Significance
Research in the past decade indicates that the building blocks of morality are present very early in development. However, little is known about the neural and environmental mechanisms underlying their emergence. Here we show, for the first time, to our knowledge, using multiple levels of analysis, the complex integration of bottom–up and top–down processes in sociomoral evaluation. Brain wave differences elicited by witnessing the prosocial and antisocial behaviors of others predicted infants and toddlers’ subsequent attraction to prosocial others and were associated with parental values of justice and fairness. This developmental social neuroscience perspective provides empirical and theoretical clarity to the biological basis and brain–behavior processes involved in moral sensitivity.
Keywords: early sociomoral evaluation, developmental social neuroscience, moral cognition, EEG/ERP, parental value transmission
Abstract
The nature and underpinnings of infants’ seemingly complex, third-party, social evaluations remain highly contentious. Theoretical perspectives oscillate between rich and lean interpretations of the same expressed preferences. Although some argue that infants and toddlers possess a “moral sense” based on core knowledge of the social world, others suggest that social evaluations are hierarchical in nature and the product of an integration of rudimentary general processes such as attention allocation and approach and avoidance. Moreover, these biologically prepared minds interact in social environments that include significant variation, which are likely to impact early social evaluations and behavior. The present study examined the neural underpinnings of and precursors to moral sensitivity in infants and toddlers (n = 73, ages 12–24 mo) through a series of interwoven measures, combining multiple levels of analysis including electrophysiological, eye-tracking, behavioral, and socioenvironmental. Continuous EEG and time-locked event-related potentials (ERPs) and gaze fixation were recorded while children watched characters engaging in prosocial and antisocial actions in two different tasks. All children demonstrated a neural differentiation in both spectral EEG power density modulations and time-locked ERPs when perceiving prosocial or antisocial agents. Time-locked neural differences predicted children’s preference for prosocial characters and were influenced by parental values regarding justice and fairness. Overall, this investigation casts light on the fundamental nature of moral cognition, including its underpinnings in general processes such as attention and approach–withdrawal, providing plausible mechanisms of early change and a foundation for forward movement in the field of developmental social neuroscience.
A dramatic shift in the study of morality has occurred, moving away from dissociable notions of moral development toward more integrated theories. An explosion of empirical research in psychology, anthropology, biology, economics, and neuroscience has resulted in an attempt to more clearly define and investigate the concept of morality across domains. Work in these various academic disciplines suggests that human moral sensibility emerges from a complex social, emotional, and cognitive integration, shaped by cultural exposure, and can therefore be seen as a product of our biological, evolutionary, and cultural history, representing an important adaptive element for social cohesion and cooperation (1, 2).
Among the most exciting findings is the accumulation of evidence for early emerging capacities for social evaluations in infants and toddlers, interpreted as precursors to complex moral cognition (3). As demonstrated through a variety of techniques such as preferential looking-time, violation-of-expectation tasks, and behavioral observations, children under 2 y of age appear to both act prosocially and prefer prosocial to antisocial others (4). For example, 3-mo-olds preferentially attend to a character who previously acted in a prosocial manner toward another (5), suggesting a bias toward those that “do good things.” By 6 mo of age, this visual preference is expanded to the realm of behaviors; infants not only selectively attend to prosocial agents but also selectively approach them over antisocial or neutral characters (3). Within the first year, infants’ preferences are sensitive to the mental states of both the agents and the recipients of prosocial and antisocial acts as well as to the context in which such behaviors occur (3, 6, 7).
In the second year of life, children’s reactions to social stimuli evolve from personal affective arousal to actual behavior such as helping, sharing, and comforting. Children 14 to 18 mo old help by fetching objects that an experimenter seems to want but cannot reach (8). Children between the ages of 1 and 2 y of age comfort others who are in distress and may go so far as to give up their favorite objects to soothe another (9). Children 18 to 25 mo old exhibit more concern for the victim of a moral transgression than for the transgressor, even if the victim did not show any behavioral signs of distress (10), suggesting that toddlers do not simply react to others’ emotional displays but actively interpret the intentions and feeling states of others. Although there is obviously considerable development in prosocial and moral abilities between infancy and childhood, it is less clear what motivates this change. There is some evidence that an improvement in one prosocial ability may relate to increased abilities in other prosocial areas. For example, 15-mo-old infants who chose to share a toy they preferred (compared with a nonpreferred toy or no toy at all) with an experimenter also attended significantly longer to a third-party interaction in which the allocation of resources among conspecifics was unequal (11). However, another body of literature suggests that early prosocial abilities are not necessarily related (12).
Taken together, precursors to moral evaluation and prosocial behavior appear very early in development. However, the nature of these propensities remains highly contentious (13–15). Interpreting preverbal infants and toddlers’ behaviors and preferences has historically fallen into two camps: one advocating for lean interpretations based on rudimentary abilities/computations (16) and the other for rich interpretations based on complex cognitive and social cognitive processes (17). In the domain of morality, some argue that infants and toddlers possess a “moral sense” based on core knowledge of the social world (18), and others suggest that social evaluations are hierarchical in nature and the product of an integration of rudimentary general processes such as attention and approach and avoidance (19). Moreover, the development of these biologically prepared minds is embedded in and dependent upon specific dynamic social environments that are highly variable (20).
Establishing neurological methods within a developmental framework has the potential to provide a more comprehensive account of morality, bridging the gap between behaviors and their underlying cognitive mechanisms. Neuroscience research is critical to clarify the computational systems that mediate early social evaluations and behaviors, often considered a prerequisite for moral thought. For example, examining the spatiotemporal dynamics of the neural processing when young children view social interactions can help us to better understand the contribution of domain-general processes to early moral thought. Our current knowledge of the brain circuits involved in the development of moral cognition is based on a limited number of studies with young children using electroencephalography (21–23), functional MRI (24), and lesion studies (25). Due to the methodological constraints of most neuroimaging methods, no study has yet investigated the link between the online neural processing of the perception of prosocial and antisocial others and actual moral preferences and prosocial behaviors in infants and toddlers, as well as their link to parental values.
