Significance
Regulation of protein activity by redox state changes of a disulfide bond between two intrinsic cysteine residues requires both reductive and oxidative signals, yet very little is known of the oxidative pathway. Deciphering the oxidative pathway is further complicated by an increased level of reactive oxygen species when plants are stressed. Here, we show that the chloroplastic atypical thioredoxin (ACHT4) participates under homeostasis conditions in an oxidative signal that diminishes AGPase activity, the first committed enzyme of starch synthesis, during the transition from day to night and attenuates AGPase activity in a dynamic response to small fluctuations in light intensity during the day.
Keywords: oxidative signal, homeostasis, light intensity regulation, starch synthesis, chloroplast
Abstract
The regulatory mechanisms that use signals of low levels of reactive oxygen species (ROS) could be obscured by ROS produced under stress and thus are better investigated under homeostatic conditions. Previous studies showed that the chloroplastic atypical thioredoxin ACHT1 is oxidized by 2-Cys peroxiredoxin (2-Cys Prx) in Arabidopsis plants illuminated with growth light and in turn transmits a disulfide-based signal via yet unknown target proteins in a feedback regulation of photosynthesis. Here, we studied the role of a second chloroplastic paralog, ACHT4, in plants subjected to low light conditions. Likewise, ACHT4 reacted in planta with 2-Cys Prx, indicating that it is oxidized by a similar disulfide exchange reaction. ACHT4 further reacted uniquely with the small subunit (APS1) of ADP-glucose pyrophosphorylase (AGPase), the first committed enzyme of the starch synthesis pathway, suggesting that it transfers the disulfides it receives from 2-Cys Prx to APS1 and turns off AGPase. In accordance, ACHT4 participated in an oxidative signal that quenched AGPase activity during the diurnal transition from day to night, and also in an attenuating oxidative signal of AGPase in a dynamic response to small fluctuations in light intensity during the day. Increasing the level of expressed ACHT4 or of ACHT4ΔC, a C terminus-deleted form that does not react with APS1, correspondingly decreased or increased the level of reduced APS1 and decreased or increased transitory starch content. These findings imply that oxidative control mechanisms act in concert with reductive signals to fine tune starch synthesis during daily homeostatic conditions.
The ever-fluctuating environment in nature has driven the nonmotile plants to evolve homeostasis control mechanisms for optimal growth. Homeostasis regulation is important during the diurnal transitions from night to day and day to night, when photosynthesis commences or diminishes, correspondingly, and concomitantly chloroplast metabolism is drastically reconfigured. Similarly, rapid changes in light intensity throughout the day serve as environmental cues fine tuning such processes. Perhaps because of the short response time required to keep photosynthesis-derived reactive oxygen species (ROS) from becoming harmful, plants use redox signals as a direct and dynamic means of regulating multiple posttranscriptional events and alleviating damage (1–7).
For a redox change of a protein to function as a regulatory signal, it must meet certain criteria: The half-life of the signal should be long enough to elicit a response, and, for reversible regulation, the signal has to be quenched by a counteracting activity (8). Although the activating reductive pathway of the chloroplast has been extensively investigated, very little is known of the oxidative pathway. In the reductive regulatory pathway, a small fraction of photosynthetic reducing equivalents are channeled for regulatory purposes from photosystem I to several chloroplast thioredoxins (Trxs). The Trxs subsequently reduce disulfide bonds in regulatory allosteric sites of target chloroplast proteins, thereby modulating their activity (5). It is important to note that, to perceive the Trx reductive signal, the allosteric disulfide site of its target protein must be in an oxidized form (8), which is also the form required to turn off the protein activity at the end of day.
During the day, a portion of plant photosynthates accumulate in the chloroplasts as transitory starch, which is used at night for respiration and export of reduced carbon to sink organs (9, 10). Starch turnover is regulated such that it is almost consumed by dawn, thereby maximizing investment in growth, while avoiding reduced carbon starvation. Starch synthesis inhibition at night is required to avoid a futile cycle of degradation and resynthesis (9, 10). AGPase, which catalyzes the first committed step in the starch synthesis pathway, is allosterically regulated by its substrate, 3-phosphoglycerate, and inhibited by its product, orthophosphate (11). The small subunit of Arabidopsis AGPase (APS1) is redox-activated both in the light and in response to sucrose accumulation, via reversible reduction of an intermolecular disulfide between two small subunits (12–15). Light-dependent activation of APS1 presumably depends on ferredoxin-dependent reduction of Trx-f1 (16). The NADPH-dependent Trx reductase C (NTRC) has been implicated in the sugar-induced redox activation of AGPase (17). In leaves, these mechanisms and additional mechanisms integrate and coordinate end-product synthesis with the rate of CO2 assimilation and control the partitioning of photoassimilates between sucrose and starch. The combined regulation results in a homeostatic mechanism that balances immediate export for growth of sink organs with the need to retain adequate reserves in the leaf to last through the night (9, 18).
The Arabidopsis thaliana atypical cysteine histidine-rich Trxs (ACHTs) constitute a small family of plant-specific and chloroplast-localized Trxs that have an atypical redox-active site, and a less reducing redox midpoint potential than that of canonical chloroplast Trxs, and that were shown to be good catalysts of 2-Cys Prx reduction, but not of malate dehydrogenase, in vitro (19). Study of the thylakoids-associated ACHT1 revealed that it transmits a disulfide-based oxidative signal in feedback regulation of photosynthesis under homeostasis growth conditions (6). ACHT1 oxidation is driven by 2-Cys Prx, a peroxidase with a low Km for hydrogen peroxide (20–22) that is conceivably required for the low level of ROS that are typical in signaling events. ACHT1 undergoes rapid redox state changes during the transition of plants from dark to light and afterward, such that, after a short period of net reduction, the ACHT1 pool is maintained in a partially oxidized state in the light. Thus, a mechanism through which ACHT1, together with 2-Cys Prx, transmits an oxidative signal in response to illumination of plants with normal light intensity was proposed (6).
