Significance
Duchenne muscular dystrophy (DMD) is a lethal, degenerative muscle disease for which there is no effective treatment. Statins have been used for decades to improve cardiovascular health. In addition to lowering blood cholesterol levels, statins also reduce inflammation, oxidative stress, and fibrosis. These pathogenic processes all contribute to functional decline in DMD muscles. Therefore, we reasoned that statins could be a beneficial treatment for dystrophic muscles. In this study, we show that simvastatin dramatically improves muscle strength and fatigue resistance in DMD (mdx) mice. This result was accompanied by significantly reduced inflammation, oxidative stress, and fibrotic deposition in old, degenerated mdx muscle. These findings indicate that simvastatin is a promising, novel therapeutic approach for DMD and related muscle disorders.
Keywords: statin, muscular dystrophy, fibrosis, inflammation, muscle force
Abstract
Duchenne muscular dystrophy (DMD) is a lethal, degenerative muscle disease with no effective treatment. DMD muscle pathogenesis is characterized by chronic inflammation, oxidative stress, and fibrosis. Statins, cholesterol-lowering drugs, inhibit these deleterious processes in ischemic diseases affecting skeletal muscle, and therefore have potential to improve DMD. However, statins have not been considered for DMD, or other muscular dystrophies, principally because skeletal-muscle-related symptoms are rare, but widely publicized, side effects of these drugs. Here we show positive effects of statins in dystrophic skeletal muscle. Simvastatin dramatically reduced damage and enhanced muscle function in dystrophic (mdx) mice. Long-term simvastatin treatment vastly improved overall muscle health in mdx mice, reducing plasma creatine kinase activity, an established measure of muscle damage, to near-normal levels. This reduction was accompanied by reduced inflammation, more oxidative muscle fibers, and improved strength of the weak diaphragm muscle. Shorter-term treatment protected against muscle fatigue and increased mdx hindlimb muscle force by 40%, a value comparable to current dystrophin gene-based therapies. Increased force correlated with reduced NADPH Oxidase 2 protein expression, the major source of oxidative stress in dystrophic muscle. Finally, in old mdx mice with severe muscle degeneration, simvastatin enhanced diaphragm force and halved fibrosis, a major cause of functional decline in DMD. These improvements were accompanied by autophagy activation, a recent therapeutic target for DMD, and less oxidative stress. Together, our findings highlight that simvastatin substantially improves the overall health and function of dystrophic skeletal muscles and may provide an unexpected, novel therapy for DMD and related neuromuscular diseases.
Duchenne muscular dystrophy (DMD) is a degenerative muscle disease caused by the absence of dystrophin, a large protein that links the cytoskeleton to the surface membrane in muscle cells. Loss of dystrophin causes widespread effects on muscle signaling and metabolic pathways, leading to cell death and progressive replacement of functional muscle fibers with fibrotic connective tissue. This process results in profound muscle weakness, usually leaving DMD boys wheelchair-bound by their early teenage years and leading to death from the consequences of respiratory and/or cardiac muscle failure by age 20–30. Current treatments, such as corticosteroids, slow disease progression only marginally (1), whereas gene-based approaches, such as exon-skipping, although promising in preclinical studies, will need to overcome many technical and regulatory hurdles, as well becoming affordable, before they are a widely available therapy for DMD patients (2). Therefore, efficacious pharmaceutical agents that are cost-effective and already approved for human use are particularly attractive candidates for the current treatment of DMD.
In DMD patients and dystrophin-deficient (mdx) mice, muscle degeneration has been attributed to a number of pathogenic processes; however, chronic inflammation, oxidative stress, and fibrosis certainly have major impacts on the disease progression and functional impairment (3–5). Therefore, therapeutic approaches targeting these pathways would likely provide significant improvement in DMD muscles. HMG CoA-reductase inhibitors (statins) are the most commonly prescribed drugs for treating high blood LDL cholesterol levels and associated cardiovascular diseases. A number of studies have shown that statins improve the cardiovascular system both by lowering circulating LDL cholesterol levels and through cholesterol-independent or “pleiotropic” mechanisms that lead to reduced oxidative stress, inflammation, and fibrosis (6, 7). Therefore, we reasoned that if statins inhibited these same pathogenic pathways in dystrophic muscle, these drugs would result in reduced muscle damage and improved physiological function.
Before the present study, statins had never been tested in DMD or any other neuromuscular disease. Indeed, statins are typically avoided in patients with muscle diseases because of the rare, muscle-related side effects that occur with statin use in the general population. The overall prevalence of statin-induced myopathy is unclear; however, a recent systematic review of several clinical trials found the incidence of adverse muscle symptoms among statin users was <1% compared with placebo controls (8). Moreover, long-term follow-up studies of statin use in children 10 y or older—the most relevant patient cohort for DMD—indicated that statins are well tolerated and have minimal side effects, including those relating to skeletal muscle (9, 10). Indeed, many of the major risk factors for statin myopathy, such as female sex, older age, and intense exercise (11), are not relevant for DMD boys. The causes of statin-induced myopathy are unclear, although several mechanisms have been postulated, including increased oxidative stress (12), activation of the atrogin-1 muscle atrophy pathway (13), and increased susceptibility to RyR1-induced Ca2+ leak in a malignant hyperthermia mouse model (14). Despite the fact that statins are often considered to be toxic to skeletal muscle, the opposite is true in ischemic limb diseases, such as diabetes, for which several studies have shown that statins provide substantial improvement to overall skeletal muscle health (15–19). These improvements are associated with reduced oxidative stress and inflammatory cell infiltration, which attenuates muscle necrosis (16–19). Given that dystrophic muscles are also subject to chronic oxidative stress (20), inflammation (21), and exercise-induced ischemia (22), all of which contribute to muscle damage and functional impairment, there is a strong rationale that statins could be efficacious in DMD.
In the present study, we treated DMD (mdx) mice with simvastatin, a lipophilic statin known to be effectively transported into skeletal muscle fibers. Using a simvastatin concentration that equates to a moderate daily dose in humans, we show that both long- and short-term treatment in mdx mice provides robust improvement in muscle pathology and physiological function, which was accompanied by reduced inflammation, oxidative stress, and fibrosis. We also provide evidence that statin treatment in dystrophic mice does not impede muscle regeneration or induce pathways thought to cause statin myopathy in humans. To our knowledge, these results demonstrate for the first time that statins are beneficial in a degenerative skeletal muscle disease. This finding suggests that statins have great potential to improve muscle health and function in DMD and possibly related neuromuscular diseases.
