Significance
Neuronal output is modulated by inhibition onto axons and dendrites by diverse inhibitory synapses comprising distinct receptor subunits. Factors that regulate the in vivo maturation of these synapses across cell-compartments are not well understood. We discovered that axonal GABAA receptors are down-regulated whereas dendritic GABAA receptors are up-regulated on retinal bipolar cells in the absence of vesicular GABA release. Deleting the γ2 subunit of GABAA receptors specifically in bipolar cells only alters axonal GABAA receptor expression, suggesting that axonal and dendritic GABAA receptors have distinct subunit compositions that are regulated independently. Moreover, vesicular GABA release from presynaptic amacrine but not horizontal interneurons is important. Thus, regulation of inhibitory synapse maturation across the bipolar cell is input-type, receptor-type, and cell-compartment-type specific.
Keywords: GABA receptor, retina, synaptic inhibition, axon-dendrite
Abstract
Neuronal output is modulated by inhibition onto both dendrites and axons. It is unknown whether inhibitory synapses at these two cellular compartments of an individual neuron are regulated coordinately or separately during in vivo development. Because neurotransmission influences synapse maturation and circuit development, we determined how loss of inhibition affects the expression of diverse types of inhibitory receptors on the axon and dendrites of mouse retinal bipolar cells. We found that axonal GABA but not glycine receptor expression depends on neurotransmission. Importantly, axonal and dendritic GABAA receptors comprise distinct subunit compositions that are regulated differentially by GABA release: Axonal GABAA receptors are down-regulated but dendritic receptors are up-regulated in the absence of inhibition. The homeostatic increase in GABAA receptors on bipolar cell dendrites is pathway-specific: Cone but not rod bipolar cell dendrites maintain an up-regulation of receptors in the transmission deficient mutants. Furthermore, the bipolar cell GABAA receptor alterations are a consequence of impaired vesicular GABA release from amacrine but not horizontal interneurons. Thus, inhibitory neurotransmission regulates in vivo postsynaptic maturation of inhibitory synapses with contrasting modes of action specific to synapse type and location.
Interneurons of the CNS control neuronal excitability through release of γ-aminobutyric acid (GABA) and glycine. How inhibition modifies neuronal output depends largely on the types of presynaptic interneurons making synapses onto a postsynaptic cell, and the location and densities of these synapses (1–3). Moreover, inhibitory receptor types with distinct transmitter affinities and kinetics present on the axon or dendrites of an individual neuron can critically shape its output (3–6). Although much is known about how different inhibitory synapses shape the spatiotemporal activity patterns of mature neurons, it is less clear what factors regulate the expression of inhibitory receptors at these synapses during development in vivo. Is the expression of distinct inhibitory receptor types within a cellular compartment (axon or dendrite) regulated coordinately or independently? Conversely, is the expression of the same receptor type at different cellular compartments of an individual neuron regulated by common or separate factors?
To answer these questions, we assessed expression of inhibitory receptors on the axon and dendrites of individual glutamatergic retinal neurons in mice with genetically suppressed inhibition. We generated retina-specific knockouts of the vesicular inhibitory amino acid transporter (VIAAT), which mediates uptake of GABA or glycine into synaptic vesicles (7, 8). We perturbed inhibition because it has been found previously to influence pre- and postsynaptic maturation of GABAergic synapses (9–12). However, whether inhibitory receptor expression at the “input” and “output” compartments of an individual neuron is coordinately regulated by activity remains unknown. We focused on retinal bipolar cells (BCs) because of the rich variety of inhibitory synapses found on these neurons. Moreover, the many types of BCs enabled us to determine whether inhibitory transmission plays a uniform or diverse role in regulating inhibitory synapses across cell types that signal in parallel. We compared the postsynaptic maturation of GABA- and glycinergic synapses on cone BCs (CBCs) versus rod BCs (RBCs) that operate at different light levels. Among CBCs, we analyzed both ON and OFF BC types, which depolarize or hyperpolarize to light increments, respectively (13).