Recently, preschool-age children were shown to have both automatic and controlled neural differentiations when perceiving third-party harm and help (22). Moreover, the later controlled differences were predictive of children’s own sharing behavior. The association between neural computations involved in the processing of perception of harm and actual behaviors in children is beginning to be established, yet little is known about the early emergence of simple evaluations, particularly using neuroscience investigations with infants and toddlers. Some preliminary evidence suggests that infant’s post hoc evaluations of previously helping and hindering characters are marked by midrange attentional event-related potential (ERP) differences (P400) (23). However, although these findings have begun to illuminate the complexity of the neural underpinnings of early social sociomoral evaluations and prosocial behaviors, it is unclear whether these two fields (social evaluations and behaviors) are related in their emergence and whether there is online neural differentiation to the processing of harmful and helpful actions of others.
There is accumulating evidence for early social evaluation, particularly between helping and hindering agents (3), but the social, cognitive, and affective processes and their interaction behind these early evaluations need to be identified (1). For instance, cognitive processes are required to understand the goals of actions and representation of agency. However, social evaluations of help and harm are also associated with relatively automatic reactions in the observer, potentially triggering basic approach versus avoidance mechanisms (26).
Research on children and adult’s emotion regulation and social competencies has consistently shown that frontal power density asymmetry (particularly in 5–8 Hz frequency band for children and 8–13 Hz band for adults) during rest and when viewing emotional stimuli is related to individual differences in emotion regulation, motivational processes, and social behavior (27). For instance, asymmetries in resting state power density of the left and right frontal cortex are differentially involved in the processing of the emotions of others and are also related to one’s own emotional reactivity and approach–withdrawal behavior (28). This body of research has shown that greater frontal power density in the left hemisphere (vs. right hemisphere) is related to approach behaviors and positive affect and greater frontal power density in the right hemisphere is related to withdrawal and negative affect (29). Resting state measures have also been used to predict individual differences in social cognitive and prosocial development. For instance, an infant’s frontal and temporal power density asymmetries during resting state, collected at 14 mo of age, independently predicted comforting and helping behaviors several months later (24 and 18 mo of age, respectively) (30). In studies of preschool representational theory of mind, resting state frontal power densities in the alpha frequency band source localized to the right temporal–parietal junction and dorsal medial prefrontal cortex predicted theory of mind competency (31). Taken together, these studies suggest resting state frontal power density is an early index of social cognitive and emotion development, both competencies crucial for the development of morality.
Infant temperament, parental socialization, and childhood environment are early antecedents of emotional regulation (32). Moreover, the complex interaction of child dispositions and the quality of parent–child relationships have long-term impacts, including social and cognitive development in childhood, adolescence, and adulthood (33). Furthermore, there is a growing body of evidence in adults that dispositional sensitivity to justice and fairness modulate online neural response to the perception of interpersonal harm in regions of the prefrontal cortex involved in cognitive control and decision-making (34, 35). Therefore, it is possible that these dispositions in parents will shape children’s prosocial behavior and neural responses during third-party evaluations of social interactions. Such parent/child value transmission is not the sole product of social learning but is rather a complex Gene × Environment interaction (36).
Several time-locked neural responses in infants and children have been associated with differences in early visual differentiation of stimuli and relatively automatic responding (Nc) and controlled cognitive processes (positive slow wave, PSW) (37, 38). Each of these waveforms serves as a proxy for domain-general mechanisms of controlled and automatic processing in the developing brain. Moral sensitivity, in later development, is actually a careful integration of both an early automatic emotional component and a later cognitive reappraisal of stimuli, which can be explored in the temporal mechanical investigations of neural processing (1, 39). By combining EEG with eye-tracking, behavioral measures, and parental and children’s dispositions, the present study investigated the mechanisms at play during third-party social evaluations of prosocial and antisocial behaviors and their developmental trajectories. Two paradigms were used to assess implicit moral evaluations. One relied on a modified version of the helper versus hinder task (social evaluation task, SET), where children witness a character attempting to climb a hill and another character approaching to either assist or prevent the first character (40, 41). The other task was an infant-friendly version of the Chicago moral sensitivity task (CMST), developed by Cowell and Decety (22), in which two characters are interacting in a variety of prosocial (e.g., sharing, helping) and antisocial (e.g., shoving, tripping, hitting) ways. Consistent with developmental research, it was hypothesized that even the youngest infants would differentiate between characters that helped another and characters that hindered another, as indexed by differences in frontal power density asymmetries during the SET, and several key ERP components in the CMST. Specifically, as previous investigations of EEG frontal asymmetry have related relatively higher left power density to right power density as indexing emotional withdrawal (42), infants’ left density in the SET was expected to be greater than the right density in the perception of hindering condition, where infants would withdraw from the negatively valenced stimuli. Conversely, as frontal EEG asymmetries greater for right than left are related to approach behaviors (28), increased power densities in right compared with left frontal areas were expected in the perception of helping condition. Greater densities in the right versus left frontal areas (approach to prosocial agent) and preferential looking to helping versus hindering characters (as indexed by eye-tracking measures) were expected to directly relate to the child’s preferential reaching for a character that previously helped versus hindered another.
Moreover, consistent with studies in older children, infants’ early, automatic, and later controlled time-locked neural responses to the perception of social interactions of others were expected in the CMST (21, 22). Infants were hypothesized to show greater amplitudes for good actions than bad actions in the Nc component, a central negativity between 300 and 500 ms poststimulus that has been previously linked to automatic resource allocation. The youngest infants (12 mo) were not expected to show later controlled differences between the processing of good and bad actions (in the PSW, 600–1,000 ms), however with age and the development of more elaborate cognitive processes, older toddlers (18–24 mo old) were expected to show greater amplitudes in the PSW for the perception of good actions, than bad actions, akin to the late positive potential (LPP) differences seen in preschool children engaged in similar implicit moral evaluations (24). Furthermore, individual differences in the later component were anticipated to predict both children’s sharing propensities and preference for the prosocial character. Finally, parental values regarding fairness and justice were expected to be reflected in early and late time-locked neural differences elicited by the perception of interpersonal harm.
Results
Behavioral.