Results and Discussion
Long-Term Simvastatin Treatment Protects Dystrophic Muscle from Damage and Inflammation While Improving Diaphragm Function.
DMD is a chronic, progressively degenerative disease, and consequently, potential treatments need to be effective over many years. Therefore, we first evaluated long-term simvastatin treatment in mdx mice both to determine its effectiveness on the disease pathogenesis and assess any side effects. Simvastatin was orally administered for 8 mo starting from 3 wk of age, which is just before the onset of muscle damage in mdx mice (5). The calculated dose given to the mice was between 5 and 10 mg/kg per d. This dose would equate to ∼20–40 mg/d for a 10-y-old DMD boy weighing 30 kg (23), based on mouse-to-human equivalence calculations (24), which is within the recommended dose range of statins for children (10). Whole-body muscle health was dramatically improved in simvastatin-treated mdx (mdx Sim) compared with control mdx mice (mdx Con), as evidenced by an 85% reduction in plasma creatine kinase (CK) activity level, a widely used clinical marker of muscle damage (Fig. 1A). This measure of improved muscle health was validated by histological assessment of the tibialis anterior (TA) muscle, which showed less inflammation in mdx Sim mice (Fig. 1B). Of note, simvastatin had no effect on CK levels or gross muscle histology of WT mice (Fig. 1 A and B), indicating that there was no measureable muscle damage in normal mice at this moderate dose. Next, we quantified the extent of inflammation—a key feature of the dystrophic muscle pathology—by immunofluorescence of TA muscle sections with CD68, a marker of macrophages and other inflammatory cells (20). As highlighted in Fig. 1C, simvastatin substantially reduced inflammatory cell levels by close to 70% compared with mdx Con. These results are consistent with the known anti-inflammatory effects of statins in skeletal muscles of ischemic limbs (17, 18).
Fig. 1.

Long-term simvastatin treatment minimizes muscle damage and inflammation, shifts fiber type, and increases diaphragm muscle force in mdx mice. In these experiments, mice were treated with simvastatin, starting from weaning (3 wk of age) for a total of 8 mo. (A) Whole-body muscle damage was measured by the levels of plasma CK activity. ***P < 0.001 for mdx Con (n = 10) compared with mdx Sim (n = 9). (B) Representative H&E-stained images of TA sections from mdx Con, mdx Sim, WT Con, and WT Sim mice. Note the inflammatory cell infiltration in the mdx Con section. (Scale bar: 50 µm.) (C, Upper) Representative images showing inflammation for mdx Con and mdx Sim using a CD68 antibody (green). The sarcolemma is labeled with a Caveolin-3 antibody (red). (Scale bar: 20 µm.) (C, Lower) Quantification of the CD68 levels for the two groups (n = 9) is shown. *P < 0.05. (D, Upper) Representative images showing myosin heavy chain 2B (green), myosin heavy chain 2A (red), and myosin heavy chain 2X (unstained, black) in an mdx Con and mdx Sim muscle section. (D, Lower) Pooled values for the percent of each fiber type are shown for mdx Con (n = 8), mdx Sim (n = 7), WT Con (n = 5), and WT Sim (n = 5) mice. ***P < 0.001; **P < 0.01 compared with mdx Con. (Scale bar: 200 µm.) (E) Diaphragm force normalized to cross-sectional area (specific force) was measured over a range of stimulation frequencies for mdx Con and mdx Sim mice. **P < 0.01 (n = 9 for both groups). Inset shows representative specific force traces from an mdx Con and mdx Sim mouse, during stimulation at 120 Hz.
The dystrophin homolog utrophin is up-regulated and expressed along the sarcolemma in mdx muscles. An increase in utrophin can partially compensate for the loss of dystrophin and provide protection against muscle damage, making it a therapeutic target for DMD (25). Therefore, we sought to determine whether the beneficial effects of simvastatin were related to enhanced expression of utrophin and/or other members of the dystrophin protein complex (DPC). However, Western blotting results showed no additional increase in the expression levels of utrophin, other members of the dystrophin complex, or associated proteins such as nNOSµ and Caveolin-3 (Fig. S1). These data indicate that the beneficial effects of simvastatin are not attributable to increased expression of utrophin or the dystrophin-associated protein complex.
Fig. S1.
Simvastatin does not increase utrophin or other dystrophin complex proteins in mdx muscle. In these experiments, mice were treated with simvastatin starting from weaning (3 wk of age) for a total of 8 mo. (A) Representative Western blots showing expression levels of utrophin and other members of the DPC in quadriceps muscles from mdx Con and mdx Sim mice. (B) Pooled data showing the expression levels of the proteins shown in A.
Recent evidence has shown that metabolic changes can provide protection against damage in dystrophic muscle. In particular, shifting muscle fiber type from glycolytic type 2B fibers to more oxidative type 2A/X or very oxidative type 1 fibers by pharmacological or genetic approaches reduces muscle damage and improves physiological function (26, 27). Therefore, we measured the fiber type composition of TA muscle sections from mdx Con and mdx Sim mice. As shown in Fig. 1D, compared with mdx Con mice, TA muscles of mdx Sim mice displayed a significant fiber type shift of 15% from fast glycolytic 2B fibers to more oxidative, type 2X fibers. In addition, there was no difference in fiber type composition between mdx Sim and both WT groups (Fig. 1D). Interestingly, voluntary wheel running in mice also causes a fiber-type shift from 2B to 2A/X, which is associated with improved endurance and enhanced oxidative metabolism (28). Therefore, although the mechanism for the fiber type shift in mdx Sim mice is currently unclear, it likely contributes to the improvement in overall muscle health in these mice.
In mdx mice, the diaphragm is the most severely affected skeletal muscle (4), and diaphragm dysfunction is a major cause of respiratory failure in DMD. Therefore, we tested whether simvastatin could improve diaphragm strength in mdx mice. Using isolated diaphragm muscle strips, specific muscle force (force normalized to muscle cross-sectional area) was significantly higher (20–25%) in mdx Sim mice compared with mdx Con, over the full range of stimulation frequencies (Fig. 1E), indicating a robust improvement in diaphragm physiological performance. As expected, diaphragm force of WT mice was significantly higher compared with mdx, but there was no difference between values for WT Con and WT Sim (Fig. S2).