Results
In the mouse retina, GABAA (α1–3, β1–3 with γ2 or an auxiliary subunit), GABAC (ρ1–3 subunits), and glycine receptors (α1–4 with a β subunit) (5, 14, 15) are present at nonoverlapping synapses. However, the complement of inhibitory receptor types and their relative expressions on the axons and dendrites of individual BCs has not been compared across BC types. We thus generated transgenic mouse lines to visualize ON and OFF BCs. We previously showed that ON BCs, especially type 6 CBCs and RBCs, express tdTomato in Grm6-tdTomato mice (ref. 16; SI Appendix, Fig. S1A). To label OFF CBCs, we cloned and used the Vsx1 promoter to drive expression of cerulean fluorescent protein. In one Vsx1-cerulean mouse line, we found sparse labeling of OFF CBCs, particularly type 1 and 2 CBCs (SI Appendix, Fig. S1A). These transgenic lines enabled us to compare the inhibitory receptor types expressed in the dendrites and axons of four distinct BC types and to determine how this expression relied on the vesicular release of inhibitory neurotransmitters.
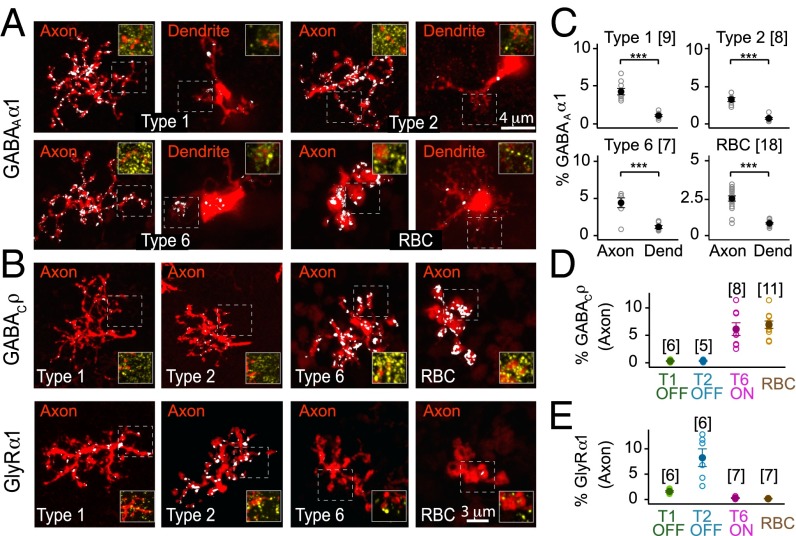
α1-Subunit Containing GABAA Receptors Are Expressed on Axons and Dendrites of Mouse Retinal Bipolar Cells.
GABAergic and glycinergic amacrine cells innervate BC axon terminals, whereas BC dendrites are contacted by GABAergic horizontal cells and interplexiform amacrine cells (refs. 13, 17, and 18, and SI Appendix, Fig. S1B). Previous electrophysiological studies suggest that GABAA receptors are abundant on axon terminals of BCs (4, 19). Using subunit-specific antibodies, we determined the expression of GABAA α-subunits on the axons and dendrites of type 1 and 2 OFF CBCs, type 6 ON CBCs, and RBCs (Fig. 1 and SI Appendix, Fig. S2). For all BCs examined, we found α1-subunit-containing GABAA receptors (GABAAα1) at both axon terminals and dendrites. We quantified receptor expression by calculating the % of the volume of the axon terminal or dendritic arbor occupied by immunolabeled receptors, and called the measure % occupancy (SI Appendix, Fig. S1 C–F and SI Materials and Methods). GABAAα1 receptors were more abundant at BC axons relative to dendrites (Fig. 1C). GABAAα3 receptor expression was low compared with GABAAα1 expression at both ON and OFF BC axon terminals (SI Appendix, Fig. S2B). We did not immunolabel for GABAAα2 subunits because they are not localized to BCs (15). As found previously (4, 19, 20), our immunolabeling analysis showed that ON, but not OFF BC axon terminals express substantial amounts of GABAC receptors (Fig. 1 B and D). Also OFF but not ON BC axons express abundant α1-subunit-containing glycine receptors (GlyRα1), with type 2 CBC axons expressing more GlyRα1 compared with type 1 CBCs (P = 0.0035, Fig. 1 B and E). Our immunolabeling thus reveals expression patterns of inhibitory receptors across BC axon types consistent with previous electrophysiology. Moreover, we discovered that although the axons of different BC types express distinct combinations of GABA and glycine receptors, their dendrites all have GABAAα1 receptors.
Fig. 1.