Following the SET, children were shown two characters: the prosocial helper and the antisocial hinderer. Children’s preferential reaching (from 54 children who explicitly reached for one character or the other) for helper versus hinderer did not significantly vary from chance, with 27 children reaching for the helper and 27 children reaching for the hinderer. Additionally results from a binary logistic regression indicate that age (in months) does not predict preference for the helper or hinderer (β = –0.049, nonsignificant).
Children also engaged in a sharing task where they were given two toys to play with, one desirable and one less desirable. After playing with the toys, when children were prompted with “Can I have one?” 15 children shared both toys immediately, 11 shared their preferred toy, 7 shared their nonpreferred toy, 4 additional children shared a toy but showed no preference for one toy over the other, and 34 children did not share either toy. As a dichotomy (sharing/not sharing), there were no significant differences in the number of children who shared a toy (n = 37) and the number who did not share (n = 34). Again, age (in months) was not a predictor of sharing behaviors (β = 0.036, nonsignificant).
Child and Parents’ Dispositions.
Three aspects of children’s temperament were calculated from a commonly used measure in early childhood, the Early Childhood Behavior Questionnaire-Very Short Form (ECBQ-VSF): surgency (level of positive affect), negative affect, and effortful control (an index of early self regulation). Children’s effortful control was directly related to the probability of children sharing (r = 0.269, P < 0.05), after controlling for age-related differences. However, children’s surgency and negative affect were unrelated to sharing behaviors or reaching preferences.
Parental disposition in perspective taking from the Interpersonal Reactivity Index (IRI) was positively related to the probability of children sharing (r = 0.284, P < 0.05). Parental personal distress (IRI) was inversely related to children’s probability of sharing (r = –0.317, P < 0.05).
Spectral Densities and Preferential Looking During Early Social Evaluation.
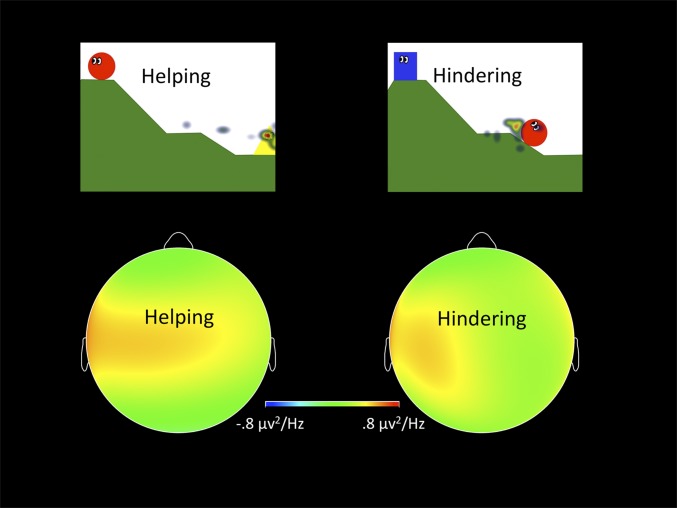
Global neural resource allocation differences for helping versus hindering scenes were examined using a 2 (Laterality, Left/Right) × 2 (Region, Frontal/Parietal) × 2 (Condition) repeated-measures analysis of variance (ANOVA) on 5–8 Hz EEG power density, controlling for age during the SET. A significant main effect of condition was observed, F(1, 19) = 4.470, P < 0.05, η2 = 0.190, with the global power density of 5–8 Hz for helping scenes less than the global power density of 5–8 Hz for hindering scenes, indicating greater underlying cortical activation for helping than hindering. Frontal 5–8 Hz asymmetries were examined with a 2 (Frontal Laterality, Left/Right) × 2 (Condition) repeated-measures ANOVA with 5–8 Hz EEG power density as the dependent variable, controlling for age. A significant Laterality × Condition interaction was detected, F(1, 23) = 4.83, P < 0.05, η2 = 0.174, with greater asymmetry (left frontal 5–8 Hz power density – right frontal 5–8 Hz power density) when observing hindering compared with helping scenarios (Fig. 1). Results from a 2 (Condition) × 2 (Agent/Target) repeated-measures ANOVA on overall percent looking-time show no significant main effect of condition but a significant interaction of Condition × Character, F(1, 36) = 39.73, P < 0.001. The interaction was driven by a significantly greater fixation time to the agent of helping than the beneficiary of helping, t(37) = 6.978, P < 0.001 (Fig. 1).
Fig. 1.
Scalp plots of the power density in the 5–8 Hz range for SET, characterized by a significant difference in asymmetry for hindering versus helping scenes. On the top are representative heat maps of toddlers’ visual fixations. Children fixated significantly longer on the helping agent than on the beneficiary of helping.
Continuous EEG Asymmetries During Evaluation and Resting State, and Attentional Differences as They Relate to Behavior.
Resting state 5–8 Hz power density frontal asymmetries did not predict reaching preferences (r = –0.199, nonsignificant) nor sharing (r = –0.247, nonsignificant). Additionally, frontal power density asymmetry during the SET did not predict character reaching preference (r = 0.029, nonsignificant) or sharing behavior (r = –0.088, nonsignificant). Moreover, differential fixation to helping scenes versus hindering scenes, as assessed with eye-tracking, was not related to reaching preference (r = –0.249, nonsignificant) or sharing behavior (r = 0.146, nonsignificant).
Neural Temporal Dynamics of Implicit Moral Evaluations.