Fig. S2.
Diaphragm-specific force for WT mice. In these experiments, mice were treated with simvastatin starting from weaning (3 wk of age) for a total of 8 mo. Diaphragm force normalized to cross-sectional area (specific force) was measured over a range of stimulation frequencies for WT Con and WT Sim mice (n = 5 for both groups).
Simvastatin Treatment Enhances mdx Hindlimb Muscle Force, Which Correlates with Reduced NADPH Oxidase 2 Expression.
Muscle degeneration and loss of function in DMD begins very early in the disease and progressively worsens over time (3). Consequently, useful therapeutic agents must be effective when administered at various stages of the disease. Therefore, we investigated whether simvastatin could improve muscle function when given several months after the onset of muscle damage in mdx mice. Simvastatin treatment was started when mice were 3 mo of age, and measurements were performed 3 mo later. Hindlimb (TA) muscle physiology was measured in situ. This method has the advantages of direct nerve stimulation and intact blood circulation, which provides an essentially in vivo approach for measuring muscle function. Remarkably, specific muscle force increased by 40% (P < 0.001) for mdx Sim compared with mdx Con mice (Fig. 2 A and B), a dramatic increase for a pharmacological agent. In fact, this 40% increase in specific force with simvastatin is comparable to that provided by the most effective gene-based therapeutic approaches, including a minidystrophin gene therapy construct containing the neuronal NOS (nNOS) binding region (29) and antisense oligonucleotides (exon skipping), which led to homologous expression of a slightly truncated dystrophin protein throughout mdx TA muscle (30). Therefore, our data demonstrate that, as a nongenetic approach, simvastatin provides a substantial improvement in contractile performance of dystrophic muscle. Interestingly, statin treatment also increases skeletal-muscle-specific force in an animal model of hindlimb ischemia (19), again emphasizing the point that statins augment muscle force production in specific disease conditions that are characterized by severe muscle damage and functional impairment.
Fig. 2.

Simvastatin treatment enhances TA muscle force and reduces NOX2 expression in mdx mice. In these experiments, mice were treated with simvastatin from 3 mo up to 6 mo of age. (A) Representative traces showing specific force values during 120-Hz isometric contractions at the optimum muscle length for mdx and WT mice, with or without simvastatin treatment. (B) Pooled values of TA-specific force for mdx Con (n = 6), mdx Sim (n = 6), WT Con (n = 5), and WT Sim (n = 5) mice. ***P < 0.001 compared with mdx Con; ###P < 0.001 compared with both mdx groups. (C) Representative Western blot showing NOX2 expression from TA (Inset) and the pooled values for each group. Values were normalized to GAPDH, which was used as a loading control. **P < 0.01 compared with mdx Con; ***P < 0.001 compared with mdx Con; ###P < 0.001 compared with both mdx groups; ##P < 0.01 compared with WT Con. (D) Values for NOX2 expression plotted against TA-specific force for mdx and WT mice with or without simvastatin treatment. A linear regression line has been fitted to the data (R2 = 0.76, P < 0.001).
Recent evidence by us and others has shown that oxidative stress due to increased reactive oxygen species (ROS) production by NADPH oxidase 2 (NOX2) is a major cause of muscle weakness in mdx mice (5, 31, 32). Because statins are known to inhibit NOX2-derived ROS in the cardiovascular system (33, 34), we measured the NOX2 expression levels in TA muscles. Compared with mdx Con, mdx Sim mice had a significant reduction in NOX2 expression levels. NOX2 levels were also decreased in WT Sim mice, possibly suggesting a common inhibitory effect of simvastatin on skeletal muscle NOX2 protein levels (Fig. 2C). Importantly, there was a strong negative correlation between NOX2 levels and TA-specific force values (R2 = 0.76; P < 0.001, Fig. 2D). These data are consistent with a recent finding showing improved force production by mdx muscle after genetic ablation of a NOX2 regulatory subunit (32).
Simvastatin Improves Resistance to Muscle Fatigue in mdx Mice.
In addition to the loss of specific force, increased muscle fatigue and slowed force recovery are significant causes of muscle weakness in DMD (4, 35). Muscle fatigue in TA was measured during repetitive tetanic contractions (every 2 s) for a total of 2 min. For mdx Con mice, force declined rapidly during the early part of the fatigue and then much less steeply for the remainder of the contractions (Fig. 3A). In contrast, the force decline for mdx Sim was very similar to the WT groups, with a slow force drop over the first minute and then a greater decline over the last minute (see Fig. 3A). After 1 min of fatigue, mdx Sim had significantly greater force than mdx Con mice (68% vs. 53% of initial force, P < 0.05; Fig. 3B). After 2 min, there was no difference between any groups, including WT mice (see Fig. 3A).
Fig. 3.

Simvastatin protects against muscle fatigue and improves force recovery in mdx mice. In these experiments, mice were treated with simvastatin from 3 mo up to 6 mo of age. (A) Representative traces of TA muscle force for mdx and WT mice with or without simvastatin treatment during 2 min of fatiguing contractions (every 2 s). (B) Pooled values of TA force after 1 min of fatigue. **P < 0.01; ***P < 0.001 compared with mdx Con. (C) Pooled values of TA force recovery after fatigue from 2 to 10 min. *P < 0.05; **P < 0.01 for mdx Con vs. all other groups.
Recovery from fatigue was measured up to 10 min after fatigue (Fig. 3C). For mdx Con mice, the average recovery at 2 min was 73% of the initial, prefatigue force, compared with 80.9% for mdx Sim mice. By 10 min, there was no further recovery for mdx Con, but values for mdx Sim mice increased to 86.9% and were significantly different (P < 0.01). Values for mdx Sim mice were not statistically different from both WT groups, indicating that simvastatin normalizes muscle fatigue and recovery to WT levels in dystrophic mice. The cellular mechanisms of muscle fatigue are complex; however, ROS are known to be an important cause of force loss during muscle fatigue (36). As for specific force, we also found that NOX2 levels (see Fig. 2C), negatively correlated with muscle force after 1 min of fatigue (R2 = 0.48, P < 0.001). Therefore, it is likely that reduced ROS production from NOX2 contributes to the improved fatigue resistance in simvastatin-treated mdx mice.
