouracil (5-FU) is one of the most widely used chemotherapeutic drugs in the treatment of malignant gastrointestinal cancers[1-7], but its use has been limited by is systemic toxicities, i.e. severe gastrointestinal toxicities, hematological side effects, and severe disturbance in bone marrow[8,9]. Moreover, 5-FU has a serum half-life of only 10 minutes[10-13], further limiting its usefulness. To maximize the therapeutic effect of 5-FU and minimize any adverse effect, we developed silicone as a polymer matrix, and 5-FU as a model drug to prepare a controlled-release implant of 5-fluorouracil (5-FUCI, Figure 1) for delivering agents directly to the site of the tumor. Tube-type pellets made of silastic elastomer were of 2 mm in outer diameter, 0.5 mm in wall thickness and 25 mm in length. Per pellet contained 13.2 mg of 5-FU. The daily delivery dose of 5-FU was approximately 30 μg·d-1, zero-order release profiles lasted for 24 weeks in vitro (unpublished data). This study investigated its toxicities as compared with standard systemic therapy, the biodistribution of 5-FU after 5-FUCI was implanted in the liver of rats, and therapeutic effect on the Walker-256 carcinosarcoma in the liver of Wistar rats. Two experiments were performed.
Figure 1.
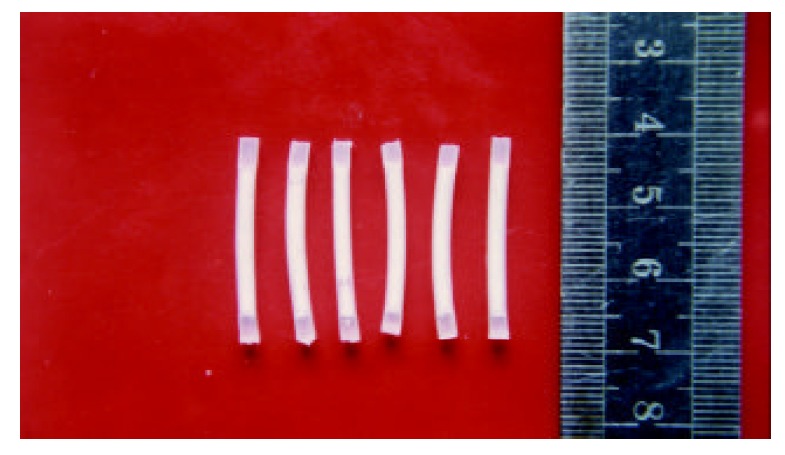
5-FUCI.
MATERIALS AND METHODS
Experiment 1: Toxicity evaluation and biodistribution of 5-FUCI
5-FUCI and blank implant 5-FUCI and blank implants were prepared and kindly provided by Prof. Huaiying Wu (from Shanghai Medical University, China). 5-Fu was purchased from Shanghai Haipu Pharmaceutical Company.
Animals Adult male Wistar rats, weighing 300-320 g, were obtained from the Experimental Animal Center of Wuhan University, China. They were housed in stock cages (3 rats per cage) and given free access to water and the commercial laboratory rodent diet. Twelve hours prior to experiment, the rats were fasted, but allowed free access to water.
Implantation and treatment The rats were anesthetized with i.p. of sodium pentobarbital (40 mg·kg-1). All procedures were carried out aseptically. Then, they were randomly divided into three groups (27 rats per group) and received implantations.
Group A: A blank implant was implanted in left lobe of the liver. Subcutaneous injection of 1 ml saline solution was administered (daily×7, weekly×7).
Group B: A blank implant was implanted in left lobe of the liver. 5-FU (10 mg·kg-1) was subcutaneously injected (daily×7, weekly ×7).
Group C: 5-FUCI was implanted in left lobe of the liver. 1 ml saline solution was subcutaneously injected (daily×7, weekly ×7).
Counts of WBC and bone marrow nucleated cells Three rats per group were respectively anesthetized weekly, counts of the peripheral white blood cells and bone marrow nucleated cells were assessed. The jugular vein was isolated and received injection of 1% Evan’s blue solution (1.0 mg·kg-1). The rats were killed by cervical dislocation at 45 min postinjection.