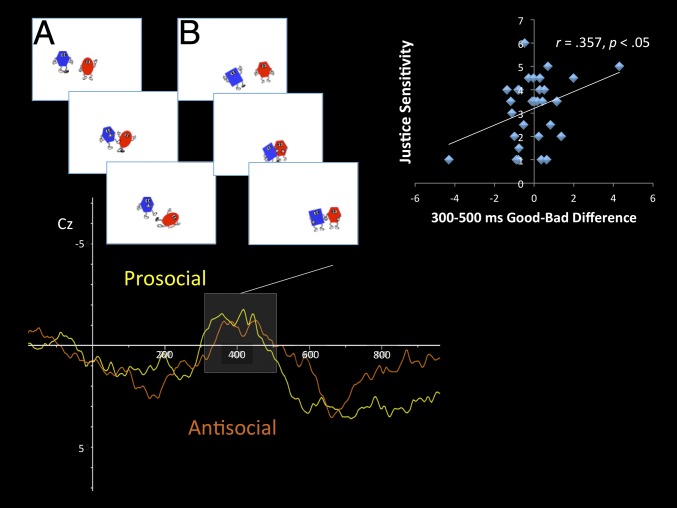
In the CMST, three time windows of interest—an early, 200–300 ms; a midrange, 300–500 ms; and a late, 600–1,000 ms—were identified based on previous research with toddlers and preschool children (43). Results from a comparison of average voltage in the 300–500 ms range over parietal electrodes (Pz), and controlling for age-related change, revealed a main effect of condition, F(1, 23) = 4.28, P < 0.05, η2 = 0.163, with a greater amplitude for the perception of prosocial scenes than antisocial scenes (see Fig. 2 for grand-averaged waveforms). Results from two (frontal, F3, Fz, F4; parietal, P7, Pz, P8) repeated-measures ANOVAs, controlling for age, on the early time window (200–300 ms) found no significant differences by condition in frontal, F(1, 15) = 0.079, nonsignificant, or parietal, F(1, 15) = 0.075, nonsignificant, regions. Similarly, no significant differences between the perception of harmful and helpful scenes, after controlling for age, were found in the late time window (600–1,000 ms) in either frontal, F(1, 22) = 0.138, nonsignificant, or parietal, F(1, 15) = 0.023, nonsignificant, regions.
Fig. 2.
Grand-averaged waveform of perceiving antisocial (orange, A for an example) and prosocial (yellow, B for an example) actions in the CMST at electrode Cz. Individual differences in the 300–500 ms poststimulus range were predicted by parental values toward injustice for others.
Individual Differences in Neural Temporal Dynamics Predict Social Preferences.
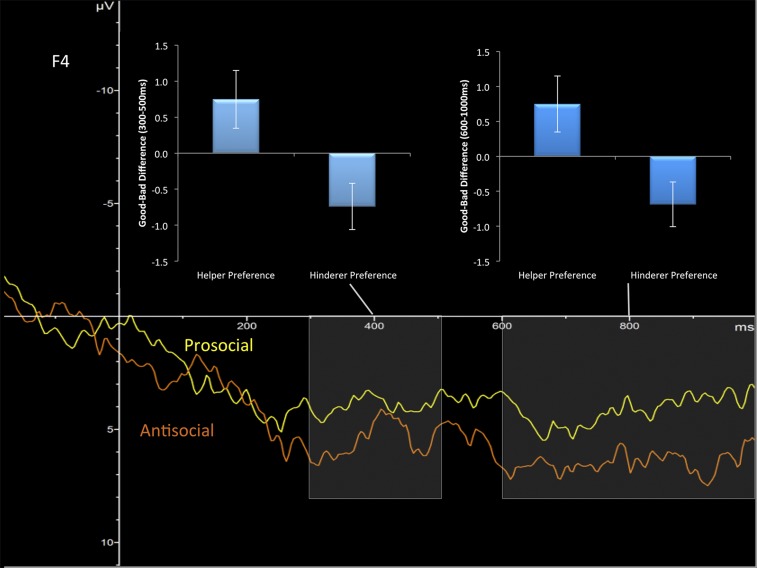
Individual differences between average neural activation to the perception of helping versus harmful scenes during the CMST in two windows—middle (300–500 ms) and late (600–1,000 ms)—were related to reaching preference for a prosocial over an antisocial character (Fig. S1). Relations were maximal over frontal areas in both middle (F4, r = 0.503, P < 0.001) and late waveforms (F4, r = 0.466, P < 0.05).
Fig. S1.
The grand-averaged waveform of perceiving prosocial (yellow) and antisocial (orange) actions in the CMST at electrode F4 is plotted. Individual differences in the 300–500 ms range poststimulus and 600–1,000 ms range for prosocial versus antisocial actions were significantly different for those who reached for the helper versus hinderer in the SET. Bars represent SE; negative values in the ERP are plotted up.
Children’s Temporal Neural Dynamics of Moral Evaluation Are Related to Parental Values.
Parental sensitivity to injustice for others was directly predictive of infants and toddlers’ ERP differences in the perception of helping versus harmful scenes in the CMST between 300 and 500 ms (Cz, r = 0.357, P < 0.05; Fig. 2). Individual differences in the 600–1,000 ms range were not significantly related to parental individual dispositions toward justice.
Discussion
Combining neurophysiolgical measures with sociomoral evaluations and sharing behavior in infants and toddlers is both a challenging and exciting endeavor. Such a multilevel approach has the potential to provide theoretical and empirical clarity into the nature of the precursors to and ontogeny of the foundations of morality. In this study, we also investigated how social and parental values shape the online neural underpinnings of early social evaluation in infants and toddlers and expressed sharing behavior.
Infants and toddlers between the ages of 12 and 24 mo exhibited neural differentiation between the perception of harmful and helpful behaviors of others in two paradigms. First, spectral asymmetries were robustly present during the viewing of both types of behaviors in a well-established behavioral task for assessing infants’ social evaluation (40). EEG frontal asymmetry has previously been linked to withdrawal/avoidance when left power density is greater than right power density in response to emotionally valenced stimuli (28). Consistent with our hypotheses, relative asymmetry for left versus right was greater for the perception of hindering than helping, implying the engagement of domain-general mechanisms of withdrawal/avoidance when confronted with aversive stimuli and the reduction of withdrawal when viewing positive stimuli. Thus, EEG markers, in the SET, support the privilege of negative social information in early development, as demonstrated by habituation paradigms with 3-mo-old infants (5). Preferential reaching for a helper over a hindering agent has been documented in younger children in some studies (41) but not in another using a similar paradigm (14). The current study failed to find such a preference in older toddlers, suggesting early and strong individual, rather than group, differences in preferring a helper that are directly related to the neural dynamics processing of perceiving prosocial and antisocial characters. This result provides a more nuanced view of developing sociomoral evaluations.