Plasma LDL Cholesterol Is Higher in mdx Mice but Not Reduced by Simvastatin.
In contrast to humans, statins are usually ineffective at reducing the naturally low, circulating LDL cholesterol levels in mice, except when specific genetic and/or dietary changes are made (37). Nevertheless, to determine whether the beneficial effects of simvastatin on TA muscle function were associated with reduced circulating cholesterol levels, we measured the plasma LDL and very-low-density lipoprotein (VLDL) as well as HDL cholesterol concentrations. Interestingly, mdx Con mice had significantly higher LDL/VLDL levels than WT mice, in accordance with elevated serum cholesterol levels in DMD individuals (38). However, simvastatin did not lower LDL/VLDL in mdx or WT mice (Fig. S3A). HDL cholesterol was not significantly different among any groups (Fig. S3B). These data indicate that the improved muscle function in mdx Sim mice is not attributable to a plasma cholesterol-lowering effect of simvastatin.
Fig. S3.
Plasma cholesterol measurements in simvastatin-treated and untreated mdx and WT mice. In these experiments, mice were treated with simvastatin from 3 mo up to 6 mo of age. Plasma LDL and VLDL (LDL/VLDL) and HDL fractions were separated and quantified by using a fluorescent assay. LDL/VLDL concentration (A) and HDL concentration (B) are shown. ***P < 0.001 vs. mdx Con; **P < 0.01 vs. mdx Sim; ###P < 0.001 vs. both mdx groups.
Simvastatin Improves Diaphragm Muscle Function in Old mdx Mice and Reverses Fibrosis.
In mdx mice, the diaphragm most closely recapitulates the functional deficits and fibrotic deposition that occur in DMD (4). Therefore, we treated old mdx mice with simvastatin to determine whether it could improve diaphragm force and reduce or reverse preexisting fibrosis. As with younger mice (see Fig. 1A), old mdx mice treated with simvastatin for 2 mo had significantly lower plasma CK levels, indicating protection against ongoing muscle damage (Fig. 4A). Diaphragm-specific force was also significantly improved by 20–30% over a wide range of stimulation frequencies (10–120 Hz), compared with untreated mdx mice (Fig. 4B).
Fig. 4.
Simvastatin treatment in old mdx mice attenuates muscle damage, improves diaphragm force, and reduces fibrosis. In these experiments, mice were treated with simvastatin starting at 12 mo of age for a total of 2 mo. (A) Whole-body muscle damage in old mice was measured by the levels of plasma CK activity. *P < 0.05 compared with mdx Con. (B) Pooled specific force values of diaphragm muscle strips as measured at different stimulation frequencies for mdx Con and mdx Sim mice. *P < 0.05; **P < 0.01 (n ≥ 6). (C) Representative sections showing connective tissue levels in diaphragm muscles by fibronectin (green) immunostaining. The sarcolemma is outlined by Caveolin3 (red), and nuclei are stained with DAPI (blue). (Scale bar: 100 µm.) (D) Quantification of Fibronectin immunofluorescence from diaphragm muscle cross-sections. **P < 0.01 compared with mdx Con. (E) Collagen I levels in homogenized diaphragm muscles were determined by the Hydroxyproline assay. *P < 0.05 compared with mdx Con.
The replacement of muscle fibers with fibrotic connective tissue is a major cause of impaired muscle force generation in DMD (3) and therefore an important therapeutic target. First, we evaluated fibrosis by fibronectin immunofluorescence of diaphragm sections (Fig. 4C), which revealed a dramatic (50%) attenuation of fibrosis in mdx Sim mice compared with mdx Con (Fig. 4D). Quantification of total collagen I levels in diaphragm muscles by hydroxyproline assay also indicated a 50% reduction in fibrosis for mdx Sim mice (Fig. 4E). Because diaphragm connective tissue deposition is already extensive in mdx mice at this age, our data suggest that simvastatin likely reversed some of the preexisting fibrosis, consistent with findings of statin treatment in fibrotic cardiac muscle (39).
Physiological Concentrations of Simvastatin Do Not Impair Muscle Regeneration or Myogenesis in mdx Muscle.
It has been suggested that statins, including simvastatin, impair muscle regeneration by impeding myoblast differentiation (40). However, the statin concentrations required to induce these deleterious effects in vitro are typically 1 µM or greater. These concentrations are considerably (100–1,000 times) higher than those found in vivo in mice and humans (41). In the present study, we measured the plasma levels of simvastatin in treated mdx mice, which were, on average, 403 ± 108 nM (n = 7). In treated rats, the simvastatin concentration in skeletal muscle relative to plasma is 30% (42). Therefore, we would expect the muscle levels of simvastatin in our mice to be ∼120 nM. At this concentration, we found that muscle regeneration in vivo was unaffected in mdx Sim mice, because they had a comparable number of centrally nucleated (regenerated) muscle fibers to mdx Con mice after long-term (8-mo) treatment (Fig. S4 A and B). We then carried out an in vitro experiment using an immortalized mdx myoblast cell line (kindly provided by Terry Partridge, Children’s National Medical Center, Washington). At the start of differentiation, we treated the cells with a range of simvastatin concentrations for 3 d and found that muscle differentiation, in terms of myotube formation, appeared similar to untreated cells at concentrations ranging from 50 to 500 nM (Fig. S4C). In accord with previous studies, at doses of 1 µM or higher, simvastatin became more toxic, and the number of viable cells progressively decreased over 3 d of treatment. Again, this finding highlights the point that simvastatin concentrations within the normal, in vivo physiological range do not impair myogenesis in dystrophic muscle cells, and deleterious effects only occur with exposure to much higher doses.
Fig. S4.
Simvastatin does not impair muscle regeneration or myogenesis in mdx muscle. (A) Representative images showing Caveolin-3 (green) and DAPI (red) from mdx Con and mdx Sim muscles after 8 mo of simvastatin treatment. In both groups, note the large number of muscle fibers with one or more central nuclei. (Scale bar: 20 µm.) (B) Pooled data showing the number of fibers with central nuclei as a percentage of the total fibers (central and peripheral nuclei). (C) Representative H&E images of differentiated myotubes, after 3 d of treatment without or with simvastatin concentrations ranging from 0.05 to 0.5 µM. (Scale bar: 50 µm.)
