Abstract
AIM: To describe the distribution of micrometastases in the surrounding liver of patients with primary liver cancer (PLC), and to describe the minimal length of resection margin (RM) for hepatectomy.
METHODS: From November 2001 to March 2003, 120 histologically verfied PLC patients without macroscopic tumor thrombi or macrosatellites or extrahepatic metastases underwent curative hepatectomy. Six hundreds and twenty-nine routine pathological sections from these patients were re-examined retrospectively by light microscopy. In the prospective study, curative hepatectomy was performed from November 2001 to March 2003 for 76 histologically verfied PLC patients without definite macroscopic tumor thrombi or macrosatellites or extrahepatic metastases in preoperative imaging. Six hundreds and forty-five pathological sections from these patients were examined by light microscopy. The resected liver specimens were minutely examined to measure the resection margin and to detect the number of daughter tumor nodules, dominant lesions, and macroscopic tumor thrombi inside the lumens of the major venous system. The paraffin sections were microscopically examined to detect the microsatellites, microscopic tumor thrombi, fibrosis tumor capsules, as well as capsule invasion and the distance of histological spread of the micrometastases.
RESULTS: In the retrospective study, 70 micrometastases were found in surrounding liver in 26 of the 120 cases (21.7%). The farthest distance of histological micrometastasis was 3.5 mm, 5.3 mm and 6.0 mm in 95%, 99% and 100% cases, respectively. Macroscopic tumor thrombi or macrosatellites were observed in 18 of 76 cases, and 149 micrometastases were found in the surrounding live in 25 (43.1%) of 58 cases with no macroscopic tumor thrombi. The farthest distance of histological micrometastasis was 4.5 mm, 5.5 mm and 6.0 mm in 95%, 99% and 100% cases, respectively. Two hundred and sixty-seven micrometastases were found in surrounding liver in 14 (77.8%) out of 18 cases with macroscopic tumor thrombi or macrosatellites. The farthest distance of histological micrometastasis was 18.5 mm, 18.5 mm and 19.0 mm in 95%, 99% and 100% cases, respectively.
CONCLUSION: The required minimal length of RM is 5.5 mm and 6 mm respectively to achieve 99% and 100% micrometastasis clearance in surrounding liver of PLC patients without macroscopic tumor thrombi or macrosatellites, and should be greater than 18.5 mm to obtain 99% micrometastasis clearance in surrounding liver of patients with macroscopic tumor thrombi or macrosatellites.
Keywords: Primary liver cancer, Micrometastases, Resection margin, Hepatectomy
INTRODUCTION
Primary liver cancer (PLC) is the fifth most common cancer in the world. The number of new cases is estimated to be 564 000 per year. About 80% of all cases are found in Asia. Hepatocellular carcinoma (HCC) accounts for more than 80% of all PLCs, while intrahepatic cholangiocarcinoma (ICC) and hepatocellular-cholangiocarcinoma (HCCC) account for less than 20%. Most patients with PLC also suffer from concomitant cirrhosis, which is the major clinical risk factor for hepatic cancer. Overall, 80% of PLCs can be attributed to chronic hepatitis B virus infection in Asia, especially in China. Hepatic resection and liver transplantation are considered the only curative treatment for PLC. For most cirrhotic patients who fulfill the Milan criteria, liver transplantation is the ultimate choice of treatment, but its application is limited due to the lack of donors[1]. Hepatic resection remains the treatment of choice for PLC despite unsatisfactory outcomes due to the high incidence of intrahepatic recurrence[2,3]. Resection margin (RM), which refers to the shortest distance from the edge of the lesion to the line of parenchymal transection margin[4], is vital to a safe operation and a complete clearance of micrometastases in surrounding liver. Because of underlying chronic liver diseases, the optimal RM in radical hepatectomy of PLC remains controversial and ambiguous[4-20] and has not been well illustrated theoretically. Although there were prospective studies on micrometastases in 55 patients[17], 36 patients[18] and 23 patients[19] and surgical margin in 40 patients[20] with PLC, they did not distinguish patients with macroscopic tumor thrombi or macrosatellites from those without macroscopic tumor thrombi or macrosatellites. To ensure a complete clearance of micrometastases in surrounding liver, the minimal length of RM depends on the farthest distance of histological micrometastasis. This study was to describe the distribution of micrometastases in the surrounding liver of patients with PLC, and the minimal length of resection margin for hepatectomy.