Toxicity evaluation of small intestine Two cm sections of the proximal jejunum were cut and weighed, and treated with 40% trichloroacetic acid solution (1:8, w/v), then left at 25 °C overnight and centrifuged at 1500 g for 10 min. The upper aqueous phase was collected and extraction of Evan’s blue was measured by spectrophotometer.
Morphological changes of small intestinal mucosa The small intestine segments were collected, and haematoxylin and eosin stained slides were used. The area of small intestinal mucosal cells and integrated optical density (IOD) of inner mucosal surface of the small intestine were measured by using image analysis system (IBAS, Kontron Co. Ltd., Germany).
Assay of element F in group C To evaluate the biodistribution of 5-FU postimplantation, major organs (left/right lobe of the liver, heart, kidney) in group C were resected, adherent blood was removed and weighed. The contents of F were measured by an electron probe analyzer (JCXA-733, JEDL Co. Ltd., Japan).
Experiment 2: Antitumor activities of 5-FUCI
Preparation of TB rats Walker-256 carcinosarcoma cells were obtained from Chinese Center of Culture Preservation. On day 0 following laparotomy, 107 tumor cells of approximately 0.1 ml of cell suspension were transplanted in left lobe of the liver under slight ketamine anesthesia.
5-FUCI transplantation and treatment On day 6 after tumor transplantation, the tumor-bearing (TB) rats were randomly assigned to three groups (12 rats per group) for treatment and reoperated for insertion of 5-FUCI or blank implant.
Group D: The TB rats were treated by intratumoral implantation of a blank implant in left lobe of the liver.
Group E: 5-FUCI was implanted in right lobe of the liver.
Group F: The TB rats were treated by intratumoral implantation of 5-FUCI in left lobe of the liver.
Tumor volume and survival days On day 21, six rats per group were sacrificed, solid tumors were excised, weighed and the rate of tumor inhibition was calculated by the formula: (the tumor volume of control group-tumor volume of experimental group)/(the tumor volume of control group)×100%. The life span of rest rats was investigated.
Pathological examination The tumor tissues taken from control group and experimental group were embedded in paraffin, and 5 μm sections were taken, stained with hematoxylin-eosin, and observed under light microscope to examine the therapeutic effect of 5-FUCI.
Statistical analysis
The differences between the mean values were analyzed for significance using the Student’s t test for independent samples. Survival time was examined with time sequence examination. P ≤ 0.05 was considered to be statistically significant.
RESULTS
Changes of counts of WBC and bone marrow nucleated cells before and after treatment
Counts of the peripheral white blood cells in group B decreased from 11.5 g·L-1 before chemotherapy to 2.0 g·L-1 3 weeks after treatment, but the data were not significantly different in group C compared with control group (group A, P > 0.05). Counts of the bone marrow nucleated cells after subcutaneous injection of 5-FU decreased from 41.4 × 109·L-1 to 24.0 × 109·L-1, and the data were normal in groups A and C (P > 0.05).
Small intestinal mucosal image analysis
Morphological changes of small intestinal mucosa are listed in Table 1 and Table 2.
Table 1.
Changes of area of small intestinal mucosal cells (μm2)
| Time (weeks) | Group A | Group B | Group C |
| 2 | 38.98 ± 8.16 | 47.16 ± 3.29 | 31.32 ± 7.76b |
| 4 | 35.07 ± 5.70 | 42.25 ± 4.42 | 28.81 ± 1.98b |
| 6 | 38.11 ± 1.89 | 36.97 ± 3.44 | 30.17 ± 5.00 |
| 8 | 29.91 ± 1.34 | - | 29.72 ± 0.97 |
| Mean | 35.68 ± 5.78 | 42.13 ± 5.48a | 30.00 ± 4.15ab |
P < 0.05 vs group A;
P < 0.05 vs group B.
Table 2.