Infants and toddlers also expressed preferential looking (as measured with average fixation time to two different predefined areas of interest around the agent and recipient/victim of each scenario) toward a prosocial agent, rather than the beneficiary of an action. Importantly, no global fixation differences were detected between the observation of prosocial and antisocial actions, only the aforementioned interaction. Previous investigations of infant social evaluation (40, 41) have relied on researcher-coded looking behavior, wherein the specificity of an infant’s visual fixations is limited. Some have argued that the resulting visual preference data and procedures vary across studies (20). The use of eye-tracking technologies in infants has become more commonplace, allowing for an expanded scope in testing, but is challenging to use (44). In the present study, infants and toddlers’ fixations for agents versus beneficiaries/victims of social interactions could be disentangled. Infants clearly demonstrate a focus on the agent of prosocial actions, rather than the beneficiary. This result expands one recent ERP study with 6-mo-old infants wherein greater P400 amplitudes (thought to index attention) for the memory of a previously prosocial agent compared with an antisocial agent were evoked (23). Thus, infants and toddlers exhibit a distinct focus on actors who help others and appear to be unmotivated to focus on a beneficiary of a positive action. This pattern of gaze fixation, together with recent empirical findings in developmental science, provide a window into how infants make sense of the behavior of intentional agents engaging in morally laden actions (3).
Using an elaborate time-locked third-party implicit social evaluation paradigm affords a more precise investigation of the mechanisms underlying moral computations. Clear neural differentiations between the perception of positive and negative social interactions were detected in infants and toddlers with the CMST ERP paradigm. Interestingly, the condition-level difference that reached significance was in an early to midrange 300–500-ms window that has previously been associated with relatively automatic allocation of cognitive resources to salient visual stimuli (37, 43). The mean amplitude for the perception of prosocial behaviors exhibited a greater absolute amplitude than that of antisocial behaviors, reflecting increased neural engagement in response to positive social interactions and is consistent with behavioral evidence suggesting the allocation of additional computational resources to evaluate prosocial or fair behaviors. Given the wealth of developmental work on a negativity bias in the perception of social behaviors, this pattern of response was expected; that is, perceived negative social interactions are more readily processed, because they trigger avoidance and defense mechanisms, which require less cognitive control and associated neural resources (45). An alternative interpretation of an infant’s social evaluations, consistent with this ERP result, is that good behaviors are more novel to them and thus elicit additional computations, whereas bad behaviors are more common and prompt an automatic response. These results seem to indicate that there is an increased recruitment of resources/computations in processing the anticipation of prosocial outcomes compared with antisocial outcomes. Such a processing difference has also been documented in preschoolers and adult participants when viewing good versus bad actions or interpersonal help versus interpersonal harm, respectively (22, 26, 34, 35).
Moreover, children’s time-locked neural differentiations between these good and bad actions in the CMST predicted their character preference (preferential reaching toward a hindering or helping character). Previous investigations with children and adults examining brain–behavior and brain–dispositional links in the domain of morality have demonstrated a consistent pattern. Individual differences in modulations to later, controlled ERPs (P300, LPP) predict actual sharing behaviors in children (22) and are thought to be related to prosocial dispositions (46) and cognitive empathy and perspective-taking in adults (34). In the present study, infants’ and toddlers’ modulations in Nc (300–500 ms) and LPP/PSW (600–1,000 ms) over frontal electrodes were predicted by their previous preference for helpful over hindering characters (from the SET). These results illustrate continuity in early social evaluation. Those infants who prefer prosocial characters in one behavioral task show the greatest neural differentiation to prosocial versus antisocial actions in an implicit moral evaluation task. Additionally, infants’ and toddlers’ individual differences in Nc (300–500 ms) for viewing positive versus bad actions or in LPP/PSW (600–1,000 ms) were not related to the expression of sharing behaviors. Although sharing behaviors in infancy and toddlerhood appear to be common (47), many have questioned whether such early expressions are actually representative of morality or rather early self-regulation, compliance, and effortful control (48, 49). Consistent with the latter argument, our results suggest that sharing propensity is directly related to children’s temperamental effortful control.
Remarkably, the values of parents regarding justice and their cognitive empathic dispositions already influence toddlers’ own neural processing of these morally laden scenarios and their propensity to share, respectively. Empathetic disposition and justice motivation are distinct constructs. Recent work using both personality trait measures combined with moral judgment (50), functional MRI during moral evaluation (35), and high-density EEG/ERPs also during moral evaluation (34) indicates that justice sensitivity is largely guided by reasoning and computations from the dorsolateral prefrontal cortex and is related, in the same individual, to their own moral cognition but not necessarily their own behavior. Similarly, in the current study, parental attitudes toward justice are related to online neural moral evaluation in their children but not behavior. Relatively automatic differences (300–500 ms) toward prosocial versus antisocial actors in infants’ and toddlers’ ERPs were predicted by their parents’ sensitivity to injustice for others. Children of those parents who reported higher dispositions in justice motivation demonstrated the greatest neural difference. Moreover, parental cognitive empathy predicted children’s sharing (or compliance) behavior. Several potential explanations for these parent–child value transmissions are conceivable, including passive, active, or evocative genes by environmental effects. Parents may directly teach their children these priorities, they may set up environments that allow their children to obtain these values, or children’s characteristics may, in and of themselves, evoke similar environments to those in which their parents were reared (36). These previously unidentified and intriguing findings warrant further investigation to decipher what contributes to such an early parent–child reflection of values, which may either be based on biological or socioenvironmental influences, or more likely a dynamic and complex developmental, interactional process between the two.
The notion that observed behavioral differences in infants’ evaluations of prosocial and antisocial agents are markers of or precursors to more complex moral cognition remains highly contentious (13, 15). This study provides compelling evidence that infants and toddlers discriminate between the prosocial and antisocial actions of others. Specific neural computations can be identified, including assymetrical frontal spectral power densities during social evaluation, eye-tracking differences in social evaluation, and time-locked condition differences between 300 and 500 ms in the CMST. However, these results suggest that this differentiation is relatively basic in nature, rooted in approach/withdrawal tendencies and rudimentary resource allocation to relevant stimuli. These findings indicate that domain-general self-regulation and attention systems underlie early social and moral evaluation, supporting mounting evidence from the neuroscience of morality (2). It should be noted, however, that although the processes and mechanisms of implicit social and moral evaluation in infants and toddlers appear to rely on domain-general processes, the distinction between prosocial and antisocial characters does modulate the relative engagement of these systems, suggesting a possible domain specificity to relative recruitment.