Here, we studied the role of a second thylakoids-associated member, ACHT4 (19), and found that it also reacted in plants with 2-Cys Prx, suggesting that its oxidation in vivo is mediated by a common disulfide exchange mechanism to that of ACHT1. In contrast to ACHT1, ACHT4 also reacted with an additional major target protein that was identified as APS1, suggesting that it transfers disulfides it receives from 2-Cys Prx to APS1 to deactivate AGPase. We found that a deletion of ACHT4 C terminus (ACHT4ΔC) abolished its reaction with APS1 but not with 2-Cys Prx. Analyses of the APS1 redox state and of the intermolecular disulfide intermediates of the ACHT4 reaction with APS1 or with 2-Cys Prx in planta suggested that ACHT4 participated in the oxidative signal that attenuates AGPase during the diurnal transition from night to day. The ACHT4 disulfide exchange reaction with APS1 and APS1 redox state also changed concomitantly with small fluctuations in light intensity during the day. Increasing the level of expressed ACHT4 or of ACHT4ΔC, correspondingly, increased or decreased the level of oxidized APS1 during the day and decreased or increased transitory starch content at the end of the day, further corroborating the oxidative role of ACHT4 in the intricate regulation of starch synthesis in daytime.
Results
Reoxidation of ACHT4 by 2-Cys Prx Shortly After Illumination.
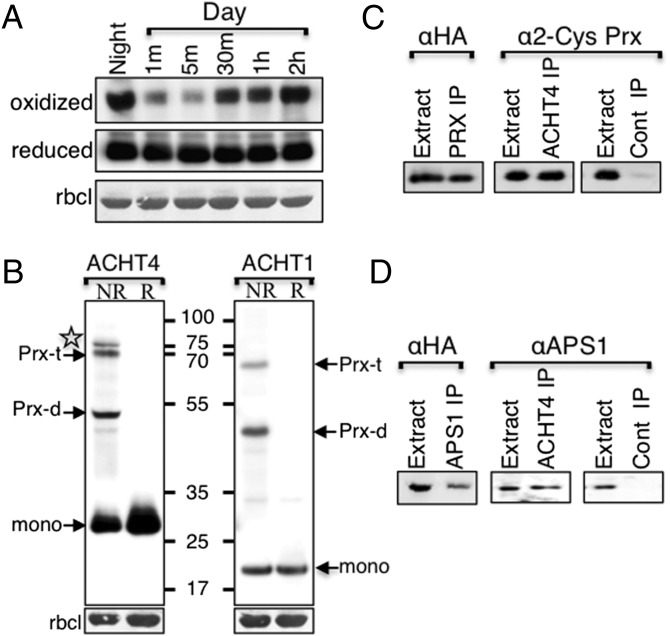
To examine whether other thylakoid-associated members of the ACHT family have similar or unique roles to that of ACHT1, we analyzed the redox state changes of the ACHT4 catalytic site after the onset of growth light (80–100 µE⋅m−2⋅s−1) after a typical 16-h night period in plants expressing ACHT4 (19). The catalytic site of ACHT4 was found to be mostly disulfide-bonded at the end of the night and to undergo rapid reduction within 1 min of exposure to the light (Fig. 1A). As reported for ACHT1, significant oxidation of the ACHT4 catalytic site was observed within 30 min of illumination and counteracted its reductive state. Thereafter, the ACHT4 redox state seemed to be in a dynamic quasi-oxidized state. A control experiment demonstrated that the total amount of ACHT4 did not change significantly (Fig. 1A, reduced) under the experimental conditions.
Fig. 1.
(A) Immunoblot assay showing the oxidized state of ACHT4 active-site Cys residues captured in plants expressing ACHT4 fused with HA-tag (19) at the end of the night (oxidized) and at 1 min (m), 5 min, 30 min, 1 h, and 2 h after beginning of illumination. Analysis of the purified proteins under reducing conditions (reduced) indicated that the changes in the oxidized level of ACHT4 were not the result of altered protein content. Equal loading was verified by ribulose-1,5-bis-phosphate carboxylase/oxygenase (RBCL) levels. (B) Immunoblot assay showing the ACHT4 intermolecular disulfide complexes, 2-Cys Prx heterotrimeric (Prx-t) and heterodimeric (Prx-d), and a unique additional complex (marked with a star) extracted under nonreducing conditions (NR) from plants expressing either ACHT4MT or ACHT1MT. The conversion of the complexes to the monomer (mono) by chemical reduction (R) indicated the disulfide nature of the complexes. Reciprocal immunoprecipitation identified 2-Cys Prx (C) and APS1 (D) as the intermolecular disulfide partners of ACHT4. Immunoblot assay of proteins immunoprecipitated with anti-HA (ACHT4 IP), anti-2-Cys Prx (Prx IP), and anti-APS1 (APS1 IP) affinity matrixes or with a nonspecific matrix (Cont IP) from plants expressing ACHT4MT. Purified proteins were run under reducing conditions and blotted with antibodies specific to the HA-tagged ACHT4 (αHA), 2-Cys Prx (α2-Cys Prx), or APS1 (αAPS1).
To investigate the identity of the proteins that ACHT4 exchanges disulfides with in planta, we captured, as in ref. 6, its intermolecular disulfide reaction intermediates. Protein blot analysis of denatured, but not reduced, plant extracts identified three intermolecular disulfide-linked ACHT4-containing protein complexes, verified by their susceptibility to chemical reduction by DTT (Fig. 1B, ACHT4). A comparison with the ACHT1 intermolecular disulfide-linked protein complexes (Fig. 1B, ACHT1) showed that two of the main ACHT4 complexes, with estimated sizes of ∼55 kDa and ∼70 kDa, corresponded to the combined molecular mass of the previously characterized heterodimeric and the heterotrimeric ACHT1 and 2-Cys Prx complexes, respectively (6). Interestingly, an additional third intermolecular disulfide ACHT4 complex (marked with a star in Fig. 1B), with a higher molecular mass than that of the 2-Cys Prx-ACHT4 heterotrimer, and which did not form in ACHT1 extracts, was identified as well.