Changes of IOD of inner mucosal surface of small intestine
| Time (weeks) | Group A | Group B | Group C |
| 2 | 0.406 ± 0.020 | 0.248 ± 0.069 | 0.453 ± 0.051 |
| 4 | 0.417 ± 0.110 | 0.290 ± 0.017 | 0.409 ± 0.024b |
| 6 | 0.379 ± 0.042 | 0.335 ± 0.055 | 0.453 ± 0.081 |
| 8 | 0.508 ± 0.083 | - | 0.352 ± 0.041a |
| Mean | 0.427 ± 0.080 | 0.295 ± 0.051a | 0.417 ± 0.063b |
P < 0.05 vs group A;
P < 0.05 vs group B.
Gastrointestinal toxicity
Gastrointestinal toxicity was positive by correlated with extraction of Evan’s blue from gastrointestinal tract. The results of extraction of Evan’s blue from small intestine are shown in Table 3.
Table 3.
Changes of extraction of Evan’s blue from small intestine (μg·g-1)
| Time (weeks) | Group A | Group B | Group C |
| 0 | 0.860 ± 0.051 | 0.840 ± 0.045 | 0.870 ± 0.035 |
| 1 | 0.903 ± 0.045 | 0.980 ± 0.145 | 0.847 ± 0.083 |
| 2 | 0.797 ± 0.086 | 1.123 ± 0.172 | 1.243 ± 0.169a |
| 3 | 0.880 ± 0.240 | 1.273 ± 0.231 | 0.813 ± 0.081b |
| 4 | 0.917 ± 0.349 | 1.275 ± 0.230 | 0.823 ± 0.067b |
| 5 | 0.793 ± 0.100 | 1.320 ± 0.110a | 0.863 ± 0.115b |
| 6 | 0.993 ± 0.276 | 1.427 ± 0.227 | 0.813 ± 0.099b |
| 7 | 0.890 ± 0.226 | 1.010 ± 0.014 | 0.923 ± 0.250 |
| 8 | 0.873 ± 0.116 | - | 0.853 ± 0.131 |
| Mean | 0.881 ± 0.183 | 1.253 ± 0.323a | 0.898 ± 0.179b |
P < 0.05 vs group A;
P < 0.05 vs group B.
Biodistribution of 5-FU after implantation
After 5-FUCI implantation, the local high accumulation of 5-FU was demonstrated in the adjacent tissue surrounding 5-FUCI implant (in left lobe of the liver). On the other hand, the concentration of 5-FU was lower in right lobe of the liver, heart and kidney (Table 4).
Table 4.
Contents of element F in group C in major organs (%)
| Time (weeks) | Left lobe of liver | Right lobe of liver | Heart | Kidney |
| 2 | 7.800 ± 0.804 | 0.782 ± 0.084a | 0.143 ± 0.050ab | 0.174 ± 0.144ab |
| 4 | 6.560 ± 0.694 | 0.645 ± 0.233a | 0.300 ± 0.131a | 0.272 ± 0.136a |
| 6 | 6.269 ± 1.383 | 0.784 ± 0.362a | 0.288 ± 0.232a | 0.141 ± 0.061ab |
| 8 | 4.414 ± 0.482 | 0.334 ± 0.143a | 0.162 ± 0.067a | 0.184 ± 0.120a |
P < 0.05 vs left lobe of the liver;
P < 0.05 vs right lobe of the liver.
Observations of the anticancer effect
Changes of tumor volume and survival time in cancer-bearing rats are listed in Table 5. One animal in group F survived to the end of the experiment (50 days) and no viable tumor was found.
Table 5.
Changes of tumor volume and survival days in tumor-bearing rats
| Group |
Tumor volume (cm3) |
Tumor inhibition rates (%) | Survival days (d) | |
| 6 days of transplantation | 21 days of transplantation | |||
| Group D | 0.021 ± 0.010 | 2.762 ± 1.384 | - | 28.0 ± 2.2 |
| Group E | 0.030 ± 0.014 | 1.844 ± 0.904 | 33.2 | 30.0 ± 3.2 |
| Group F | 0.027 ± 0.012 | 0.102 ± 0.117ab | 96.3b | 38.7 ± 6.7ab |
P < 0.05 vs group D,
P < 0.05 vs group E.