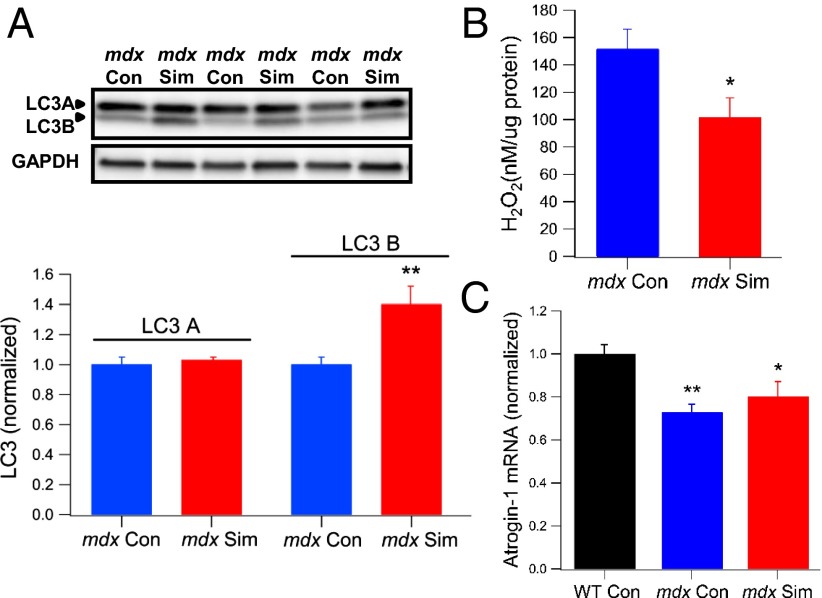
Simvastatin Enhances Autophagy and Reduces Oxidative Stress but Does Not Simulate Atrogin-1 in mdx Muscle.
Autophagy is an important cellular pathway for degrading damaged proteins, organelles, and protein aggregates, which are then recycled by the cell for energy use. Impaired autophagy can trigger cell death, and in skeletal muscle, proper regulation of autophagy is essential for normal cellular and physiological function (43). Both mdx and DMD muscles show evidence of reduced autophagy, and treatment of mdx mice with a low-protein diet triggered autophagy, which reduced inflammation and fibrosis and enhanced muscle function (43). Simvastatin was recently shown to enhance autophagy in arterial myocytes (44), and therefore, we postulated that increased autophagy might contribute to the improved muscle health in mdx mice. A key protein marker of autophagic flux is the microtubule-associated protein 1A/1B light chain 3 (LC3), which has a cytosolic form (LC3A) and a lipidated form (LC3B). An increased level of LC3B relative to LC3A is indicative of enhanced autophagic flux (43). We measured LC3A and LC3B expression in the diaphragm of simvastatin-treated and untreated mdx mice, using an antibody that detects both protein isoforms. As shown in Fig. 5A, the levels of LC3A were not different between the groups; however, LC3B was significantly increased by 40% in mdx Sim mice (P < 0.01), signifying enhanced autophagy. This result is consistent with the increased levels of LC3B by simvastatin in arterial myocytes (44).
Fig. 5.
Simvastatin treatment in old mdx mice enhances autophagy, attenuates ROS levels, and does not induce atrogin-1. In these experiments, mice were treated with simvastatin starting at 12 mo of age for a total of 2 mo. (A, Upper) Western blot showing the levels of the autophagy proteins LC3A (upper band) and LC3B (lower band) for mdx Con and mdx Sim. GAPDH is shown as a loading control. (A, Lower) Pooled data for LC3A and LC3B are shown. **P < 0.01 compared with mdx Con. (B) Hydrogen peroxide (H2O2) levels in diaphragm muscle homogenates, as quantified with a fluorescent amplex red assay. *P < 0.05 compared with mdx Con. (C) Atrogin-1 mRNA levels in quadriceps muscles were quantified by qPCR and normalized to the internal control (HPRT). *P < 0.05; **P < 0.01 compared with WT Con.
Interestingly, recent evidence revealed that NOX2-derived ROS play a key role in reducing autophagy in mdx muscle (32). Therefore, our data showing reduced NOX2 expression by simvastatin is consistent with its autophagy enhancement of dystrophic muscle. To further explore this idea, we also measured the levels of the ROS (H2O2) in diaphragm muscle homogenates. We found that mdx Sim muscles had ∼30% less H2O2 compared with mdx Con (Fig. 5B), indicative of reduced oxidative stress. This finding highlights important differences between dystrophic muscle, where statins reduce oxidative stress derived from NOX2, and normal muscle susceptible to statin myopathy, where mitochondrial ROS increases oxidative stress (12).
Another pathway that has been implicated in statin myopathy is muscle atrophy mediated by atrogin-1, a ubiquitin-protein ligase that stimulates protein breakdown (13). Atrogin-1 is induced by statin treatment in animal models and humans with statin-induced myopathy (13). However, in DMD, atrogin-1 levels are consistently lower than in normal muscle and are not increased at any stage of the disease (21). We quantified mRNA levels of atrogin-1 by real-time quantitative RT-PCR (qPCR) in quadriceps muscles of old mice and also found that levels in mdx Con were significantly lower than for WT (Fig. 5C). Interestingly, values for mdx Sim mice were also lower than WT and not significantly different from mdx Con (Fig. 5C). These data indicate that atrogin-1 is not induced in dystrophic muscle by simvastatin. Again, this finding emphasizes the opposite effect of simvastatin on a pathogenic pathway in dystrophic skeletal muscle compared with normal muscle.
Conclusions
In summary, to our knowledge, our results reveal for the first time that treatment of dystrophic mdx mice with simvastatin provides a dramatic reduction in inflammation, oxidative stress, and fibrosis, key pathogenic pathways that mediate skeletal muscle damage and functional impairment in DMD. Most importantly, these mechanistic effects translated into a substantial improvement in skeletal muscle physiological function, both in terms of specific force production and protection from muscle fatigue. Although our results may initially seem unexpected, based on the general perception that statins can be myotoxic, they are accordant with extensive evidence demonstrating statin-mediated inhibition of these pathogenic pathways in both the cardiovascular system and ischemic skeletal muscle. Thus, our data are consistent with the idea that statins are highly beneficial to skeletal muscles afflicted with an underlying disease that involves ischemia, oxidative stress, and inflammation. Further studies are now required to delve into the cellular and molecular mechanisms that mediate these positive effects. From a clinical perspective, several statins, including simvastatin, are already FDA-approved for the treatment of familial hypercholesterolemia in children as young as 10 y of age. Thus, our novel findings indicate that simvastatin and possibly other statins have great potential to provide a readily available therapy for DMD and related neuromuscular diseases.