To verify their authenticity, the 2-Cys Prx-ACHT4 intermolecular disulfide complexes were pulled down in a reciprocal analysis performed under nonreducing denaturing conditions, with either anti-HA (for ACHT4) or anti–2-Cys Prx sera. Protein blot analysis of denatured and reduced samples identified the 2-Cys Prx in the anti-HA pulled down complexes and ACHT4 in the anti–2-Cys Prx pulled down complexes (Fig. 1C). As expected, mass spectrometry analysis identified both ACHT4 and 2-Cys Prx in the gel slice containing the heterotrimer complex but not in a corresponding gel slice containing extracts that were separated under reducing conditions (Table S1). We concluded that ACHT4 exchanged disulfides with the 2-Cys Prx in plants, in a similar manner as that reported for ACHT1.
Table S1.
Identification of 2-Cys Prx and APS1 as ACHT4 targets by mass spectrometry
| Protein name | Queries matched | Score | Protein sequence coverage, % | Protein length, aa |
| 2-CYS-PRX | APDFEAEAVFDQEFIK (3) | 478 | 44 | 266 |
| LNTEVLGVSVDSVFSHLAWVQTDR (2) | ||||
| SGGLGDLNYPLISDVTK (2) | ||||
| SFGVLIHDQGIALR (2) | ||||
| GLFIIDK (1) | ||||
| EGVIQHSTINNLGIGR (1) | ||||
| TLQALQYIQENPDEVCPAGWKPGEK (2) | ||||
| APS1 | LIDIPVSNCLNSNISK (1) | 572 | 37 | 520 |
| IYVLTQFNSASLNR (2) | ||||
| NEGFVEVLAAQQSPENPNWFQGTADAVR (4) | ||||
| ETDADITVAALPMDEQR (1) | ||||
| VDTTILGLDDQR (1) | ||||
| EMPFIASMGIYVVSR (1) | ||||
| NQFPGANDFGSEVIPGATSLGLR (3) | ||||
| VQAYLYDGYWEDIGTIEAFYNANLGITK (1) | ||||
| KPVPDFSFYDR (1) | ||||
| MLDADVTDSVIGEGCVIK (1) | ||||
| IINSDNVQEAAR (1) |
The mass spectrometry analysis was performed by the Biological Mass Spectrometry Unit at the Weizmann Institute of Science by online reversed-phase nano-liquid chromatography, electrospray ionization tandem mass spectrometric analyses. Survey scans were recorded in the Fourier transformed (FT) mode followed by data-dependent collision-induced dissociation of the seven most intense ions in the linear ion trap. Raw spectra were processed using open-source software DTA SuperCharge. The data were searched with MASCOT (Matrix Science) against a Swissprot or National Center for Biotechnology Information database. Control samples treated with DTT or derived from WT plants allowed for the subtraction of nonspecific background proteins. Numbers in parentheses represent the number of times the peptide was identified in the analysis.
APS1 Is a Unique Target of ACHT4.
The comparison of the intermolecular disulfide complexes formed in planta by ACHT4 and ACHT1 uncovered a major disulfide linked complex unique to ACHT4 (Fig. 1B). A mass spectrometry analysis identified APS1 in the gel slice containing the unique complex of ACHT4 but not in a corresponding reduced gel slice (Table S1). To verify these findings, the protein intermolecular disulfide complexes were reciprocally pulled down under nonreducing denaturing conditions with either anti-HA, for ACHT4, or anti-APS1. Protein blot analyses identified the APS1 in the anti-HA pulled-down complexes and ACHT4 in the anti-APS1 pulled-down complexes (Fig. 1D), suggesting that APS1 is a target protein of ACHT4. These findings also implied that, although ACHT1 and ACHT4 share a similar mode of oxidation by 2-Cys Prx, they differ in at least one major target, suggesting that they may serve to regulate distinctive processes.
ACHT4 Participates in the Diurnal Redox Regulation of AGPase.
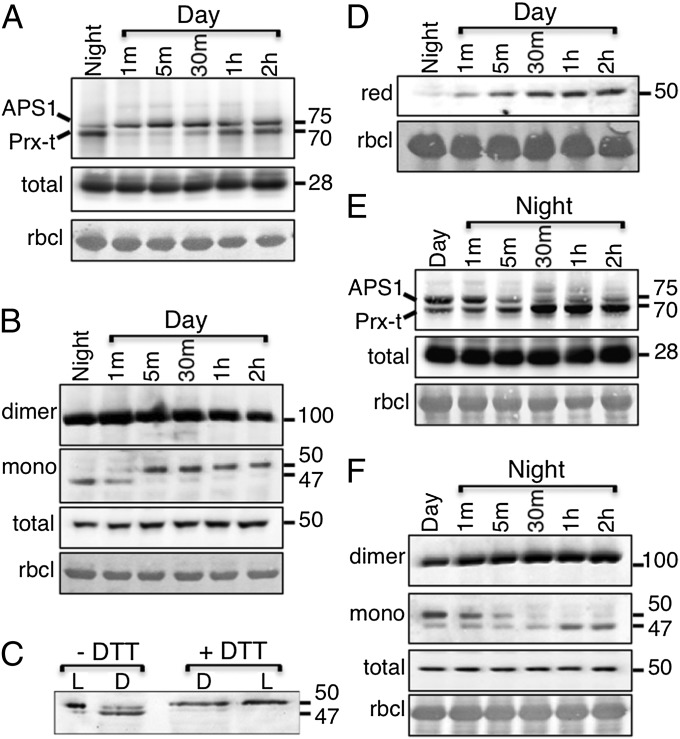
The trapping in vivo of APS1-ACHT4 and 2-Cys Prx-ACHT4 disulfide exchange reaction intermediates (RIs) opened the possibility of studying the environmental stimuli that influence ACHT4-driven AGPase redox control. First, we analyzed the changes in the 2-Cys Prx-ACHT4 and APS1-ACHT4 RIs and the corresponding changes in the APS1 redox state upon light onset of plants grown under an 8/16-h light/dark regime. Intriguingly, the low APS1-ACHT4 RI level and the high 2-Cys Prx-ACHT4 level in the dark contrasted each other (Fig. 2A). Because the 2-Cys Prx RIs were high in the dark also with ACHT1 (6), it suggested that the ACHT4 disulfide transfer reaction with APS1 differs from the reducing reaction of either ACHT1 or ACHT4 with 2-Cys Prx (19). Because the dark is a relatively stable condition (i.e., changes in the redox state of reacting proteins are not expected), these findings could conceivably reflect an opposite directionality of the disulfide transfer reaction, ACHT4 to APS1 versus 2-Cys Prx to ACHT4, which could possibly be derived from dissimilar redox midpoint potentials of the proteins. Consequently, the contrasting levels of ACHT4 RIs in the dark would be a result of the levels of its two substrates, a high level of oxidized 2-Cys Prx and low level of reduced APS1. Notably, the increase in the level of APS1-ACHT4 RIs upon illumination (Fig. 2A) matched the increase in reduction of APS1 at this time period, further supporting this notion. The APS1-ACHT4 RI level approached a steady-state level after a 30-min transition period in the light. The 2-Cys Prx-ACHT4 RI levels (Fig. 2A) showed a similar pattern to those of 2-Cys Prx-ACHT1 (6), also during illumination, a transient decrease, and then an increased level, which reached a steady state after the 30-min transition period in the light.