Taken together, the convergent evidence from multiple measures used in the present study identified specific neural computations underpinning early sociomoral evaluations and their relation to moral preferences as well as parental dispositions, which goes beyond what observational and behavioral assessments can provide. This demonstrates the potential of developmental social neuroscience to provide productive, new, and exciting directions for the study of moral development, when stemming from an integration of neurobiology, behavior, and social environment (51, 52).
Methods
Participants.
Seventy-three children between the ages of 12 and 24 mo (M = 18.69 mo, SD = 6.35; n = 37 females) were recruited from a large Midwestern city. All parents were compensated for their children’s inclusion in the study, and infants were given a small gift (for sample size on each measure, see EEG/ERP Collection and Data Analysis).
Procedure.
Upon arrival, parents provided consent and were asked to fill out a series of questionnaires measuring their own dispositions toward empathy and sensitivity to fairness and justice as well as basic demographics. Children were acclimatized to the laboratory and then brought to the EEG testing room and seated on their parent’s lap. Parents were instructed to remain silent during the testing and had a pair of blinding glasses. A 17-channel EEG cap (EasyCap, Brain Products) was applied, and impedances were reduced to less than 30 kOhm. Following impedance reduction, infants and toddlers watched the SET, completed a preferential reaching paradigm, had 4 min of resting state EEG collected, then viewed harmful and helping scenarios in the CMST, and finally played an infant sharing game. Eye-tracking was continuously monitored during the two EEG tasks with an SMI red-M device (120 Hz). The study was approved by the University of Chicago institutional review board.
Dispositional Measures.
Parents were asked to fill out several questionnaires about their children and themselves to assess dispositional empathy: the ECBQ-VSF (53), the short form of the Sensitivity to Justice Scale (54), and the IRI (55). These standard scales are widely used in many studies of social behavior in young children and adults (50).
Resting State EEG.
Resting state EEG assessment was modeled after a common method used with infants, continuously spinning a large bingo ball cage with colored balls (56). The cage was spun for a 1-min period, followed by a break (repeated four times), collecting a total of 4 min of continuous EEG data per infant.
SET.
In this variant of the task, children observed a shape (e.g., a circle) attempting to climb a hill (40, 41). They then saw another shape either hinder (block the shape from moving upward) or help (push the shape up the hill). Each scene lasted 15 s on the screen and is repeated at least four times for hindering and four times for helping, randomly, with the experimenter regaining the child’s attention between trials, then manually forwarding. Children received one of two versions of the task to account for shape/color preferences. Continuous EEG was recorded from 17 scalp sites during the presentations as well as eye-tracking. Following the task, the experimenter put two physical versions of the characters (helper and hinderer) just out of reach of the child, and the infant’s preferred reaching was documented.
CMST.
In the CMST (22), children watched dynamic visual scenarios of characters intentionally performing either prosocial acts (e.g., sharing food, helping another character after they fall) or antisocial acts, predominately those where a character harms another (e.g., hitting another character, shoving another character). The EEG version of the CMST included 70 trials (35 prosocial and 35 antisocial) 3 s in duration, randomly presented, with a jittered intertrial interval of 500–1,500 ms.
Sharing Game.
In this game, children were given two toys to play with (11). One toy was a very desirable ring, which makes sounds while shaking it and has colorful paintings on the other side. The other toy was also a ring but was a single color and made no sound. Both toys were handed to the toddler, and the child was given 1 min to play. Children’s preference for toys was documented. Following the minute of free play, an experimenter approached the toddler and said “Can I have one?” Sharing proclivity was assessed in two ways: first, the toddler’s sharing of any toy, and second, the toddler’s willingness to share the preferred toy.
EEG/ERP Collection and Data Analysis
Data were collected from a 17-channel (Fz, Cz, Pz, Oz, F3, F4, F7, F8, P3, P4, P7, P8, Fp1, Fp2, TP9, TP10, and ground) active electrode system (actiCHamp, Brain Products) at 2,000 Hz, referenced to Cz, and all electrode impedances were kept below 30 kOhm. Electrophysiological data analysis was performed using Brain Vision Analyzer 2 software (Brain Products). Data were downsampled offline (256 Hz) and rereferenced to the average of all electrodes. EEG data were filtered using an IIR filter of 0.1–30 Hz and a notch filter of 60 Hz, and artifact rejection was carried out using a –200 µV to 200 µV threshold and visual inspection and rejection by trained researchers. Gratton–Coles ocular artifact correction was performed on EEG data to identify and correct ocular movements, seeded off electrode Fp1.
SET and Resting State Spectral Density and Eye-Tracking Analyses.
For the SET, the entrance of the helping or hindering character was the starting point for spectral analyses. Continuous EEG during the helping or hindering actions was subdivided into 500 ms windows with 50% overlap. Using a Fast Fourier Transform with a Hanning window, power densities were computed for each segment. Segments were then averaged for a grand average per individual for each condition. Based off previous literature, the 5–8 Hz band was used for all analyses. For global 5–8 Hz analyses, frontal (F7, Fz, and F8) and parietal (P7, Pz, and P8) electrodes were used. For frontal asymmetry analyses during the task, average power density for left (F3) and right (F4) 5–8 Hz activity was natural log-transformed and subtracted (left–right). Only infants with at least 60 artifact-free segments were included in all analyses (n = 25). Moreover, infants and toddlers’ attention was tracked using an SMI red-m from the entrance of the helping or hindering character 7 s into the 15-s trial. The 8-s social evaluation period was subdivided into 300-ms segments, and areas of interest were defined surrounding the helping or hindering agent and the beneficiary/victim of the action. Average fixation time to each area of interest was exported. Children with average fixations less than 10 ms were eliminated from group analyses.