Inhibitory receptor types expressed on the axons and dendrites of mouse retinal bipolar cells. (A) α1-subunit-containing GABAA (GABAAα1) receptors (white) on the axons and dendrites (red) of types 1, 2, and 6 cone bipolar cells and rod bipolar cells (RBCs). Insets show raw images of receptor labeling (yellow) for axonal or dendritic processes within the boxed region. (B) ρ-subunit-containing GABAC receptors (GABACρ) and α1-subunit-containing glycine receptors, GlyRα1, (white) on the axon terminals of OFF (type 1 and 2) and ON (type 6 and rod) bipolar cells (red). Axon terminals of ON rather than OFF bipolar cells robustly express GABAC receptors, whereas OFF bipolar cells abundantly express GlyRα1. (C) GABAAα1 occupancy (%) at the axons and dendrites (Dend) of bipolar cells (see SI Appendix, SI Materials and Methods and text for definition of % occupancy). (D and E) GABACρ and GlyRα1 receptor occupancy (%) in bipolar cell axon terminals. All error bars represent SEM. Number of cells is shown in parentheses; for all cell types, n > 3 animals. ***P < 0.001.
Perturbing GABA Release Exerts Cell-Type-Specific Effects on Bipolar Cell Morphology and Cone Connectivity.
VIAAT is expressed by both mouse horizontal cells and amacrine cells (21). To assess the importance of GABA and glycine release on the formation and maintenance of inhibitory synapses on BCs, we perturbed vesicular release of inhibitory neurotransmitters specifically in the retina by abolishing VIAAT expression using αPax6-Cre (22) mice (VIAAT KO). In the KO, VIAAT immunoreactivity is virtually eliminated from both the outer and inner plexiform layer (OPL and IPL respectively, SI Appendix, Fig. S3A). To confirm the lack of inhibitory neurotransmission in the VIAAT KO, we performed whole-cell voltage clamp recordings from OFF BCs (SI Appendix, Fig. S3B). Indeed, OFF BCs in the VIAAT KO retina displayed a dramatically reduced frequency of spontaneous inhibitory postsynaptic currents (sIPSCs, SI Appendix, Fig. S3B), compared with BCs of the same type in littermate controls (control). Light-evoked inhibitory responses of OFF CBCs were also abolished in the VIAAT KO retina (SI Appendix, Fig. S3C).
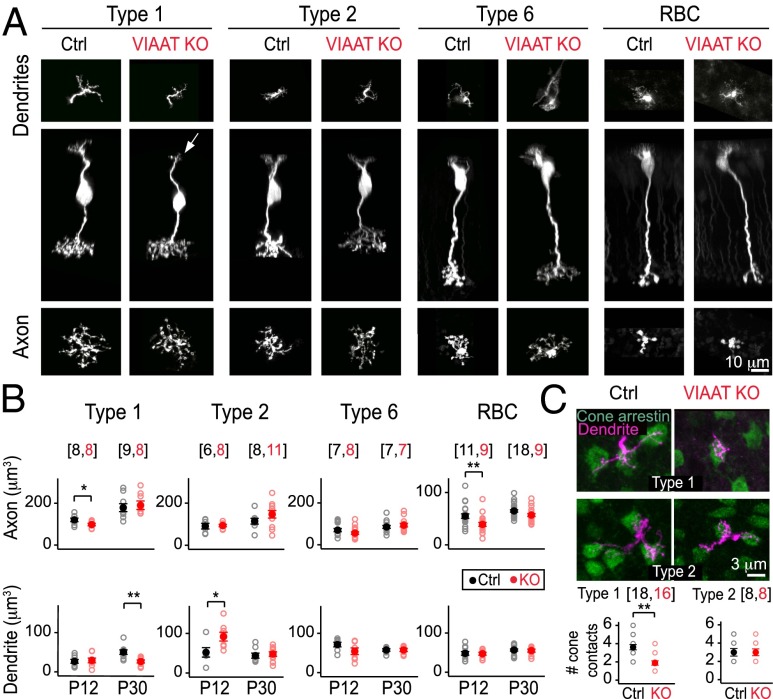
Because GABA can act as a trophic signal to influence morphogenesis (23, 24), we examined the axonal and dendritic morphologies of BCs in the VIAAT KO retina before eye-opening and at maturity (Fig. 2). In the mature retina [postnatal day (P) 30], the axonal and dendritic arbors of all BC types examined were unaffected in the VIAAT KO, with the exception that type 1 CBC dendritic arbors in the KO were smaller than normal (Fig. 2 A and B). Before eye-opening (P12), some BCs in the VIAAT KO retina exhibited transient differences in arbor sizes compared with controls (Fig. 2B). However, unlike in control retina, type 1 CBCs in the VIAAT KO did not increase their dendritic arbor size with maturation (control cells: P = 0.007, P12 versus P30; KO: P = 0.75, P12 versus P30; Fig. 2 B and C). Type 1 CBCs contacted fewer cone photoreceptors, as might be expected from their reduced dendritic territory (P = 0.003, Fig. 2C). Because type 1 dendritic arbors were normal in size early in development (Fig. 2B), the failure to contact more cones with maturation may be due to slowed dendritic growth and/or a failure to stabilize elaborating dendrites. Type 1 axon terminal sizes were normal in adult VIAAT KO retina (Fig. 2B), suggesting that their smaller dendritic arbors are not the result of a general impairment to cell growth. Together, these observations indicate a cell-type-specific role for VIAAT-mediated transmitter release in regulating the development and synaptic connectivity of BC dendrites.