Fig. 2.
Immunoblot assay with HA Ab showing the ACHT4 intermolecular disulfide complexes during the transition from night to day (A) and from day to night (E). Immunoblot assay showing APS1 redox state during the transition from night to day (B) and from day to night (F). The panels of APS1 dimer and the monomer were taken from the same immunoblot exposure. (C). Immunoblot assay with APS1 Ab of gel slice of 50-kDa monomer extracted from plants in the dark (D) or in the light (L) before (−DTT) and after (+DTT) chemical reduction with DTT. (D). Immunoblot assay with APS1 Ab showing the reduced 50-kDa APS1 monomer (red). Equal loading was verified as in Fig. 1. The results shown are representative of three independent experiments.
Similarly to previous findings (12–15), the analysis of the concomitant changes in the redox state of APS1 showed that APS1 was resting in the inactive intermolecular disulfide form in the dark (Fig. 2B). In addition, in dark conditions, a monomeric form of APS1, with slightly lower molecular weight (MW) that was converted to the monomeric form with the expected MW during the first 5 min of illumination, was observed. The conversion of the faster migrating form to the slower form upon illumination suggested that the lower MW monomer could be a compacted APS1 form bearing an intramolecular disulfide, the reduction of which creates a stretched molecule in the light; to verify that, we compared the migration of the APS1 monomer, purified from dark protein extracts and chemically reduced with DTT in vitro, with the monomer purified from light extracts (Fig. 2C). The DTT-reduced monomer derived from dark extracts migrated parallel to the monomer from light extracts whereas the DTT-reduced monomer isolated from light extracts did not alter its migration, indicating that an APS1 intramolecular disulfide indeed participated in the redox control of APS1. Thus, we analyzed the monomeric APS1 via a methodology that exclusively measures the levels of reduced monomer. In this method, the reduced cysteines are first blocked with N-ethyl maleimide (NEM), and the disulfides are then chemically reduced and reacted with methoxypolyethylene glycol-maleimide (mPEG). We found that the level of reduced monomer was barely detectable in the dark, gradually increased during the 30-min transition period, and reached a steady-state level thereafter (Fig. 2D). In parallel, 2-Cys Prx-ACHT4 RI levels, indicative of an ACHT4 oxidative signal, were low during the transition period and also reached relatively stable values only thereafter (Fig. 2A), suggesting that the oxidative signal of ACHT4 dynamically counteracted APS1 reduction by Trx-f1 and approached a dynamic equilibrium after 30 min in the light.
Both 2-Cys Prx ACHT4 and APS1 RI levels and the APS1 redox state after the light was switched off (i.e., the period in which oxidation is expected to turn off AGPase) were inverse to those observed after the onset of light. The 2-Cys Prx-ACHT4 RI remained stable during the first 5 min and then increased, suggesting increased oxidation by 2-Cys Prx during that time period (Fig. 2E). Levels of the reduced monomeric APS1 form gradually decreased alongside a concomitant increase in the oxidized, intramolecular disulfide-bearing monomer and intermolecular disulfide-linked dimer (Fig. 2F). At the same time, the level of the APS1 reaction intermediate, which was high at the end of the day, gradually decreased and reached levels similar to those observed before the beginning of the day (Fig. 2E). These results are consistent with an increased oxidation rate of APS1 by ACHT4 during the transition from day to night, resulting in diminishing APS1 activity.
ACHT4 Participates in the Regulation of APS1 During Fluctuations in Light Intensity.