MATERIALS AND METHODS
Specimens
From November 2001 to March 2003, 120 histologically verfied PLC patients without macroscopic tumor thrombi or macrosatellites or extrahepatic metastases underwent curative hepatectomies (Table 1). Six hundred and twenty-nine routine pathological sections from these patients were re-examined retrospectively by light microscopy. In the prospective study, hepatectomy was performed from March to November 2003 for 76 histologically verfied PLC patients without definite macroscopic tumor thrombi or macrosatellites or extrahepatic metastases in preoperative imaging (Table 1). Six hundred and forty-five pathological sections from these patients, including 389 routine pathological sections, were examined by light microscopy. A computerized database was used to collect clinicopathological data of all patients in the prospective group, including the macroscopic width and histological involvement of surgical margin assessed by pathologists. Any postoperative recurrence was entered into the database immediately after diagnosis. No difference was found in age, sex, HBsAg (+) and tumor size between the two groups (P > 0.05).
Table 1.
Perioperative data of 196 cases of primary liver cancer
|
Retrospective group |
Prospective group |
|||
| Without Mt or Ms | Total | Without Mt or Ms | With Mt or Ms | |
| Cases | 120 | 76 | 58 | 18 |
| Age (yr) | 49.6 ± 11.5 | 50.8 ± 11.4 | 52.4 ± 11.0 | 45.4 ± 11.2 |
| (30-81) | (24-78) | (31-78) | (24-65) | |
| Sex (male/female) | 100/20 | 63/13 | 47/11 | 16/2 |
| TBIL (μmol/L) | 12.8 ± 4.9 | 13.1 ± 4.5 | 11.9 ± 5.8 | |
| PALB (mg/L) | 219 ± 59 | 222 ± 59 | 208 ± 58 | |
| PT (s) | 12.5 ± 1.1 | 12.5 ± 1.0 | 12.6 ± 1.2 | |
| HBsAg (+) | 98 (81.7%) | 65 (85.5%) | 49 (84.5%) | 16 (88.9%) |
| Liver cirrhosis or fibrosis | 103 (85.8%) | 76 (100%) | 58 (100%) | 18 (100%) |
| Size of tumor (mm) | 58.4 ± 42.6 | 54.9 ± 35.2 | 43.4 ± 23.9 | 92.0 ± 40.6 |
| Tumor volume (cm3) | 107 ± 203 | 54 ± 111 | 275 ± 320 | |
Mt: macroscopic tumor thrombus; Ms: macrosatellite. TBIL: total bilirubin; PALB: pre-albumin; PT: prothrombin time.
All surgical procedures were performed by the same surgical team in Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University. All pathology slides were reviewed independently by two hepatobiliary pathologists.
Preparation of fresh specimens
The resected liver specimens were photographed and then examined to measure the RM. Macroscopically, the number of daughter tumor nodules, besides the dominant lesion, were recorded, and the presence of macroscopic tumor thrombi inside the lumens of the major venous system and the level of its infiltrated venous branches were also noted. The size of tumors and vertical, transverse and anteroposterior dimensions of the specimens were documented according to their different shapes and photographed before 3-6 rectangle specimens were cut in the portal vein direction, hepatic vein direction and other directions, which measured approximately 2 mm by 10 mm in thickness and width, including 3-5 mm tumor and 10-25 mm liver parenchyma in length. The specimens were fixed in 10% formalin and stained with hematoxylin and eosin for microscopic examination.
In the presence of a multinodular lesion, the nodule with the largest diameter was taken as the dominant nodule except that 2-3 nodules were considered synchronous multicentric liver carcinogenesis if they located in different hepatic lobes with no significant difference in size, at a distance beyond 5 cm and had no macroscopic tumor thrombi. All the remaining macroscopically evident tumor nodules, or daughter nodules, macroscopic tumor thrombi and micrometastases were assumed to have radially disseminated from the dominant nodule without other preferred direction except for portal vein and hepatic vein directions.