Fig. 2.
Loss of VIAAT influences the morphology of ON and OFF bipolar cells in a cell-type-specific manner. (A) Types 1, 2, and 6 cone bipolar cells and rod bipolar cells (RBCs) in mature VIAAT knockout (KO) and littermate control (Ctrl) retinas. En face view of the dendrites and axon terminals of individual bipolar cells are displayed above and below the side view of the cell, respectively. Arrow points to the smaller dendritic arbor of a type 1 OFF cone bipolar cell in KO retina. (B) Quantification of the axonal (top plots) and dendritic (bottom plots) volumes of immature (postnatal day 12, P12) and mature (P30) bipolar cells in KO (red) and Ctrl (black) retinas. (C, Upper) Colabeling for cone arrestin (green) and dendrites of types 1 and 2 (magenta) OFF bipolar cells in adult retina. (Lower) Quantification of the number of cones contacted by type 1 and type 2 OFF bipolar cells in KO (red) and Ctrl (black) retinas. All error bars represent SEM. Number of cells in parentheses; for all cell types, n > 3 animals. Statistics for different cell types shown for Ctrl versus KO comparisons at each age. *P < 0.05; **P < 0.01.
GABA Receptor Expression on Axons and Dendrites of maturing Bipolar Cells Is Regulated Separately, and by Vesicular GABA Release from One Presynaptic Input Type.
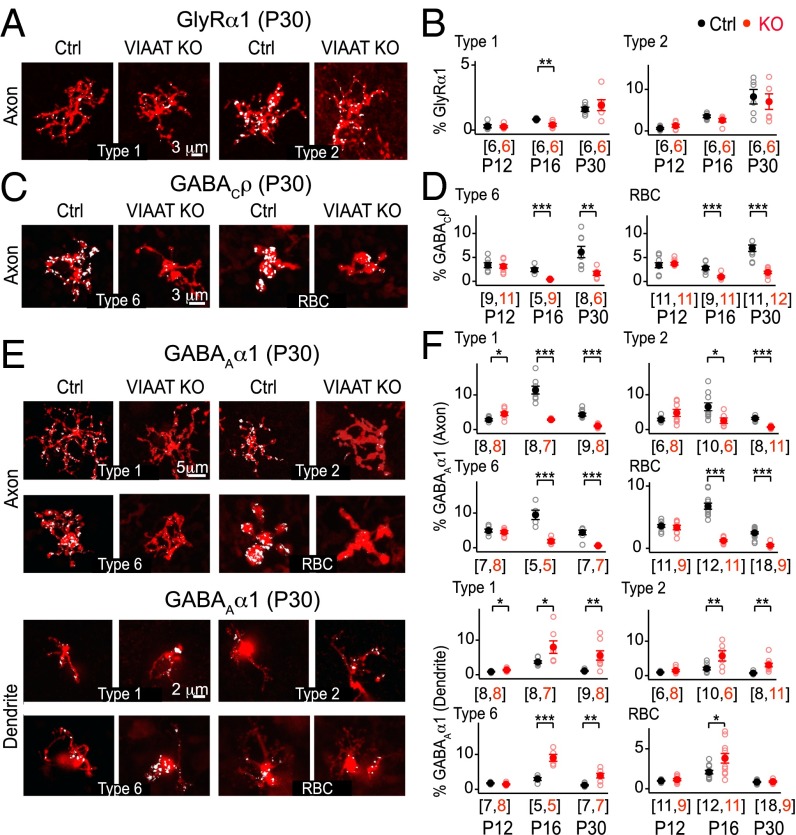
Does loss of VIAAT-mediated transmitter release perturb BC GABA or glycinergic synapse development in a receptor-type-specific and/or BC-type-specific manner? Could cell-compartment-specific alterations in receptor distribution occur on the axon and dendrites of BCs in VIAAT KO retina? To answer these questions, we analyzed GlyRα1 receptors on OFF BC (type 1 and 2) axons, GABAC receptors on ON BC (type 6 and RBC) axons, and GABAAα1 receptors on the axon and dendrites of all four BC types in VIAAT KO and control retinas (Fig. 3). GlyRα1 receptor expression on the axon terminals of OFF CBCs was unaffected in the P30 VIAAT KO retina (Fig. 3 A and B). In contrast, GABAC receptors were reduced on type 6 CBC and RBC axon terminals in VIAAT KO retina at this age (Fig. 3 C and D). Similarly, GABAAα1 receptor expression was diminished on the axon terminals of P30 RBCs and the CBC types studied in the VIAAT KO retina (Fig. 3 E and F, Top). However, CBC dendrites showed increased GABAAα1 receptor expression in the P30 VIAAT KO retina (Fig. 3 E and F). In contrast, RBC dendrites in the KO maintained GABAAα1 receptors at levels comparable to control (Fig. 3 E and F). Thus, impaired vesicular release of inhibitory neurotransmitters GABA and glycine in the retina causes disparate effects on GABA and glycine receptor distribution across BC types and cell compartments.
Fig. 3.