Results of pathologic examination
5-FUCI implantation had no apparent pathological changes in the heart, liver, kidney and small intestine in group C. Some local effects of 5-FUCI were observed. The tumor cell proliferation in group D was very active (Figure 2). A number of focal necrosis were observed in group E (Figure 3) Most necrotic tissues were found at a distance of up to 1 cm from the implant in group F(Figure 4).
Figure 2.
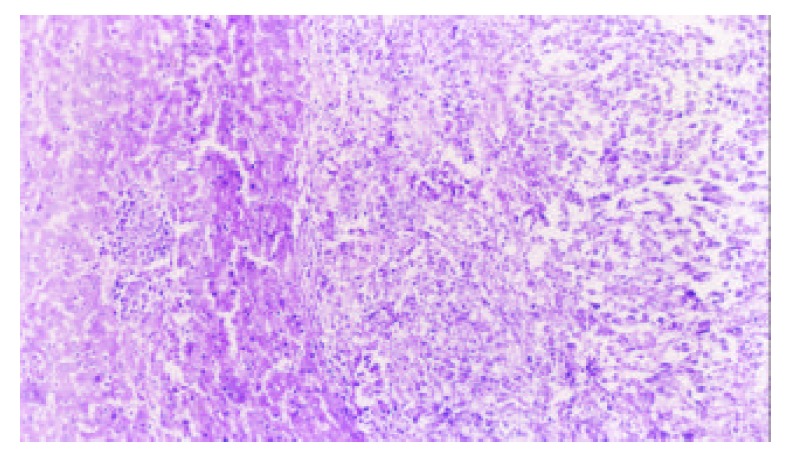
Tumor section (HE×100): tumor cell proliferation was active in group D.
Figure 3.
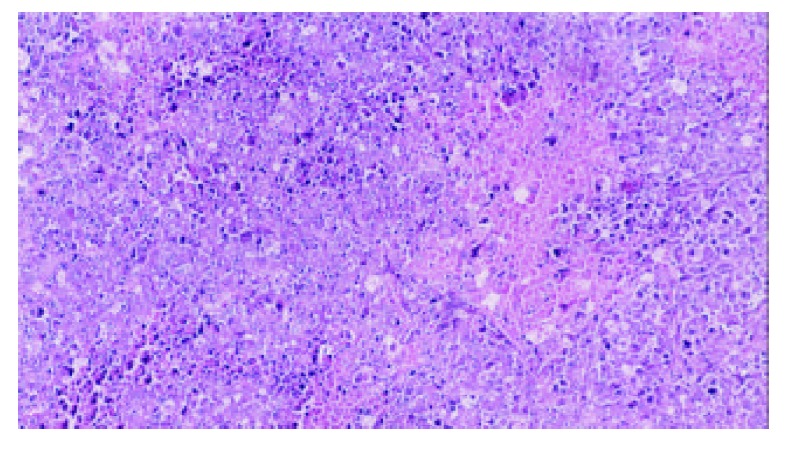
Tumor section (HE×100): a number of focal necrosis observed in group E.
Figure 4.
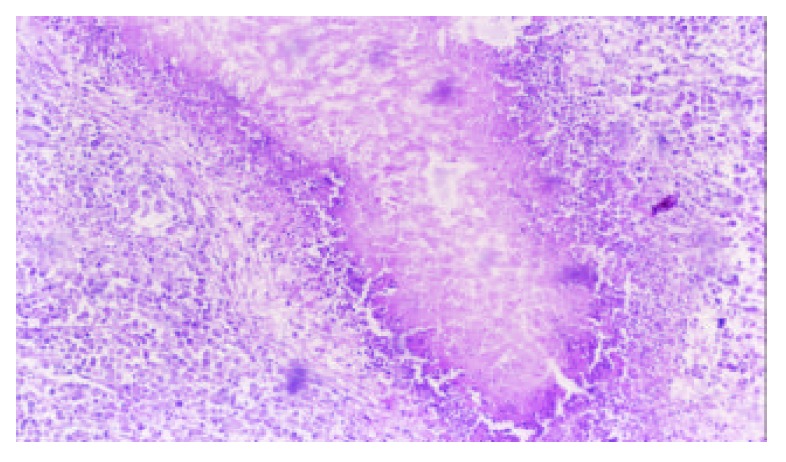
Tumor section (HE×100): the island of necrosis and lymphocytes infiltrated around the necrotic tissue found in group F.