Correction for shrinkage
The tissue shrinkage rate secondary to the process of histological slide preparation was estimated by comparing the width of specimens from non-tumor liver in its final state on the slide and its fresh state before formalin immersion.
Documentation of pathological features
Various pathological features were studied, including the presence and absence of microsatellites, microscopic tumor thrombi, fibrosis tumor capsules, and capsule invasion (whether the tumor capsule was infiltrated partially or completely by the tumor), or liver invasion (whether the tumor infiltrated directly into the adjacent non-tumor liver), and the distance of micrometastases.
Measurement of micrometastases
The size of all micrometastases detected in the adjacent non-tumor liver was estimated by the microscope scale. The shortest distance between the edges of the dominant nodule and the farthest micrometastasis was considered the distance of histological spread.
Statistical analysis
SPSS10.0 for Windows was used to compute the distribution of frequencies and SAS6.12 System to compute the statistical significance of difference for unpaired data. Time of recurrence and survival after recurrence were determined by Kaplan-Meier analysis, and the relation between micrometastases and clinicopathological characteristics was compared using the T stat test or Wilcoxon test. P < 0.05 was considered statistically significant.
RESULTS
Micrometastasis in liver parenchyma surrounding the lesion
Of the 120 cases, 24 (20%) had no encapsulation, 54 (45%) had incomplete encapsulation and 42 (35%) had almost complete encapsulation. Seventy micrometastases were found in the liver parenchyma surrounding the lesions in 26 cases (21.7%), among which 27 microsatellites were found in 16 (1-10 per case), 12 microscopic tumor thrombi in 6 (1-3 per case), and 26 microscopic tumor thrombi in 4 (3-15 per case) and microsatellites in 5 (1-2 per case). The farthest distance of micrometastasis was 3.5 mm, 5.3 mm and 6.0 mm in 95%, 99% and 100% cases, respectively (Table 2).
Table 2.
Distribution of micrometastases in surrounding liver of 178 cases of primary liver cancer without macroscopic tumor thrombi or macrosatellites
|
Retrospective group (120 cases) |
Prospective group (58 cases) |
|||||
| Distance (mm) | Cases | Percent | Accumulative percent | Cases | Percent | Accumulative percent |
| 0 | 94 | 78.3 | 78.3 | 35 | 60.3 | 60.3 |
| 1 | 9 | 7.5 | 85.8 | 8 | 13.8 | 74.1 |
| 2 | 10 | 8.3 | 94.2 | 4 | 6.9 | 81 |
| 3 | 2 | 1.7 | 95.8 | 6 | 10.3 | 91.4 |
| 4 | 4 | 3.3 | 99.2 | 3 | 5.2 | 96.6 |
| 5 | 1 | 0.8 | 100.0 | 2 | 3.4 | 100.0 |
Of the 76 cases, 12 (15.8%) had no encapsulation, 55 (72.4%) had incomplete encapsulation and 9 (11.8%) had almost complete encapsulation. Among the 58 cases free of macroscopic tumor thrombi or macrosatellites, 25 (43.1%) exhibited 149 micrometastases in the liver parenchyma surrounding the lesions, among which, 9 microsatellites were found in 5 (1-3 per case), 69 microscopic tumor thrombi in 12 (1-20 per case), and 37 microscopic tumor thrombi in 8 (1-12 per case) and microsatellites in 34 (1-20 per case) (Figure 1A-C). The farthest distance of micrometastasis was 4.5 mm, 5.5 mm and 6.0 mm in 95%, 99% and 100% cases, respectively (Table 2). In 18 cases with macroscopic tumor thrombi or macrosatellites, 267 micrometastases were found in 14 (77.8%), 3 micrometastases in 1, 56 microscopic tumor thrombi in 5 (1-18 per case), and 154 microscopic tumor thrombi in 8 (6-33 per case) and microsatellites in 54 (2-11 per case) (Figure 1D and E). The farthest distance of micrometastasis was 18.5 mm, 18.5 mm and 19.0 mm in 95%, 99% and 100% cases, respectively (Table 3). As only 18 cases had macroscopic tumor thrombi or macrosatellites and it was impossible to obtain the liver parenchyma surrounding the lesion beyond 2 cm, the practical farthest distance should be greater than 18.5 mm. The tissue shrinkage rate was 89.7% ± 5.6%.