For resting state asymmetries, continuous EEG from the 4 min was segmented into 1,000-ms segments with 50% overlap. The same process used in the social evaluation spectral analyses was implemented, however a left frontal pooled average of power density from F3 and F7 and a right frontal pooled average from F4 and F8 were calculated and subtracted. Continuous left–right power density “dispositions” were then related to behavioral measures. Children with at least 100 artifact-free segments (M = 400 segments) were included in further analyses (n = 43).
CMST ERP Analyses.
Following basic EEG preprocessing, data were segmented according to stimulus type (prosocial/antisocial). Epochs were created with 200-ms baselines and 1,000-ms stimulus presentation following the second stimulus presentation (the frame that shows the moral action). Epochs were average by trial type and baseline corrected. Corrected averages per individual were combined for a grand average, and ERPs were analyzed across subjects by trial types. Due to exhaustion, not all participants completed all trials of the CMST or the SET. Only children who had at least 15 artifact-free trials for each condition (prosocial/antisocial) in the CMST were included in the grand average (n = 21). For measures of individual differences, children with at least 10 artifact-free trials for each condition (n = 25) were included.
Acknowledgments
We are grateful to Kat Bovbjerg, Ariel Cheng, Martha Fahlgren, Megan High, Alexa Karczmar, Angela Liu, and Eliana Polimeni, who assisted us at the Child Neurosuite in recruitment and data collection. We also thank the many families and children who participated in this project. This research was supported by a grant from the John Templeton Foundation (Science of Philanthropy Initiative).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.M. is a guest editor invited by the Editorial Board.
See Commentary on page 12551.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508832112/-/DCSupplemental.
References
- 1.Decety J, Howard LH. The role of affect in the neurodevelopment of morality. Child Dev Perspect. 2013;7(1):49–54. [Google Scholar]
- 2.Young L, Dungan J. Where in the brain is morality? Everywhere and maybe nowhere. Soc Neurosci. 2012;7(1):1–10. doi: 10.1080/17470919.2011.569146. [DOI] [PubMed] [Google Scholar]
- 3.Hamlin JK. The infantile origins of our moral brains. In: Decety J, Wheatley T, editors. The Moral Brain: A Multidisciplinary Perspective. Oxford Univ Press; New York: 2015. pp. 105–122. [Google Scholar]
- 4.Baillargeon R, et al. Psychological and sociomoral reasoning in infancy. APA Handbook of Personality and Social Psychology: Vol. 1. Attitudes and Social Cognition. In: Borgida E, Bargh J, editors. American Psychological Association; Washington, DC: 2015. pp. 79–150. [Google Scholar]
- 5.Hamlin JK, Wynn K, Bloom P. Three-month-olds show a negativity bias in their social evaluations. Dev Sci. 2010;13(6):923–929. doi: 10.1111/j.1467-7687.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geraci A, Surian L. The developmental roots of fairness: Infants’ reactions to equal and unequal distributions of resources. Dev Sci. 2011;14(5):1012–1020. doi: 10.1111/j.1467-7687.2011.01048.x. [DOI] [PubMed] [Google Scholar]
- 7.Sloane S, Baillargeon R, Premack D. Do infants have a sense of fairness? Psychol Sci. 2012;23(2):196–204. doi: 10.1177/0956797611422072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warneken F, Tomasello M. The roots of human altruism. Br J Psychol. 2009;100(Pt 3):455–471. doi: 10.1348/000712608X379061. [DOI] [PubMed] [Google Scholar]
- 9.Nichols SR, Svetlova M, Brownell CA. The role of social understanding and empathic disposition in young children’s responsiveness to distress in parents and peers. Cogn Brain Behav. 2009;13(4):449–478. [PMC free article] [PubMed] [Google Scholar]
- 10.Vaish A, Carpenter M, Tomasello M. Sympathy through affective perspective taking and its relation to prosocial behavior in toddlers. Dev Psychol. 2009;45(2):534–543. doi: 10.1037/a0014322. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt MFH, Sommerville JA. Fairness expectations and altruistic sharing in 15-month-old human infants. PLoS One. 2011;6(10):e23223. doi: 10.1371/journal.pone.0023223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunfield K, Kuhlmeier VA, O’Connell L, Kelley E. Examining the diversity of prosocial behavior: Helping, sharing, and comforting in infancy. Infancy. 2011;16(3):227–247. doi: 10.1111/j.1532-7078.2010.00041.x. [DOI] [PubMed] [Google Scholar]
- 13.Tafreshi D, Thompson JJ, Racine TP. An analysis of the conceptual foundations of the infant preferential looking paradigm. Hum Dev. 2014;57(4):222–240. [Google Scholar]
- 14.Scarf D, Imuta K, Colombo M, Hayne H. Social evaluation or simple association? Simple associations may explain moral reasoning in infants. PLoS One. 2012;7(8):e42698. doi: 10.1371/journal.pone.0042698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahl A. Definitions and developmental processes in research on infant morality. Hum Dev. 2014;57(4):241–249. doi: 10.1159/000364919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haith MM. Who put the cog in infant cognition? Is rich interpretation too costly? Infant Behav Dev. 1998;21(2):167–179. [Google Scholar]
- 17.Aslin RN. Why take the cog out of infant cognition? Infancy. 2000;1(4):463–470. doi: 10.1207/S15327078IN0104_6. [DOI] [PubMed] [Google Scholar]
- 18.Bloom P. Just Babies: The Origins of Good and Evil. Crown Publishers; New York: 2013. [Google Scholar]
- 19.Prinz JJ. The Emotional Construction of Morals. Oxford Univ Press; New York: 2007. [Google Scholar]