DISCUSSION
Controlled release implant is a newly developed agent[14-17]. Different from other kinds of common or slow-released agents, it is capable of being administered through implantation to target organ, and can result in a therapeutically suitable plasma concentration of agents for an extended period of time[18-22], and exert a full antitumor action, despite the short half-life of drugs[23,24].
This study illustrated that the concentration of 5-FU in left lobe of the liver was 9.84, 28 and 34 times as much as that in right lobe of the liver, heart and kidney respectively after the implantation. It kept a high level of 5-FU in surrounding tissues of the implant, ranging from (4.414% ± 0.482%) to (7.800% ± 0.804%), for eight weeks. 5-FUCI not only provided higher drug and /or drug metabolite concentrations in the tumor than the drug administered as an aqueous solution s.c. or systemically, but also resulted in a prolonged exposure of the tumor tissue to the drug and /or its metabolites. Consequently, 5-FUCI would be expected to improve effectiveness and to minimize the systemic toxicity associated with current systemic therapy of 5-FU[25-28].
New insights indicate that the toxicity of 5-FU is dose dependent, frequent low-dose infusions are less toxic to host than less frequent higher doses. No sign of systemic toxicities of 5-FUCI was detected because the daily delivery dose of 5-FUCI was only approximately 30 μg·d-1.
In previous studies, Smith et al[25,29] demonstrated the sensitivity of tumor cells to 5-FU and dependence on drug concentration and exposure time. If the exposure time was increased, the required drug concentration could be reduced. For example, the concentration of 5-FU that inhibited growth of 50%-90% (IC50, IC90) of BxPC-3 pancreatic cancer cells was eight to ten times less with a 72-h drug exposure than with a 24-h exposure[29]. We have shown that delivery of drugs administrated in 5-FUCI resulted in growth inhibition of Walker-256 carcinosarcoma cells that could hardly be accomplished with s.c. 5-FU solution.
Controlled release implant may enhance the pharmacological alternatives for the treatment of malignant tumors. Some researches have proved that tumor cell growth could be inhibited at a distance of 0.5-5 cm from the implant[30-32]. Therefore an implant needs to be inserted in many sites for multicenter or > 5 cm neoplasms in outer diameter.
The data indicated that silicone pellets were transparent, and without fracture or apparent degradation after 8 weeks of implantation. In addition, a fibrous capsule was not detected around the pellets. Release of 5-FU from these pellets allows the drug to remain in the plasma for 1 to 8 weeks, in spite of its short plasma half-life (10 min)[10-13]. This was an improvement compared with the injection of drugs. Administration of 5-FU by implantation of silastic elastomer seems to be a good candidate for 5-FU therapy. It suggests that 5-FUCI is a novel clinical approach and can offer an effective local chemotherapy.