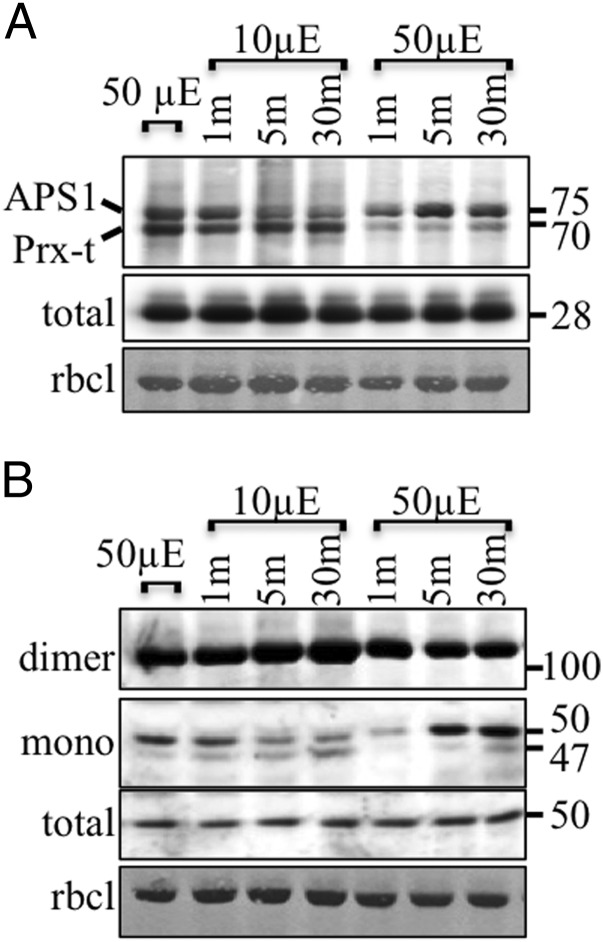
We found that ACHT4 participated in the diurnal regulation of APS1, which has been proposed to influence the day and night cycles of starch synthesis and degradation (18). The reactions of ACHT4 with APS1 and 2-Cys Prx, as judged by the levels of their disulfide exchange reaction intermediates, seemed to reach balanced levels after a transition period in the light (Fig. 2A), as manifested by APS1 activation levels (Fig. 2B), suggesting that ACHT4 oxidation of APS1 is active and dynamically counteracting its reduction by Trx-f1 during the day. Such activity might be important for the rationing of photosynthates to starch and sugars exported from the chloroplast during the day. Alternatively, as shown in the unicellular green alga Chlamydomonas reinhardtii, such activity may regulate the levels of starch to be used as a transient pool for reduced carbon in a fluctuating light environment (23). We tested this hypothesis by subjecting the plants to small fluctuations of light intensity during the day. Interestingly, when light intensity was reduced to 10 μE·m−2·s−1 after a 2-h 50-μE·m−2·s−1 light period, APS1-ACHT4 RI levels declined within 5 min and rose again when the light intensity was switched back to 50 μE·m−2·s−1 (Fig. 3A). Although 2-Cys Prx-ACHT4 RI levels remained steady upon decreased light intensity, they rapidly declined after the transfer back to the higher light intensity (Fig. 3A). In parallel, a lower ratio of reduced-to-oxidized APS1 was obtained after the transfer to the lower light and a higher ratio was observed upon increased light intensity (Fig. 3B). These results suggest that, in addition to its diurnal role, ACHT4-driven oxidation of APS1 may also participate in the dynamic regulation of AGPase activity in response to natural fluctuations in light intensity. Furthermore, the reduction of APS1 upon increased light intensity occurred rapidly in comparison with its oxidation upon decreased light intensity (Fig. 3B), suggesting that AGPase redox regulation might also play a role in stimulating a transient burst of starch synthesis to accommodate an abruptly increased light intensity, as suggested in ref. 23. The concomitant gradual decrease of both APS1-ACHT4 RIs levels and reduced APS1 upon lowering the light intensity and the abrupt increase in both upon increased intensity further supported the notion that ACHT4 reacted with the reduced APS1.
Fig. 3.
Immunoblot assay showing the ACHT4 intermolecular disulfide complexes (A) or APS1 redox state (B) in plants treated for 2 h with 50 μE·m−2·s−1 light intensity (50 µE) and after abrupt decreased (10 µE) followed by abrupt increased light intensity (50 µE). Equal protein loading was verified as in Fig. 1. The results shown are representative of three independent experiments.
The C Terminus of ACHT4 Is Important for Its Reaction with APS1.
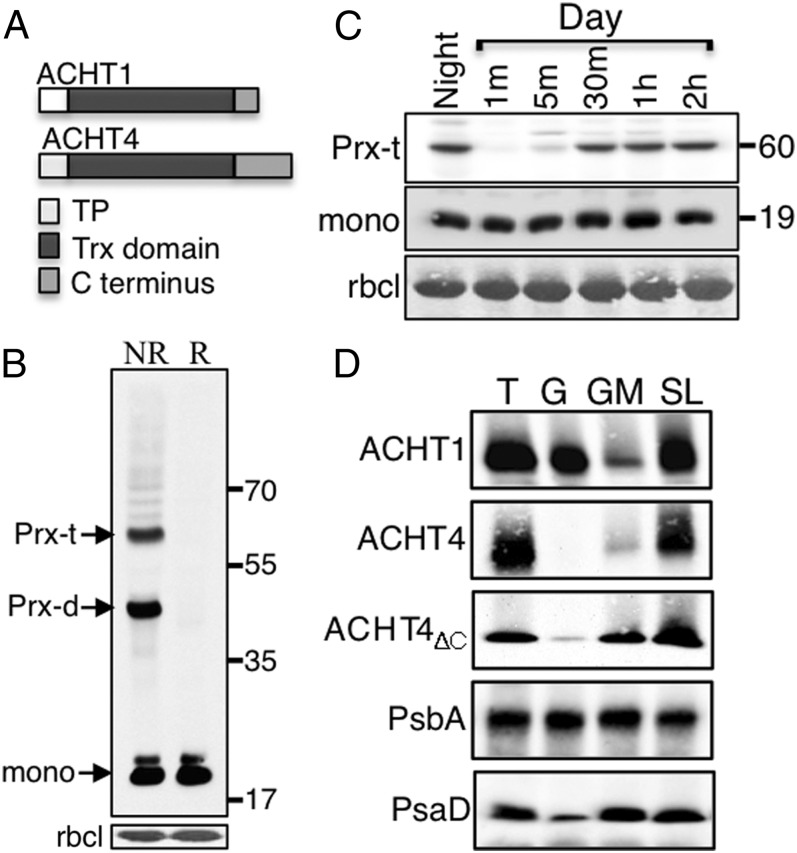
We then assessed whether the distinct 47-aa-long ACHT4 C terminus (19) (Fig. 4A) is responsible for its in planta differences from ACHT1, by analyzing the 2-Cys Prx and APS1 RIs in plants expressing ACHT4 lacking the C terminus (ACHT4ΔC). Notably, the ACHT4ΔC form reacted with 2-Cys Prx and formed the two intermolecular disulfide-linked 2-Cys Prx ACHT4 RIs, but failed altogether to interact with APS1 (Fig. 4B). The preferential reaction of ACHT4ΔC with 2-Cys Prx and not with APS1 was maintained throughout the transition from night to day (Fig. 4C). Moreover, the profile of the reaction of ACHT4ΔC with 2-Cys Prx during the first 2 h of illumination was similar to that of ACHT4, indicating that the oxidation of ACHT4ΔC by 2-Cys Prx does not involve the protein's C terminus and that the C terminus deletion affected only ACHT4-driven redox control of APS1.