Figure 1.
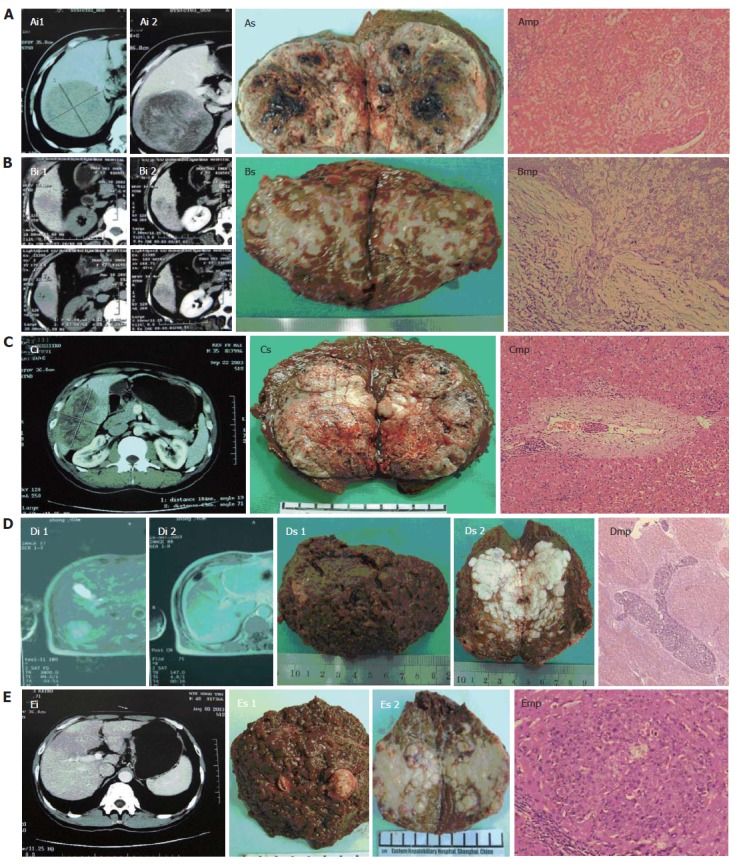
Imaging (i), specimens (s) and microscopic pathology (mp) of 5 PLC patients. A: case 24, tumor size 12.0 cm x 11.0 cm x 10.5cm, with microscopic tumor thrombi (metastatic distance 6 mm, x 100); B: case 40, tumor size 4.0 cm x 3.8 cm x 3.5 cm, with microscopic capsular infiltration (x 200); C: case 58, tumor size 10.5 cm x 6.5 cm x 6.0 cm, with microscopic tumor thrombi (metastatic distance 2 mm, x 100); D: case 38, tumor size 6.5 cm x 4.0 cm x 3.6 cm, with macroscopic tumor thrombi in the branches of right portal vein and microscopic tumor thrombi of arborization (x 16); E: case 52, tumor size 7.0 cm x 5.0 cm x 5.0 cm, with macroscopic tumor thrombi in the branches of right portal vein and microsatellites (metastatic distance 3.5 mm, x 100).
Table 3.
Distribution of micrometastases in surrounding liver of 18 cases of primary liver cancer with macroscopic tumor thrombi or macrosatellites
| Distance (mm) | Cases | Percent | Accumulative percent |
| 0 | 5 | 27.78 | 27.8 |
| 3 | 7 | 38.89 | 66.7 |
| 6 | 3 | 16.67 | 83.3 |
| 9 | 0 | 0 | 83.3 |
| 12 | 1 | 5.56 | 88.9 |
| 15 | 1 | 5.56 | 94.4 |
| 18 | 1 | 5.56 | 100.0 |
Relation between micrometastases and clinicopathological characteristics
The yield rate of micrometastasis among patients with incomplete encapsulation was statistically higher than that among patients with no or complete encapsulation (P < 0.05). The yield rate of micrometastasis in the liver parenchyma surrounding the lesion was positively correlated with the preoperative serum AFP level (P < 0.01), tumor size (P < 0.01) and presence of macroscopic tumor thrombi or macrosatellites (P < 0.01) in patients with PLC (Table 4).
