Most aerobic photosynthetic organisms have enough light-harvesting capacity so that on bright sunny days their photosynthesis is saturated for the major part of the day (1, 2). Early on in such sunny days the capacity of the reaction centers to process the absorbed solar energy is exceeded. The excess absorbed photons are potentially very dangerous. For example, if the chlorophyll-excited singlet states created in the antenna complexes cannot be used productively by the reaction centers, then these singlet states can potentially last long enough to undergo intersystem crossing to produce triplets. Excited chlorophyll triplet states can interact with molecular oxygen to produce very dangerous products, such as singlet oxygen (3). Singlet oxygen is an extremely powerful oxidizing molecule that can irreversibly damage lipids and, indeed, most major biological polymers. Cells exposed to singlet oxygen are rapidly killed (4). Oxygenic phototrophs have evolved a process, called nonphotochemical quenching (NPQ), to mitigate this problem of overexcitation (5). These phototrophs are able to control the efficiency of their light-harvesting systems. Under nonsaturating incident light intensities, the antenna complexes work as efficient light-harvesters, transferring absorbed solar energy effectively to the reaction centers. As incident light intensities become saturating, the antenna complexes switch to a quenched state, where the lifetime of their excited singlet states are strongly reduced and the efficiency of energy transfer to the reaction centers is much lower. The excess absorbed light-energy is effectively detoxified and converted harmlessly into heat.
The process of NPQ has been extensively studied in plants and algae, where the major light-harvesting complexes are integral membrane chlorophyll-containing pigment-protein complexes (6). Cyanobacteria also show NPQ, but their major light-harvesting proteins are the water-soluble phycobiliproteins, where the light absorbing pigments are bilins (linear tetrapyroles) rather than chlorophylls (7). So how does NPQ work in this very different type of light-harvesting system? The answer involves a protein called the orange carotenoid protein (OCP) (8). Under nonsaturating incident light intensities, OCPO remains orange and the phycobilisome functions as an efficient antenna system. When incident light becomes saturating, the excess light switches OCPO into its active red form, called OCPR. OCPR interacts with the allophycocyanin molecules at the base of the phycobilisome to strongly accelerate the decay of the excited singlet states of the allophycobilin’s bilin pigments, thereby shutting down the efficiency of energy transfer from the phycobilisome to the photosynthetic reaction centers in the membrane (Fig. 1B) (6). It is as if OCPR can close a “tap” at the end of the light-harvesting funnel to reduce the flow of energy through the funnel to the reaction centers in the photosynthetic membrane. The important question addressed in the paper by Gupta et al. (9) is: What are the structural changes occurring in OCP that underlie this form of NPQ?
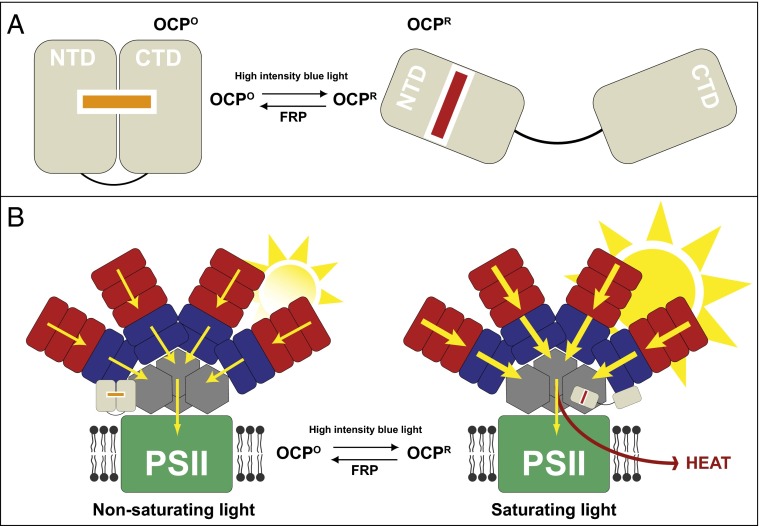
Fig. 1.
(A) Schematic representation of the reversible light-induced conversion of OCP from the inactive OCPO globular form to the active OCPR elongated form by intense blue light. OCPO contains two domains, the NTD and the CTD, which are bolted together by the carotenoid molecule. Upon conversion to OCPR, the carotenoid moves into the NTD and the CTD dissociates. (B) A schematic model for how OCP induces NPQ in cyanobacterial phycobilisomes. Under nonsaturating conditions, light energy (yellow arrows) is harvested by (red) phycoerythrin and (blue) phycocyanin pigment-protein molecules and transferred to photosystem II (PSII) via (gray) allophycocyanin. The OCPO protein is inactive and in a more globular form. When too much light is present OCPO is converted to OCPR. The NTD domain of OCPR can now bind to allophycocyanin and induce NPQ. Now the excess light energy is dissipated as heat, reducing the flow of energy to PSII, thereby preventing damage to the system. OCPR is converted back to OCPO by the fluoresence recovery protein (FRP).