Fig. 4.
(A) Schematic representation comparing ACHT1 and ACHT4 protein sequences. Transit peptide (TP). (B) Immunoblot assay of intermolecular disulfide complexes of ACHT4ΔC as in Fig 1B. (C) Immunoblot assay with HA Ab showing the ACHT4ΔC intermolecular disulfide complexes during the transition from night to day. (D) Immunoblot assay of thylakoid membranes (T), enriched grana (G), grana margin (GM), and stroma lamellae (SL) and decorated with anti-HA Ab (ACHT1, ACHT4, ACHT4ΔC), or with Ab against the PS II protein PsbA (PsbA) or the PS I protein PsaD Ab (PsaD).
Both ACHT4 and ACHT1 are thylakoid-associated proteins (19). The distinct reaction of ACHT4, and not of ACHT1, with APS1 prompted us to investigate whether the disparity might be due to different thylakoid localization, either the grana, the grana margins, or the stroma lamella. Protein blot analysis showed that ACHT1 was primarily found in both the grana and the stroma lamella domains whereas ACHT4 was mainly present in the stroma lamella domain and was undetectable in the grana (Fig. 4D), further promoting distinct roles of ACHT4 and ACHT1. The membrane association of ACHT4ΔC was then analyzed to determine whether ACHT4 localization is influenced by its unique C terminus. Only a small increase in the partitioning of ACHT4ΔC to the grana margins was observed, suggesting that the ACHT4 C terminus deletion effect on its disulfide exchange reaction with APS1 was direct and not mediated via its thylakoid domain localization.
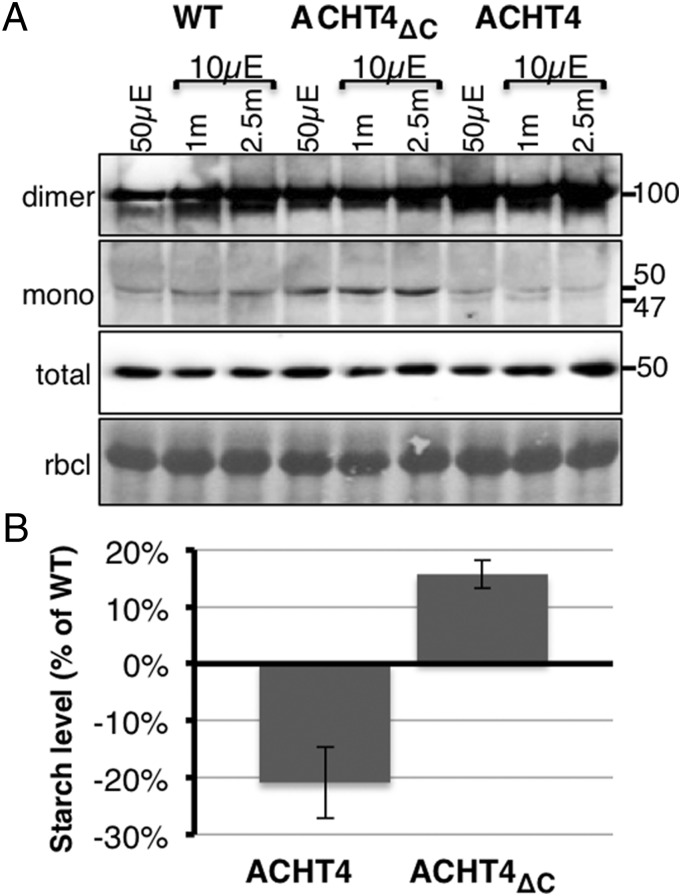
The finding that the deletion of the ACHT4 C terminus diminished its disulfide exchange reaction with APS1 (Fig. 4B) facilitated studying the effect of ACHT4 on the APS1 redox state and on transitory starch content. We first compared the APS1 redox state after 2 h in the light, representing steady-state conditions in which transitory starch is synthesized, and when light intensity was reduced, conditions in which APS1 is being oxidized (Fig. 3). Consistently with the expected oxidative role of ACHT4 and the dominant negative effect of the C terminus deletion, plants expressing increased levels of ACHT4 exhibited lower levels of reduced APS1 monomer, and plants expressing ACHT4ΔC contained higher levels of the reduced APS1 monomer, than WT plants (Fig. 5A). Next, to determine whether the influence of ACHT4 on the redox state of APS1 impacts starch accumulation, we assayed the starch content of WT ACHT4 and ACHT4ΔC plants at the end of the day (Fig. 5B). In agreement with the changes in the APS1 redox state, plants expressing ACHT4 showed reduced starch levels whereas plants expressing ACHT4ΔC contained increased starch levels. These results corroborate the notion that disulfides transferred by ACHT4 to APS1 are used in planta to fine-tune APS1 activity by counterbalancing the reduction of APS1 by the reductive type Trxs.
Fig. 5.
(A) Immunoblot assay with APS1 Ab showing APS1 redox state in WT plants (WT), plants expressing increased level of ACHT4ΔC (ACHT4ΔC), or plants expressing increased level of ACHT4 (ACHT4). Equal protein loading was verified as in Fig.1. (B) Leaf starch content of plants expressing increased level of ACHT4ΔC, or plants expressing increased level of ACHT4 relative to that of WT plants. The results shown are representative of three independent experiments.