Table 4.
Relation between micrometastases and clinicopathological characteristics in 196 cases of primary liver cancer
|
Retrospective group |
Prospective group |
|||||||||
| Parameters | Cases | Both mt and ms | One of mt and ms | None of mt or ms | P value | Cases | Both mt and ms | One of mt and ms | None of mt or ms | P value |
| Encapsulation | 120 | < 0.01 | 76 | < 0.01 | ||||||
| No | 24 | 1 | 2 | 21 | 12 | 1 | 5 | 6 | ||
| Incomplete | 54 | 3 | 17 | 34 | 55 | 16 | 17 | 22 | ||
| Complete | 42 | 0 | 3 | 39 | 9 | 0 | 0 | 9 | ||
| AFP (μmol/L) | 120 | 0.08 | 76 | < 0.01 | ||||||
| < 20 | 44 | 0 | 7 | 37 | 21 | 0 | 4 | 17 | ||
| 20-400 | 33 | 0 | 6 | 27 | 22 | 2 | 8 | 12 | ||
| > 400 | 43 | 4 | 9 | 30 | 33 | 15 | 10 | 8 | ||
| Size of tumor (mm) | 111 | 0.1 | 76 | < 0.01 | ||||||
| ≤ 20 | 10 | 0 | 1 | 9 | 7 | 0 | 1 | 6 | ||
| 20-30 | 23 | 1 | 3 | 19 | 14 | 2 | 4 | 8 | ||
| 30-50 | 30 | 1 | 6 | 23 | 29 | 4 | 8 | 17 | ||
| 50-100 | 27 | 0 | 3 | 24 | 15 | 6 | 6 | 3 | ||
| > 100 | 21 | 2 | 5 | 14 | 11 | 5 | 3 | 3 | ||
| Mt or Ms | 76 | < 0.01 | ||||||||
| Mt and Ms | 3 | 3 | 0 | 0 | ||||||
| Mt | 9 | 6 | 3 | 0 | ||||||
| Ms | 6 | 0 | 2 | 4 | ||||||
| None of both | 58 | 8 | 18 | 32 | ||||||
Mt; microscopic tumor thrombus; ms: microsatellite. Incomplete encapsulation: part encapsulation or encapsulation breakthrough or with lesion inside encapsulation.
DISCUSSION
Postoperative intrahepatic recurrence results either from residual intrahepatic metastasis or from de novo tumor due to the underlying hepatitis or liver cirrhosis[21-24]. The incidence of multicentric carcinogenesis in postoperative tumor is around 50%[24,25]. Theoretically, hepatectomy for PLC only resects the main tumor and surgical margin, the high risk area of intrahepatic metastasis[25]. Of the 6 PLC patients with only macrosatellites, 4 had no micrometastasis, which may be synchronously multicentric carcinogenic. In addition, of the 13 patients with 2-3 nodules who were clinically considered to be synchronously multicentric carcinogenic, only 4 had microsatellites without microscopic tumor thrombi, while 1 of them had microscopic tumor thrombi. Furthermore, treatment after postoperative recovery, aiming at the activity of hepatitis or liver cirrhosis, may decrease recurrence due to metachronously multicentric carcinogenesis[26-28]. Therefore, the aim of hepatectomy for PLC is not only to resect the main tumor and possible micrometastasis but also to decrease postoperative morbidity.
Up to date, prospective studies on micrometastases are only available from 55[17], 36[18] and 23 patients[19] and surgical margin in 40 patients[20] with PLC, but they did not distinguish patients with macroscopic tumor thrombi or macrosatellites from those without them, and micrometastasis from synchronously multicentric micro-foci. The farthest distance of micrometastasis[17-20] was more than 1.0 cm.
Clinical follow-up studies showed that although safety margin at resection is not a prognostic factor, patients with a surgical margin of over 1 cm[7-9] are free from tumor recurrence and a surgical margin of 0.5-1.0 cm[10-13] does not affect the prognosis and postoperative recurrence rate of hepatectomy for HCC after hepatectomy. These findings are not consistent with the reported results[17-20].