Previously, the groups of Kerfeld and Kirilovsky have collaborated to determine the X-ray crystal structures of both OCPO (10) and OCPR (11). However, they could only determine the structure of part of OCPR because the complete protein, in this form, has up until now been resistant to attempts to crystallize it. The result of comparison between these two OCP crystal structures shows that upon conversion of OCPO to OCPR the carotenoid moves by about 12 Å. In OCPO the two main structural domains, the N-terminal domain (NTD) and the C-terminal domain (CTD), are “bolted” together by the carotenoid, whose binding site extends into both domains (Fig. 1A). On conversion to OCPR the carotenoid “bolt” is withdrawn into the NTD. This then releases the CTD and a short N-terminal helix. This transition is illustrated in Fig. 1A. The absorption spectra of carotenoids are very sensitive to the polarizability of the solvent (12). The shift in color from orange to red just reflects the change in the polarizability of the carotenoid’s binding site during the conversion to OCPO to OCPR. Although it is clear that light absorbed by the carotenoid is responsible for triggering the conversion of OCPO to OCPR, the photochemical mechanism that results in the large-scale reorganization of the carotenoid remains unclear, especially because this light-driven reaction has a low quantum yield. As shown in Fig. 1A, the back reaction that converts OCPR into OCPO is catalyzed by a protein called the fluorescence recovery protein (13). The model proposed to account for how OCP is activated by intense blue light to produce OCPR was based on a comparison of the two crystal structures. However, because the structure of OCPR was only a structure of part of the molecule, there was still some uncertainty that all of the structural changes had been fully described. The results presented in the Gupta et al. report (9) address these uncertainties.
Gupta et al. (9) show very clearly, from small angle X-ray scattering data, that using the full-length OCPO, light-activation to produce OCPR is accompanied by structural changes that convert a compact globular protein into an elongated tubular conformation.
The Gupta et al. report now provides a clear structural basis for the detailed molecular mechanism of how cyanobacterial NPQ is determined.
This structural change is consistent with the crystallographic data and reflects the dissociation of the NTD and the CTD, as shown in Fig. 1A. Gupta et al. also used two separate techniques (X-ray radiolytic labeling with mass spectometry and hydrogen–deuterium exchange mass spectrometry) to show how this gross structural transition is accompanied by local changes in the organization of water molecules at the surface of OCP. The authors suggest that part of the way in which the result of “unbolting” the carotenoid is transmitted to the surface of the NTD is because of changes in chains of bound water molecules. Water often plays key roles in protein–protein interactions and it is intriguing to speculate that changes in the bound water molecules might play a major part in the subsequent interaction between the NTD and the phycobilisome. The Gupta et al. (9) report now provides a clear structural basis for the detailed molecular mechanism of how cyanobacterial NPQ is determined. One can even dream of seeing a structure of OCPR bound to the phycobilisome, where structural changes in the phycobilisomes could be correlated with precise measurements of fluorescence lifetimes, thereby fully allowing the process of NPQ to be visualized. The Gupta et al. report now makes this a real possibility.
Footnotes
The authors declare no conflict of interest.
See companion article on page E5567.
References
- 1.Niyogi KK. Photoprotection revisited: Genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 2.Ruban AV. The Photosynthetic Membrane: Molecular Mechanisms and Biophysics of Light-Harvesting. Wiley; Chichester, United Kingdom: 2013. [Google Scholar]
- 3.Foote CS, Shook FC, Abakerli RB. Characterization of singlet oxygen. Methods Enzymol. 1984;105:36–47. doi: 10.1016/s0076-6879(84)05006-0. [DOI] [PubMed] [Google Scholar]
- 4.Krinsky NI. The protective function of carotenoid pigments. In: Giese AC, editor. Phytophysiology: Current Topics. Vol 3. Academic; New York: 1968. pp. 123–195. [Google Scholar]
- 5.Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 6.Müller P, Li XP, Niyogi KK. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001;124(4):1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossmann AR, Schaefer MR, Chiang GG, Collier JL. The phyocbilisome, a light-harvesting complex responsive to environmental conditions. Microbiol Rev. 1993;57(3):725–749. doi: 10.1128/mr.57.3.725-749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt TK, Krogman DW. A carotenoid-protein from cyanobacteria. Biochim Biophys Acta. 1981;637(3):408–414. [Google Scholar]
- 9.Gupta S, et al. Local and global structural drivers for the photoactivation of the orange carotenoid protein. Proc Natl Acad Sci USA. 2015;112:E5567–E5574. doi: 10.1073/pnas.1512240112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson A, et al. Structural determinants underlying photoprotection in the photoactive orange carotenoid protein of cyanobacteria. J Biol Chem. 2010;285(24):18364–18375. doi: 10.1074/jbc.M110.115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leverenz RL, et al. Photosynthesis. A 12 Å carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science. 2015;348(6242):1463–1466. doi: 10.1126/science.aaa7234. [DOI] [PubMed] [Google Scholar]
- 12.Andersson PO, Gillbro T, Ferguson L, Cogdell RJ. Absorption spectral shifts of carotenoids related to medium polarizability. Photochem Photobiol. 1991;5(3):353–360. [Google Scholar]
- 13.Sutter M, et al. Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria. Proc Natl Acad Sci USA. 2013;110(24):10022–10027. doi: 10.1073/pnas.1303673110. [DOI] [PMC free article] [PubMed] [Google Scholar]