Discussion
Intrinsic redox changes stimulate allosteric control of the biological activity of redox-regulated proteins, such as AGPase. The reversible breakage and formation of the internal regulatory intermolecular disulfide of the APS1 subunit of AGPase are used, correspondingly, to activate and inactivate AGPase and subsequent starch synthesis (9–18). In vivo, the redox state of APS1 at any time point is the sum of each of the reductive signals, presumably driven by Trx-f1 (16) and NTRC (17), and the oxidative signals it perceives. Because the timing and the extent of the reductive APS1-activating signal are important for turning on starch synthesis, the timing and the extent of the counteracting quenching oxidative signal might be conceivably as important toward turning off the signal and a dynamic fine-tuning of starch synthesis. Our results indicate that ACHT4, together with 2-Cys Prx, participates in both the transduction of an oxidative signal during the diurnal inactivation of AGPase at the beginning of night (Fig. 2), and in a dynamic attenuation of APS1 activity in response to small fluctuations in light intensity during the day (Fig. 3). These findings portray a homeostasis control mechanism by which the APS1 redox state is dictated by a dynamic counterbalance between the activating reductive signals, carried by either Trx-f1 or NTRC, and an attenuating oxidative signal carried by ACHT4, which confers rapid alteration of APS1 redox state (Fig. 3B) and consequently starch synthesis in response to small and abrupt fluctuations in light intensity during the day. In a recent study assessing photosynthesis, metabolism, and growth responses to an increase in irradiance in the unicellular green alga C. reinhardtii, a rapid increase in starch levels was suggested to uncouple photosynthesis from growth in a fluctuating light environment, by providing a transient buffer for carbon until growth increased (23). The rapid response of the APS1 redox state in Arabidopsis plants exposed to fluctuating light intensity (Fig. 3B), is consistent with the hypothesis that APS1 redox regulation drives a transient change in starch content in response to abrupt changes in light intensity. The intriguingly intricate posttranscriptional regulation of AGPase, allosteric by PPi and AGP, protein turnover and redox control might be required for a precise control of starch synthesis, which is used both for the accumulation of the daily ration by the end of day (9) and for the transient accumulation of starch upon abrupt increased light intensity.
Accumulating evidence has indicated that low levels of ROS play a role in oxidative signaling in the chloroplast. We hypothesized that, because physiological conditions are relatively devoid of oxidative stress, they provide an ideal platform for study of the homeostatic mechanisms of low levels of oxidative signals. The rapid oxidation of APS1, driven by the 2-Cys Prx–ACHT4 interaction (Fig. 3A), in response to a small decrease in light intensity (Fig. 3B) is consistent with this hypothesis. The high catalytic efficiency of the 2-Cys Prxs with kcat/Km values of ∼107 M−1⋅s−1 and high affinity with Km values in the µM range (20–22) presumably ensure rapid oxidation kinetics of their Trx partner in response to small changes in low peroxide levels (24, 25). In turn, the identified preference of ACHT4 disulfide transfer reaction toward APS1 (Fig. 2) will result in diminishing APS1 activity. According to this view, a series of disulfide exchange reactions, the 2-Cys Prx reaction with ACHT4 and that of ACHT4 with APS1, are involved in mediating the oxidative signal of APS1, which counterbalances the reductive signals of Trx-f1 and NTRC. Our findings also indicate that, whereas ACHT4 and ACHT1 are both oxidized by 2-Cys Prx, they differ in at least one major target, APS1, suggesting that they may serve to regulate distinct processes. It is possible that their unique roles might be spatiotemporally governed by their protein sequences. This assumption is supported by the different thylakoids localization of ACHT4 and ACHT1 (Fig. 4D) and by the impact of deletion of the ACHT4 C terminus (Fig. 4). Although this spatiotemporally dictated Trx–target interaction hypothesis is also consistent with the multiplicity of the reductive and oxidative type Trxs in the chloroplast, its validation still requires extensive additional studies.
Materials and Methods
Plant Material and Growth Conditions.
A. thaliana var Columbia was grown as in ref. 19. Plants were grown under an 8/16-h light/dark cycle at 20 °C/18 °C, respectively, at 80 to 100 µE⋅m−2⋅s−1 (unless otherwise stated) for 3–4 wk. Thylakoid membranes were isolated as previously described (26).
Generation of Transgenic Plants.
ACHT4, ACHT4MT (in which the nonnucleophilic cysteine of the active site was replaced with a serine), ACHT4ΔC, and ACHT1 ORFs were ligated upstream and in frame of the HA3 affinity tag and under the control of the 35S promoter into pART7 vector as in ref. 19.
Protein Redox Assays, Immunoblot, and Affinity Purification Analyses.
The disulfide state of plant-extracted proteins, the identification of intermolecular disulfide complexes, and the isolation of the intermolecular disulfide complexes by immunoprecipitation were assayed in planta as previously described (6). The mass spectrometry (MS) analysis is detailed in Table S1. Trapped intermolecular disulfide protein complexes were incubated overnight at 4 °C in RIPA buffer (1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 10 mM Tris⋅HCl, pH 8, and 140 mM NaCl) with either anti-HA (A2095; Sigma) resin or anti-2-Cys Prx– or anti-APS1–coated protein G beads (Amersham). The proteins were eluted with either reducing or nonreducing 2× sample buffer and separated on SDS/PAGE gels for immunoblots or for MS analysis. Anti-APS1 polyclonal antibodies were raised in rabbits at GenScript HK Ltd., using a purified peptide (CILGLDDQRAKEMPF). 2-Cys Prx–specific polyclonal antibodies were as in ref. 19. Mouse monoclonal anti-HA antibodies (H9658; Sigma) were used in protein blot assays.
Starch Analysis.
Starch content of 0.8-g samples of 2-mo-old rosettes was analyzed using the Sigma starch assay kit (SA20-1KT). Every replicate included 10 rosettes.