In the present study, the farthest distance of micrometastasis was 3.5 mm, 5.3 mm and 6.0 mm in 95%, 99% and 100% patients without macroscopic tumor thrombi or macrosatellites, respectively, which is different from the reported results of other prospective studies on micrometastasis[17-20], but is in agreement with clinical follow-up studies[10-13]. Because routine pathological sections, in which the liver parenchyma surrounding the lesion obtained is relatively less (0.2-1.0 cm), are mainly used to make diagnosis, it was impossible to achieve accurate record of resection margin and integrated clinical data for all PLC patients. The result of our prospective study on micrometastasis in PLC patients without macroscopic tumor thrombi or macrosatellites or extrahepatic metastases showed that the farthest distance of micrometastasis was 5.5 mm and 6 mm in 99% and 100% cases, respectively, which was in agreement with that of our retrospective study. These findings can explain the difference found in prospective studies on micrometastases[17-20] and clinical follow-up studies[7-13].
In conclusion, the farthest distance of micrometastasis is 18.5 mm and 19.0 mm in 99% and 100% of patients with macroscopic tumor thrombi or macrosatellites, respectively. The required minimal length of RM is 5.5 mm and 6 mm respectively to achieve 99% and 100% micrometastasis clearance in surrounding liver of PLC patients without macroscopic tumor thrombi or macrosatellites, and should be greater than 18.5 mm to obtain 99% micrometastasis clearance in patients with macroscopic tumor thrombi or macrosatellites.
Footnotes
Supported by grants from Health Bureau of Shanghai, China, No. 99ZDII002
S- Editor Liu Y L- Editor Wang XL E- Editor Li JL
References
- 1.Kassahun WT, Fangmann J, Harms J, Hauss J, Bartels M. Liver resection and transplantation in the management of hepatocellular carcinoma: a review. Exp Clin Transplant. 2006;4:549–558. [PubMed] [Google Scholar]
- 2.Tang ZY, Yu YQ, Zhou XD, Ma ZC, Wu ZQ. Progress and prospects in hepatocellular carcinoma surgery. Ann Chir. 1998;52:558–563. [PubMed] [Google Scholar]
- 3.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 4.Lai EC, Ng IO, You KT, Choi TK, Fan ST, Mok FP, Wong J. Hepatectomy for large hepatocellular carcinoma: the optimal resection margin. World J Surg. 1991;15:141–145. doi: 10.1007/BF01658988. [DOI] [PubMed] [Google Scholar]
- 5.Yang GS, Wu ZQ, Wu MC. Normalize comprehensive treatment of primary hepatocellular carcinoma. Zhonghua Waike Zazhi. 2001;39:742–743. [Google Scholar]
- 6.Wang YH, Liu YX. Tumor staging scheme, radical resection criterion and prognostic index of primary liver cancer. Zhonghua Gandan Waike Zazhi. 2003;9:3–7. [Google Scholar]
- 7.Franco D, Usatoff V. Resection of hepatocellular carcinoma. Hepatogastroenterology. 2001;48:33–36. [PubMed] [Google Scholar]
- 8.Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati PL, Pigato P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer. 1998;82:1028–1036. doi: 10.1002/(sici)1097-0142(19980315)82:6<1028::aid-cncr4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, Sung JL, Hwang LY, Sheu JC, Chen DS, Lin TY, Beasley RP. Surgical treatment of 109 patients with symptomatic and asymptomatic hepatocellular carcinoma. Surgery. 1986;99:481–490. [PubMed] [Google Scholar]
- 10.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg. 2000;231:544–551. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosuge T, Makuuchi M, Takayama T, Yamamoto J, Shimada K, Yamasaki S. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology. 1993;40:328–332. [PubMed] [Google Scholar]