Footnotes
Edited by Zhang JZ and Wang XL
References
- 1.Aboagye EO, Saleem A, Cunningham VJ, Osman S, Price PM. Extraction of 5-fluorouracil by tumor and liver: a noninvasive positron emission tomography study of patients with gastrointestinal cancer. Cancer Res. 2001;61:4937–4941. [PubMed] [Google Scholar]
- 2.Xiao HB, Cao WX, Yin HR, Lin YZ, Ye SH. Influence of L-methionine-deprived total parenteral nutrition with 5-fluorouracil on gastric cancer and host metabolism. World J Gastroenterol. 2001;7:698–701. doi: 10.3748/wjg.v7.i5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu LX, Zhang WH, Jiang HC, Zhu AL, Wu LF, Qi SY, Piao DX. Arterial chemotherapy of 5-fluorouracil and mitomycin C in the treatment of liver metastases of colorectal cancer. World J Gastroenterol. 2002;8:663–667. doi: 10.3748/wjg.v8.i4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen XX, Lai MD, Zhang YL, Huang Q. Less cytotoxicity to combination therapy of 5-fluorouracil and cisplatin than 5-fluorouracil alone in human colon cancer cell lines. World J Gastroenterol. 2002;8:841–846. doi: 10.3748/wjg.v8.i5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao S, Rustum YM. Synergistic antitumor activity of irinotecan in combination with 5-fluorouracil in rats bearing advanced colorectal cancer: role of drug sequence and dose. Cancer Res. 2000;60:3717–3721. [PubMed] [Google Scholar]
- 6.Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T, Hashimoto-Tamaoki T. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001;61:1029–1037. [PubMed] [Google Scholar]
- 7.van Kuilenburg AB, Haasjes J, Richel DJ, Zoetekouw L, Van Lenthe H, De Abreu RA, Maring JG, Vreken P, van Gennip AH. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res. 2000;6:4705–4712. [PubMed] [Google Scholar]
- 8.Di Paolo A, Danesi R, Falcone A, Cionini L, Vannozzi F, Masi G, Allegrini G, Mini E, Bocci G, Conte PF, et al. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann Oncol. 2001;12:1301–1306. doi: 10.1023/a:1012294617392. [DOI] [PubMed] [Google Scholar]
- 9.van Kuilenburg AB, Haasjes J, Richel DJ, Zoetekouw L, Van Lenthe H, De Abreu RA, Maring JG, Vreken P, van Gennip AH. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res. 2000;6:4705–4712. [PubMed] [Google Scholar]
- 10.Fraile RJ, Baker LH, Buroker TR, Horwitz J, Vaitkevicius VK. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980;40:2223–2228. [PubMed] [Google Scholar]
- 11.Yi YM, Yang TY, Pan WM. Preparation and distribution of 5-fluorouracil (125)I sodium alginate-bovine serum albumin nanoparticles. World J Gastroenterol. 1999;5:57–60. doi: 10.3748/wjg.v5.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuan HY, Smith DE, Ensminger WD, Knol JA, DeRemer SJ, Yang Z, Stetson PL. Regional pharmacokinetics of 5-fluorouracil in dogs: role of the liver, gastrointestinal tract, and lungs. Cancer Res. 1998;58:1688–1694. [PubMed] [Google Scholar]
- 13.Schlemmer HP, Becker M, Bachert P, Dietz A, Rudat V, Vanselow B, Wollensack P, Zuna I, Knopp MV, Weidauer H, et al. Alterations of intratumoral pharmacokinetics of 5-fluorouracil in head and neck carcinoma during simultaneous radiochemotherapy. Cancer Res. 1999;59:2363–2369. [PubMed] [Google Scholar]
- 14.Saltzman WM, Fung LK. Polymeric implants for cancer chemotherapy. Adv Drug Deliv Rev. 1997;26:209–230. doi: 10.1016/s0169-409x(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 15.Dash AK, Cudworth GC. Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol Methods. 1998;40:1–12. doi: 10.1016/s1056-8719(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T. Development and clinical application of drug delivery systems for cancer treatment. Int J Clin Oncol. 2002;7:206–218. doi: 10.1007/s101470200032. [DOI] [PubMed] [Google Scholar]
- 17.Einmahl S, Zignani M, Varesio E, Heller J, Veuthey JL, Tabatabay C, Gurny R. Concomitant and controlled release of dexamethasone and 5-fluorouracil from poly(ortho ester) Int J Pharm. 1999;185:189–198. doi: 10.1016/s0378-5173(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 18.Itokazu M, Kumazawa S, Wada E, Wenyi Y. Sustained release of adriamycin from implanted hydroxyapatite blocks for the treatment of experimental osteogenic sarcoma in mice. Cancer Lett. 1996;107:11–18. doi: 10.1016/0304-3835(96)04337-6. [DOI] [PubMed] [Google Scholar]