- 20.Lewkowicz DJ. The biological implausibility of the nature-nurture dichotomy & what it means for the study of infancy. Infancy. 2011;16(4):331–367. doi: 10.1111/j.1532-7078.2011.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Y, Chen C, Decety J. An EEG/ERP investigation of the development of empathy in early and middle childhood. Dev Cogn Neurosci. 2014;10:160–169. doi: 10.1016/j.dcn.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowell JM, Decety J. The neuroscience of implicit moral evaluation and its relation to generosity in early childhood. Curr Biol. 2015;25(1):93–97. doi: 10.1016/j.cub.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gredebäck G, et al. The neuropsychology of infants’ pro-social preferences. Dev Cogn Neurosci. 2015;12:106–113. doi: 10.1016/j.dcn.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cereb Cortex. 2012;22(1):209–220. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- 25.Taber-Thomas BC, et al. Arrested development: Early prefrontal lesions impair the maturation of moral judgement. Brain. 2014;137(Pt 4):1254–1261. doi: 10.1093/brain/awt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decety J, Porges EC. Imagining being the agent of actions that carry different moral consequences: An fMRI study. Neuropsychologia. 2011;49(11):2994–3001. doi: 10.1016/j.neuropsychologia.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- 28.Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218(4578):1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- 29.Saby JN, Marshall PJ. The utility of EEG band power analysis in the study of infancy and early childhood. Dev Neuropsychol. 2012;37(3):253–273. doi: 10.1080/87565641.2011.614663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulus M, Kühn-Popp N, Licata M, Sodian B, Meinhardt J. Neural correlates of prosocial behavior in infancy: Different neurophysiological mechanisms support the emergence of helping and comforting. Neuroimage. 2013;66:522–530. doi: 10.1016/j.neuroimage.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Sabbagh MA, Gehring WJ, Wellman HM. Neural correlates of children’s theory of mind development. Child Dev. 2009;80(2):318–326. doi: 10.1111/j.1467-8624.2009.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochanska G. Toward a synthesis of parental socialization and child temperament in early development of conscience. Child Dev. 1993;64(2):325–347. [Google Scholar]
- 33.Sroufe LA. Attachment and development: A prospective, longitudinal study from birth to adulthood. Attach Hum Dev. 2005;7(4):349–367. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- 34.Yoder KJ, Decety J. Spatiotemporal neural dynamics of moral judgment: A high-density ERP study. Neuropsychologia. 2014;60:39–45. doi: 10.1016/j.neuropsychologia.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoder KJ, Decety J. The good, the bad, and the just: Justice sensitivity predicts neural response during moral evaluation of actions performed by others. J Neurosci. 2014;34(12):4161–4166. doi: 10.1523/JNEUROSCI.4648-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarr S, McCartney K. How people make their own environments: A theory of genotype greater than environment effects. Child Dev. 1983;54(2):424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An event-related potential and cortical source localization study. Dev Psychol. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossmann T. doi: 10.1037/bul0000002. (2015) The development of social brain functions in infancy. Psychol Bull. [DOI] [PubMed] [Google Scholar]
- 39.Rutland A, Killen M, Abrams D. A new social-cognitive developmental perspective on prejudice: The interplay between morality and group identity. Perspect Psychol Sci. 2010;5(3):279–291. doi: 10.1177/1745691610369468. [DOI] [PubMed] [Google Scholar]
- 40.Kuhlmeier V, Wynn K, Bloom P. Attribution of dispositional states by 12-month-olds. Psychol Sci. 2003;14(5):402–408. doi: 10.1111/1467-9280.01454. [DOI] [PubMed] [Google Scholar]
- 41.Hamlin JK, Wynn K, Bloom P. Social evaluation by preverbal infants. Nature. 2007;450(7169):557–559. doi: 10.1038/nature06288. [DOI] [PubMed] [Google Scholar]
- 42.Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20(1):125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- 43.Richards JE. Attention in young infants: A developmental psychophysiological perspective. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 321–338. [Google Scholar]
- 44.Oakes LM. Advances in eye tracking in infancy research. Infancy. 2012;17(1):1–8. doi: 10.1111/j.1532-7078.2011.00101.x. [DOI] [PubMed] [Google Scholar]
- 45.Vaish A, Grossmann T, Woodward A. Not all emotions are created equal: The negativity bias in social-emotional development. Psychol Bull. 2008;134(3):383–403. doi: 10.1037/0033-2909.134.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiu Loke I, Evans AD, Lee K. The neural correlates of reasoning about prosocial-helping decisions: An event-related brain potentials study. Brain Res. 2011;1369:140–148. doi: 10.1016/j.brainres.2010.10.109. [DOI] [PubMed] [Google Scholar]
- 47.Sommerville JA, Schmidt MFH, Yun J, Burns M. The development of fairness expectations and prosocial behavior in the second year of life. Infancy. 2013;18(1):40–66. [Google Scholar]
- 48.Paulus M. The emergence of prosocial behavior: Why do infants and toddlers help, comfort, and share? Child Dev Perspect. 2014;8(2):77–81. [Google Scholar]
- 49.Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Dev Psychol. 2000;36(2):220–232. [PubMed] [Google Scholar]
- 50.Decety J, Yoder KJ. Empathy and motivation for justice: Cognitive empathy and concern, but not emotional empathy, predict sensitivity to injustice for others. Soc Neurosci. 2015:1–14. doi: 10.1080/17470919.2015.1029593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulus M. Cognitive development: The neurocognitive basis of early prosociality. Curr Biol. 2015;25(1):R47–R48. doi: 10.1016/j.cub.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 52.Turiel E. The relevance of moral epistemology and psychology for neuroscience. In: Zelazo PD, Chandler M, Crone E, editors. Developmental Social Cognitive Neuroscience. Taylor & Francis; London: 2009. pp. 313–331. [Google Scholar]
- 53.Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The early childhood behavior questionnaire. Infant Behav Dev. 2006;29(3):386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baumert A, et al. Measuring four perspectives of justice sensitivity with two items each. J Pers Assess. 2014;96(3):380–390. doi: 10.1080/00223891.2013.836526. [DOI] [PubMed] [Google Scholar]
- 55.Davis MH. A mulitdimensional approach to individual differences in empathy. J Pers Soc Psychol. 1983;44(1):113–126. [Google Scholar]
- 56.Bell MA, Fox NA. Crawling experience is related to changes in cortical organization during infancy: Evidence from EEG coherence. Dev Psychobiol. 1996;29(7):551–561. doi: 10.1002/(SICI)1098-2302(199611)29:7<551::AID-DEV1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]