- 12.Wu CC, Hwang CJ, Yang MD, Liu TJ. Preliminary results of hepatic resection for centrally located large hepatocellular carcinoma. Aust N Z J Surg. 1993;63:525–529. doi: 10.1111/j.1445-2197.1993.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 13.Lai EC, Ng IO, You KT, Fan ST, Mok FP, Tan ES, Wong J. Hepatic resection for small hepatocellular carcinoma: the Queen Mary Hospital experience. World J Surg. 1991;15:654–659. doi: 10.1007/BF01789219. [DOI] [PubMed] [Google Scholar]
- 14.Tang ZY, Yu YQ, Zhou XD, Ma ZC, Yang R, Lu JZ, Lin ZY, Yang BH. Surgery of small hepatocellular carcinoma. Analysis of 144 cases. Cancer. 1989;64:536–541. doi: 10.1002/1097-0142(19890715)64:2<536::aid-cncr2820640230>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Ma ZM, Feng YZ, Zhou XR. Rational surgical approaches to the treatment of small primary liver cancer, and the prevention of postoperative recurrence. Zhonghua WaiKe ZaZhi. 1994;32:31–34. [PubMed] [Google Scholar]
- 16.Lee CS, Chao CC, Lin TY. Partial hepatectomy on cirrhotic liver with a right lateral tumor. Surgery. 1985;98:942–948. [PubMed] [Google Scholar]
- 17.Li SP, Zhang CQ, Li JQ, Feng KT. Study of clinicopathological significance of micrometastasis in hepatocellular carcinoma. Zhongguo Zhongliu Linchuang. 2002;29:77–81. [Google Scholar]
- 18.Shi M, Zhang C, Feng K, Zhang Y, Chen M, Guo R, Lin X, Li J. Micrometastasis distribution in liver tissue surrounding hepatocellular carcinoma. Zhonghua ZhongLiu ZaZhi. 2002;24:257–260. [PubMed] [Google Scholar]
- 19.Lai EC, You KT, Ng IO, Shek TW. The pathological basis of resection margin for hepatocellular carcinoma. World J Surg. 1993;17:786–790; discussion 791. doi: 10.1007/BF01659097. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida Y, Kanematsu T, Matsumata T, Takenaka K, Sugimachi K. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis. Further evaluation of limited hepatic resection. Ann Surg. 1989;209:297–301. doi: 10.1097/00000658-198903000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394–1402. doi: 10.1002/hep.510270529. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Fukuda M, Koida I, Arase Y, Chayama K, Murashima N, et al. Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus: a pilot study. Cancer. 1998;82:827–835. doi: 10.1002/(sici)1097-0142(19980301)82:5<827::aid-cncr5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Cenuşă A. Treatment of hepatocellular carcinoma: hepatic resection and liver transplantation. Rev Med Chir Soc Med Nat Iasi. 2005;109:709–712. [PubMed] [Google Scholar]
- 24.Sakon M, Umeshita K, Nagano H, Eguchi H, Kishimoto S, Miyamoto A, Ohshima S, Dono K, Nakamori S, Gotoh M, et al. Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg. 2000;135:1456–1459. doi: 10.1001/archsurg.135.12.1456. [DOI] [PubMed] [Google Scholar]
- 25.Sakon M, Nagano H, Nakamori S, Dono K, Umeshita K, Murakami T, Nakamura H, Monden M. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: analysis based on tumor hemodynamics. Arch Surg. 2002;137:94–99. doi: 10.1001/archsurg.137.1.94. [DOI] [PubMed] [Google Scholar]
- 26.Takata M, Yamanaka N, Tanaka T, Yamanaka J, Maeda S, Okamoto E, Yasojima H, Uematsu K, Watanabe H, Uragari Y. What patients can survive disease free after complete resection for hepatocellular carcinoma?: A multivariate analysis. Jpn J Clin Oncol. 2000;30:75–81. doi: 10.1093/jjco/hyd016. [DOI] [PubMed] [Google Scholar]
- 27.Bilimoria MM, Lauwers GY, Doherty DA, Nagorney DM, Belghiti J, Do KA, Regimbeau JM, Ellis LM, Curley SA, Ikai I, et al. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136:528–535. doi: 10.1001/archsurg.136.5.528. [DOI] [PubMed] [Google Scholar]
- 28.Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650–655. [PubMed] [Google Scholar]