Methods
Detailed methods can be found in SI Methods. Details of assays for Western blotting, immunostaining, hydroxyproline, RT-PCR, and plasma CK and cholesterol are available in SI Methods. Protocols for simvastatin treatment, muscle function, cell culture, and plasma simvastatin measurements are also available in SI Methods.
Male dystrophin-deficient (mdx) and WT mice on the C57BL/10ScSn background were used for all experiments, which were approved by the Institutional Animal Care and Use Committee at the University of Washington.
SI Methods
Simvastatin Treatment.
Simvastatin powder (TCI America) was formulated as a treatment in two ways: in the drinking water and in the food. The inactive form of simvastatin contains a lactone group and is relatively insoluble in water. Therefore, for the drinking-water experiments (see data for Fig. 1), simvastatin (50 mg) was initially dissolved in 1 mL of ethanol and then mixed for several minutes into 1 L of alkaline water (pH ∼10), which hydrolyzes the lactone group, enabling the active (hydroxy acid) form of simvastatin to be solubilized in water. Hydrochloric acid was then added to lower the pH to ∼2.5–3.0, which was the pH of the drinking water given to the control (untreated) mice. For formulation into the food (see data for Figs. 2–4), simvastatin (lactone form) was mixed into a standard rodent diet (D12450B) at a concentration of 80 mg/kg (Research Diets).
Skeletal Muscle Contractile Function.
Diaphragm function, ex vivo, was measured as described (4). Briefly, diaphragm strips (∼2–3 mm wide) were perfused with a physiological solution bubbled with 95% O2/5% CO2. Stimulation was provided by two platinum electrodes, attached along the sides of the chamber. A length-force curve, established by using 120-Hz isometric contractions (300-ms duration) spaced 1 min apart, established the optimum length (length producing maximum tetanic force), which was later used to calculate the specific force (force divided by cross-sectional area) (4).
TA muscle function was measured in situ. Mice were anesthetized with an i.p. injection of Avertin (625 mg/kg) and placed on a heated metal platform (∼37 °C). The distal TA tendon was dissected free and sutured to the lever arm of the force and length control system (305C-LR; Aurora Scientific). Muscle contraction was provided by stimulation of the sciatic nerve using bipolar electrodes, which were kept moist during the experiment with PBS (pH 7.4). The knee bone was firmly anchored to the platform via a steel pin. A length-force curve was generated by stimulating the muscle at 120 Hz (200 ms duration), every 90 s, from short to long muscle lengths. Isometric force at the optimum length was used to calculate the specific force, by dividing force by cross-sectional area, as described (4). After establishing the optimum length, the muscle then underwent a fatigue protocol, in which it was stimulated at 120 Hz (200-ms duration) every 2 s for a total of 2 min. Fatigue recovery was measured every 2 min up to 10 min after fatigue.
Western Blotting.
Western blotting was performed as described (5). Briefly, muscles were homogenized in a PBS buffer (pH 7.4) containing EDTA (5 mM), protease (Thermo Scientific) and phosphatase (Roche) inhibitor mixtures, and 1% Triton X-100. Samples were loaded onto 4–15% gradient gels (Bio-Rad) and transferred on PVDF membranes (Millipore). Membranes were blocked for 1–2 h with 5% (wt/vol) skim milk in PBS containing 0.1% Tween 20 (PBST) (pH 7.4) or 2.5% (wt/vol) BSA in PBST for phosphorylated proteins and then incubated with primary antibodies in blocking buffer for 1 h at room temperature or overnight at 4 °C. Primary antibodies used were as follows: NOX2, α-dystrobrevin 1 and 2, and caveolin 3 (BD Biosciences); LC3A/B (Bio-Rad), utrophin and α-syntrophin (noncommercial antibodies from Froehner laboratory); nNOS (Invitrogen); β-dystroglycan (Novocastra); and GAPDH (Santa Cruz Biotechnology). HRP-labeled secondary antibodies were then incubated for 1 h at room temperature. Membranes were incubated with enhanced chemiluminescence reagent (Amersham), and bands were detected by the FluorChem M imaging system (Protein Simple).
Immunofluorescence Images.
Muscles were imbedded in optimal cutting temperature compound and frozen in isopentane cooled in liquid nitrogen. Cryosections (10-µm thick) were placed on a glass slide. Sections were fixed with either ice-cold methanol or 2% (vol/vol) paraformaldehyde for 5–10 min. Some sections were stained with hematoxylin and eosin (H&E) using standard procedures. For immunofluorescence, sections were incubated in blocking buffer (0.8% BSA and 1% fish gelatin in PBS) for 45 min. Primary antibodies—Fibronectin (Sigma); CD68 (Abcam); Caveolin-3 (BD Biosciences); and myosin heavy chain 1, 2A, and 2B (Developmental Studies Hybridoma Bank)—were added for 1 h and 30 min at room temperature. Alexa Fluor-conjugated secondary antibodies (Life Technologies) were then added for 1 h and 30 min. In some experiments, DAPI was also added to detect nuclei. Sections were mounted with anti-fade Gold reagent (Life Technologies), coverslipped, and imaged with a Zeiss LSM510 confocal microscope (W. M. Keck Center, University of Washington) or a Zeiss Axioscop 2 fluorescent microscope. For fluorescence quantification (CD68, fibronectin, and myosin heavy chains), images were converted to grayscale in ImageJ, and a threshold was applied to calculate the area of fluorescence as a percent of the total area of the section. Fibers with central nuclei were expressed as a percent of total fibers with detectable nuclei (i.e., both central and peripheral).
Hydroxyproline Collagen I Fibrosis Assay.