Accession Numbers.
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ACHT4 (AT1G08570), 2-Cys PrxA (At3g11630), and APS1 (AT5G48300).
Acknowledgments
We thank Tevi Mehlman (Biological Mass Spectrometry, Weizmann Institute of Science) for dedicated MS analysis and Rotem Kleinberger for excellent technical assistance. This study was supported by a grant from the Israeli Science Foundation. A.D. is the incumbent of The Henry and Bertha Benson Chair, Weizmann Institute of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515513112/-/DCSupplemental.
References
- 1.Karpinski S, et al. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284(5414):654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- 2.Trebitsh T, Danon A. Translation of chloroplast psbA mRNA is regulated by signals initiated by both photosystems II and I. Proc Natl Acad Sci USA. 2001;98(21):12289–12294. doi: 10.1073/pnas.211440698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfannschmidt T. Chloroplast redox signals: How photosynthesis controls its own genes. Trends Plant Sci. 2003;8(1):33–41. doi: 10.1016/s1360-1385(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 4.Rochaix JD. Redox regulation of thylakoid protein kinases and photosynthetic gene expression. Antioxid Redox Signal. 2013;18(16):2184–2201. doi: 10.1089/ars.2012.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schürmann P, Buchanan BB. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid Redox Signal. 2008;10(7):1235–1274. doi: 10.1089/ars.2007.1931. [DOI] [PubMed] [Google Scholar]
- 6.Dangoor I, Peled-Zehavi H, Wittenberg G, Danon A. A chloroplast light-regulated oxidative sensor for moderate light intensity in Arabidopsis. Plant Cell. 2012;24(5):1894–1906. doi: 10.1105/tpc.112.097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikkanen L, Rintamäki E. Thioredoxin-dependent regulatory networks in chloroplasts under fluctuating light conditions. Philos Trans R Soc Lond B Biol Sci. 2014;369(1640):20130224. doi: 10.1098/rstb.2013.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danon A. Redox reactions of regulatory proteins: Do kinetics promote specificity? Trends Biochem Sci. 2002;27(4):197–203. doi: 10.1016/s0968-0004(02)02066-2. [DOI] [PubMed] [Google Scholar]
- 9.Stitt M, Zeeman SC. Starch turnover: Pathways, regulation and role in growth. Curr Opin Plant Biol. 2012;15(3):282–292. doi: 10.1016/j.pbi.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Mugford ST, et al. Regulatory properties of ADP glucose pyrophosphorylase are required for adjustment of leaf starch synthesis in different photoperiods. Plant Physiol. 2014;166(4):1733–1747. doi: 10.1104/pp.114.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preiss J. Biosynthesis of starch and its regulation. In: Preiss J, editor. The Biochemistry of Plants. Vol 14. Academic; New York: 1988. pp. 181–254. [Google Scholar]
- 12.Ballicora MA, Frueauf JB, Fu Y, Schürmann P, Preiss J. Activation of the potato tuber ADP-glucose pyrophosphorylase by thioredoxin. J Biol Chem. 2000;275(2):1315–1320. doi: 10.1074/jbc.275.2.1315. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Ballicora MA, Leykam JF, Preiss J. Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem. 1998;273(39):25045–25052. doi: 10.1074/jbc.273.39.25045. [DOI] [PubMed] [Google Scholar]
- 14.Lunn JE, et al. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J. 2006;397(1):139–148. doi: 10.1042/BJ20060083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendriks JHM, Kolbe A, Gibon Y, Stitt M, Geigenberger P. ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol. 2003;133(2):838–849. doi: 10.1104/pp.103.024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thormählen I, et al. Inactivation of thioredoxin f1 leads to decreased light activation of ADP-glucose pyrophosphorylase and altered diurnal starch turnover in leaves of Arabidopsis plants. Plant Cell Environ. 2013;36(1):16–29. doi: 10.1111/j.1365-3040.2012.02549.x. [DOI] [PubMed] [Google Scholar]
- 17.Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA. 2009;106(24):9908–9913. doi: 10.1073/pnas.0903559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilkington SM, et al. Relationship between starch degradation and carbon demand for maintenance and growth in Arabidopsis thaliana in different irradiance and temperature regimes. Plant Cell Environ. 2015;38(1):157–171. doi: 10.1111/pce.12381. [DOI] [PubMed] [Google Scholar]
- 19.Dangoor I, Peled-Zehavi H, Levitan A, Pasand O, Danon A. A small family of chloroplast atypical thioredoxins. Plant Physiol. 2009;149(3):1240–1250. doi: 10.1104/pp.108.128314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akerman SE, Müller S. Peroxiredoxin-linked detoxification of hydroperoxides in Toxoplasma gondii. J Biol Chem. 2005;280(1):564–570. doi: 10.1074/jbc.M406367200. [DOI] [PubMed] [Google Scholar]
- 21.Peskin AV, et al. The high reactivity of peroxiredoxin 2 with H(2)O(2) is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem. 2007;282(16):11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 22.Parsonage D, Karplus PA, Poole LB. Substrate specificity and redox potential of AhpC, a bacterial peroxiredoxin. Proc Natl Acad Sci USA. 2008;105(24):8209–8214. doi: 10.1073/pnas.0708308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mettler T, et al. Systems analysis of the response of photosynthesis, metabolism, and growth to an increase in irradiance in the photosynthetic model organism Chlamydomonas reinhardtii. Plant Cell. 2014;26(6):2310–2350. doi: 10.1105/tpc.114.124537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polle A. Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling: Computer simulations as a step towards flux analysis. Plant Physiol. 2001;126(1):445–462. doi: 10.1104/pp.126.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 26.Andersson B, Anderson JM. 1985. The chloroplast thylakoid membrane: Isolation, subfractionation and purification of its supramolecular complexes. Cell Components, Modern Methods of Plant Analysis, eds Linskens H-F, Jackson J (Springer, Berlin), Vol 1, pp 231–258. [Google Scholar]