- 19.Ramchandani M, Robinson D. In vitro and in vivo release of ciprofloxacin from PLGA 50: 50 implants. J Control Release. 1998;54:167–175. doi: 10.1016/s0168-3659(97)00113-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Tucker IG, Roberts MS, Hirst LW. In vitro and in vivo evaluation in rabbits of a controlled release 5-fluorouracil subconjunctival implant based on poly(D,L-lactide-co-glycolide) Pharm Res. 1996;13:1059–1064. doi: 10.1023/a:1016062825360. [DOI] [PubMed] [Google Scholar]
- 21.Kong Q, Kleinschmidt-Demasters BK, Lillehei KO. Intralesionally implanted cisplatin cures primary brain tumor in rats. J Surg Oncol. 1997;64:268–273. doi: 10.1002/(sici)1096-9098(199704)64:4<268::aid-jso4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Kitchell BK, Orenberg EK, Brown DM, Hutson C, Ray K, Woods L, Luck E. Intralesional sustained-release chemotherapy with therapeutic implants for treatment of canine sun-induced squamous cell carcinoma. Eur J Cancer. 1995;31A:2093–2098. doi: 10.1016/0959-8049(95)00446-7. [DOI] [PubMed] [Google Scholar]
- 23.Ning S, Yu N, Brown DM, Kanekal S, Knox SJ. Radiosensitization by intratumoral administration of cisplatin in a sustained-release drug delivery system. Radiother Oncol. 1999;50:215–223. doi: 10.1016/s0167-8140(98)00134-0. [DOI] [PubMed] [Google Scholar]
- 24.Maeda M, Moriuchi S, Sano A, Yoshimine T. New drug delivery system for water-soluble drugs using silicone and its usefulness for local treatment: application of GCV-silicone to GCV/HSV-tk gene therapy for brain tumor. J Control Release. 2002;84:15–25. doi: 10.1016/s0168-3659(02)00236-5. [DOI] [PubMed] [Google Scholar]
- 25.Smith JP, Kanekal S, Patawaran MB, Chen JY, Jones RE, Orenberg EK, Yu NY. Drug retention and distribution after intratumoral chemotherapy with fluorouracil/epinephrine injectable gel in human pancreatic cancer xenografts. Cancer Chemother Pharmacol. 1999;44:267–274. doi: 10.1007/s002800050977. [DOI] [PubMed] [Google Scholar]
- 26.Tang WX, Cheng PY, Luo YP, Wang RX. Interaction between cisplatin, 5-fluorouracil and vincristine on human hepatoma cell line (7721) World J Gastroenterol. 1998;4:418–420. doi: 10.3748/wjg.v4.i5.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng LY, Zhang YH, Xu P, Yang SM, Yuan XB. Expression of IL 1betaconverting enzyme in 5-FU induced apoptosis in esophageal carcinoma cells. World J Gastroenterol. 1999;5:50–52. doi: 10.3748/wjg.v5.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin J, Huang M, Wei HL, Liu GT. Mechanism of 5-fluorouracil required resistance in human hepatocellular carcinoma cell line Bel(7402) World J Gastroenterol. 2002;8:1029–1034. doi: 10.3748/wjg.v8.i6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JP, Stock E, Orenberg EK, Yu NY, Kanekal S, Brown DM. Intratumoral chemotherapy with a sustained-release drug delivery system inhibits growth of human pancreatic cancer xenografts. Anticancer Drugs. 1995;6:717–726. doi: 10.1097/00001813-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Harper E, Dang W, Lapidus RG, Garver RI. Enhanced efficacy of a novel controlled release paclitaxel formulation (PACLIMER delivery system) for local-regional therapy of lung cancer tumor nodules in mice. Clin Cancer Res. 1999;5:4242–4248. [PubMed] [Google Scholar]
- 31.Williams JA, Dillehay LE, Tabassi K, Sipos E, Fahlman C, Brem H. Implantable biodegradable polymers for IUdR radiosensitization of experimental human malignant glioma. J Neurooncol. 1997;32:181–192. doi: 10.1023/a:1005704913330. [DOI] [PubMed] [Google Scholar]
- 32.Yuan X, Fahlman C, Tabassi K, Williams JA. Synthetic, implantable, biodegradable polymers for controlled release of radiosensitizers. Cancer Biother Radiopharm. 1999;14:177–186. doi: 10.1089/cbr.1999.14.177. [DOI] [PubMed] [Google Scholar]