To quantify muscle fibrosis, Collagen I content was measured by using the hydroxyproline assay. Muscles were homogenized in the same PBS–Triton X-100 buffer (pH 7.4) used for Western blotting. Samples were centrifuged at 9,500 × g for 10 min. The pellet was used for hydroxyproline measurement. The supernatant was used to measure the total protein concentration (BCA assay) for later normalizing hydroxyproline values. The insoluble pellets, containing Collagen I, were placed into glass tubes with 200 µL of 6M HCl. Samples were boiled for 24 h at 120 °C in a heat block. After vortexing and centrifuging at 9,500 × g for 3 min, the supernatants were used for the hydroxyproline assay, using a commercial kit (Chondrex). Hydroxyproline values (micrograms per microliter) were normalized to the total protein content of the muscle.
Plasma CK Activity.
Plasma CK was evaluated as a measure of whole-body muscle damage. Blood was drawn via submandibular puncture, collected into EDTA-coated tubes (BD Microtainer), and centrifuged at 2,400 × g for 12 min at 4 °C. Plasma was then used to measure CK activity with a standard assay kit, according to the manufacturer’s instructions (StanBio).
Cell Culture Experiments.
Immortomouse mdx myoblasts were kindly provided by Terry Partridge (Children’s National Medical Center). Myoblasts were grown on gelatin-coated plates and until ∼70% confluence in DMEM supplemented with 10% (vol/vol) horse serum (HS), 20% (vol/vol) FBS, 0.5% chicken embryo extract, and 20 units/mL of γ-IFN. To induce differentiation into myotubes, cells were incubated with DMEM containing 5% (vol/vol) HS, with or without different concentrations of simvastatin for 3 d. Myotubes were washed in PBS (pH 7.4), fixed in ice-cold methanol for 10 min, and H&E-stained. Images of myotubes were taken with a Nikon inverted microscope (W. M. Keck Center, University of Washington).
Real-Time PCR.
Total RNA was isolated from quadriceps of WT, mdx, and simvastatin-treated mdx mice by using TRIzol (Life Technologies) and further purified by using an RNAeasy Kit (Qiagen). qPCR was performed by using TaqMan chemistry and the ABI 7000 sequence detection system with 50 ng of RNA, ABI Fast Virus 1-Step Master Mix reagents, and primer sets specific for atrogin-1 and HPRT (Applied Biosystems). Data were obtained from five mice in each treatment group and normalized to the internal HPRT control.
Plasma Cholesterol Measurements.
By using a commercial assay kit (Abcam), LDL/VLDL and HDL fractions were separated from plasma and mixed with an enzyme reaction solution containing a fluorescent probe to quantify the amount of cholesterol in the sample. Fluorescence levels were measured with a spectrophotometer in a 96-well plate. Cholesterol values for each sample were determined from a cholesterol standard curve.
Simvastatin Concentrations in mdx Mouse Plasma.
Simvastatin concentrations in the plasma of mdx mice treated from 3 to 6 mo of age were measured and analyzed by Josefin Koehn and Rodney Ho (Department of Pharmaceutics, University of Washington). Simvastatin was extracted from plasma samples with methanol and cyheptamide. Briefly, 20 μL of plasma was mixed with 50 μL of methanol and 5 μL of cyheptamide (250 μg/mL in methanol) was added. Samples were vortexed for 2 min, followed by centrifuging for 10 min at 18,600 × g. The supernatant was collected and used for analysis by HPLC/UV. The separation was performed on a Zorbax XDB-C8 column (50 × 2.1 mm; particle size, 5 μm) (Agilent). The mobile phase consisted of A, H2O; and B, MeOH. The flow rate was set to 0.6 mL/min, and simvastatin was detected at a UV wavelength of 238 nm. Plasma samples from untreated mice were used as a negative control and for setting up the standard curve.
Statistical Analysis.
All data are presented as means ± SEM. The significance level for all experiments was set at P < 0.05. Unpaired Student’s t test was used to compare values between two groups. One-way ANOVA with least significant difference post hoc test was used for analysis of more than two groups. For analysis of CD68 immunostaining, the nonparametric Mann–Whitney u test was applied. For some variables, a linear regression analysis was performed. The statistical program used was Data Desk.
Acknowledgments
We thank Prof. David Allen (University of Sydney) for his contribution to preliminary experiments that initiated the research carried out in this paper; Prof. William Catterall, Prof. Fernando Santana, and Prof. David Dichek for their helpful comments; and Prof. Terry Partridge for providing the immortomouse mdx myoblasts. Part of this research was conducted while N.P.W. was a recipient of the Weisman Postdoctoral Fellowship (Parent Project Muscular Dystrophy). M.J.K. was supported by National Heart, Lung, and Blood Institute Cardiovascular Training Grant T32HL007828. Research reported in this publication was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke Awards 1P01NS046788 (to S.C.F.) and 1R21NS088691 (to N.P.W. and S.C.F.); and by the Raymond and Beverly Sackler Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509536112/-/DCSupplemental.
References
- 1.Malik V, Rodino-Klapac LR, Mendell JR. Emerging drugs for Duchenne muscular dystrophy. Expert Opin Emerg Drugs. 2012;17(2):261–277. doi: 10.1517/14728214.2012.691965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman EP, McNally EM. Exon-skipping therapy: A roadblock, detour, or bump in the road? Sci Transl Med. 2014;6(230):230fs14. doi: 10.1126/scitranslmed.3008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desguerre I, et al. Endomysial fibrosis in Duchenne muscular dystrophy: A marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol. 2009;68(7):762–773. doi: 10.1097/NEN.0b013e3181aa31c2. [DOI] [PubMed] [Google Scholar]
- 4.Percival JM, et al. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol. 2012;228(1):77–87. doi: 10.1002/path.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehead NP, Yeung EW, Froehner SC, Allen DG. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS One. 2010;5(12):e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonopoulos AS, Margaritis M, Shirodaria C, Antoniades C. Translating the effects of statins: From redox regulation to suppression of vascular wall inflammation. Thromb Haemost. 2012;108(5):840–848. doi: 10.1160/TH12-05-0337. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, et al. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation. Arterioscler Thromb Vasc Biol. 2013;33(7):1591–1600. doi: 10.1161/ATVBAHA.112.300922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168(1):6–15. doi: 10.1016/j.ahj.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Braamskamp MJ, et al. Long-term statin treatment in children with familial hypercholesterolemia: More insight into tolerability and adherence. Paediatr Drugs. 2015;17(2):159–166. doi: 10.1007/s40272-014-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Gorman CS, Higgins MF, O’Neill MB. Systematic review and metaanalysis of statins for heterozygous familial hypercholesterolemia in children: Evaluation of cholesterol changes and side effects. Pediatr Cardiol. 2009;30(4):482–489. doi: 10.1007/s00246-008-9364-3. [DOI] [PubMed] [Google Scholar]
- 11.Bhardwaj S, Selvarajah S, Schneider EB. Muscular effects of statins in the elderly female: A review. Clin Interv Aging. 2013;8:47–59. doi: 10.2147/CIA.S29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouitbir J, et al. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: A ‘mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur Heart J. 2012;33(11):1397–1407. doi: 10.1093/eurheartj/ehr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanai J, et al. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117(12):3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knoblauch M, Dagnino-Acosta A, Hamilton SL. Mice with RyR1 mutation (Y524S) undergo hypermetabolic response to simvastatin. Skelet Muscle. 2013;3(1):22. doi: 10.1186/2044-5040-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiello FA, et al. Statin therapy is associated with superior clinical outcomes after endovascular treatment of critical limb ischemia. J Vasc Surg. 2012;55(2):371–379, discussion 380. doi: 10.1016/j.jvs.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 16.El-Azab MF, Hazem RM, Moustafa YM. Role of simvastatin and/or antioxidant vitamins in therapeutic angiogenesis in experimental diabetic hindlimb ischemia: Effects on capillary density, angiogenesis markers, and oxidative stress. Eur J Pharmacol. 2012;690(1-3):31–41. doi: 10.1016/j.ejphar.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Köksoy C, et al. Simvastatin pretreatment reduces the severity of limb ischemia in an experimental diabetes model. J Vasc Surg. 2007;45(3):590–596. doi: 10.1016/j.jvs.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 18.Cowled PA, et al. Statins inhibit neutrophil infiltration in skeletal muscle reperfusion injury. J Surg Res. 2007;141(2):267–276. doi: 10.1016/j.jss.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Dillon JP, et al. Pravastatin attenuates tourniquet-induced skeletal muscle ischemia reperfusion injury. Acta Orthop. 2006;77(1):27–32. doi: 10.1080/17453670610045669. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586(7):2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YW, et al. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65(6):826–834. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- 22.Asai A, et al. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One. 2007;2(8):e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shabanian R, et al. Myocardial performance index and atrial ejection force in patients with Duchenne’s muscular dystrophy. Echocardiography. 2011;28(10):1088–1094. doi: 10.1111/j.1540-8175.2011.01515.x. [DOI] [PubMed] [Google Scholar]
- 24.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 25.Miura P, Jasmin BJ. Utrophin upregulation for treating Duchenne or Becker muscular dystrophy: How close are we? Trends Mol Med. 2006;12(3):122–129. doi: 10.1016/j.molmed.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Ljubicic V, et al. Chronic AMPK activation evokes the slow, oxidative myogenic program and triggers beneficial adaptations in mdx mouse skeletal muscle. Hum Mol Genet. 2011;20(17):3478–3493. doi: 10.1093/hmg/ddr265. [DOI] [PubMed] [Google Scholar]
- 27.Reyes NL, et al. Fnip1 regulates skeletal muscle fiber type specification, fatigue resistance, and susceptibility to muscular dystrophy. Proc Natl Acad Sci USA. 2015;112(2):424–429. doi: 10.1073/pnas.1413021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Röckl KS, et al. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56(8):2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- 29.Lai Y, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119(3):624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godfrey C, et al. How much dystrophin is enough: The physiological consequences of different levels of dystrophin in the mdx mouse. Hum Mol Genet. 2015;24(15):4225–4237. doi: 10.1093/hmg/ddv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khairallah RJ, et al. Microtubules underlie dysfunction in Duchenne muscular dystrophy. Sci Signal. 2012;5(236):ra56. doi: 10.1126/scisignal.2002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal R, et al. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun. 2014;5:4425. doi: 10.1038/ncomms5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalaklioglu S, Sahin P, Tasatargil A, Celik-Ozenci C. Pravastatin improves the impaired nitric oxide-mediated neurogenic and endothelium-dependent relaxation of corpus cavernosum in aged rats. Aging Male. 2014;17(4):259–266. doi: 10.3109/13685538.2013.832194. [DOI] [PubMed] [Google Scholar]
- 34.Pignatelli P, et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation. 2012;126(1):92–103. doi: 10.1161/CIRCULATIONAHA.112.095554. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi YM, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456(7221):511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37 degrees C. J Physiol. 2005;564(Pt 1):189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zadelaar S, et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27(8):1706–1721. doi: 10.1161/ATVBAHA.107.142570. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava NK, Pradhan S, Mittal B, Gowda GA. High resolution NMR based analysis of serum lipids in Duchenne muscular dystrophy patients and its possible diagnostic significance. NMR Biomed. 2010;23(1):13–22. doi: 10.1002/nbm.1419. [DOI] [PubMed] [Google Scholar]
- 39.Patel R, et al. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation. 2001;104(3):317–324. doi: 10.1161/hc2801.094031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baba TT, Nemoto TK, Miyazaki T, Oida S. Simvastatin suppresses the differentiation of C2C12 myoblast cells via a Rac pathway. J Muscle Res Cell Motil. 2008;29(2-5):127–134. doi: 10.1007/s10974-008-9146-9. [DOI] [PubMed] [Google Scholar]
- 41.Björkhem-Bergman L, Lindh JD, Bergman P. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br J Clin Pharmacol. 2011;72(1):164–165. doi: 10.1111/j.1365-2125.2011.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sidaway J, et al. Statin-induced myopathy in the rat: Relationship between systemic exposure, muscle exposure and myopathy. Xenobiotica. 2009;39(1):90–98. doi: 10.1080/00498250802585539. [DOI] [PubMed] [Google Scholar]
- 43.De Palma C, et al. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis. 2012;3:e418. doi: 10.1038/cddis.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei YM, et al. Enhancement of autophagy by simvastatin through inhibition of Rac1-mTOR signaling pathway in coronary arterial myocytes. Cell Physiol Biochem. 2013;31(6):925–937. doi: 10.1159/000350111. [DOI] [PMC free article] [PubMed] [Google Scholar]