Bipolar cell Glycine and GABA receptors are differentially altered in VIAAT KO retina. (A) α1-subunit-containing glycine receptors (GlyRα1, white) on the axon terminals (red) of type 1 and type 2 OFF cone bipolar cells in adult (postnatal day (P)30), VIAAT knockout (KO) and control (Ctrl) retina. (B) Quantification of GlyRα1 occupancy (%) in type 1 and type 2 terminals in Ctrl and KO retina before eye-opening (P12), just after eye-opening (P16), and in adult (P30) retinas. (C) ρ-subunit-containing GABAC receptors (white) on the axon terminals (red) of type 6 ON cone bipolar cells and rod bipolar cells (RBCs) in P30 VIAAT KO and Ctrl retinas. (D) Quantification of GABAC receptor occupancy (%) in type 6 and RBC terminals in Ctrl and KO retinas at various ages. (E) Examples of GABAAα1 receptor immunoreactivity (white) on axons (Upper) and dendrites (Lower) of cone and rod bipolar cells (cell profile in red) in P30 Ctrl and VIAAT KO retinas. (F) Quantification of GABAAα1 expression (%) on the axonal and dendritic arbors in Ctrl and KO retina at various ages. Alterations in bipolar cell axonal and dendritic GABAAα1 receptor expression occur around eye-opening in the KO retina. Error bars represent SEM. Number of cells in parentheses; n > 3 animals for all cell types. For each cell type, Ctrl versus KO values are compared at each age, and statistical differences indicated. *P < 0.05; **P < 0.01; ***P < 0.001.
The changes we observed in GABA receptor expression in the mature VIAAT KO retina could arise from a failure to localize receptors early in development and/or an impairment of their maintenance thereafter. To distinguish between these possibilities, we examined BC GABA receptor expression in the VIAAT KO retina at two developmental time points: P12, before eye-opening and P16 after eye-opening (Fig. 3 D and F and SI Appendix, Fig. S4). Axonal GABAC receptors on ON BCs were reduced in P16 VIAAT KO retina compared with controls (Fig. 3D and SI Appendix, Fig. S4). Similarly, axonal expression of GABAAα1 receptors was reduced by P16 for all BC types studied in VIAAT KO compared with control (Fig. 3F and SI Appendix, Fig. S4). In control animals, axonal GABAAα1 receptor expression increased significantly from P12 to P16 (P < 0.03 for all four BC types), but this developmental increase did not occur in the VIAAT KO. In fact, in the KO, axonal GABAAα1 receptor expression decreased in type 1 and 6 CBCs and RBCs between P12 and P16 (P < 0.009 for the three BC types), whereas axonal receptor expression did not change between P12 and P16 in type 2 CBCs (P = 0.128). Together, these observations indicate that the initial accumulation of GABA receptors on BC axons is largely unaffected in the VIAAT KO, but the further localization and maintenance of these receptors after eye-opening is disrupted in the absence of vesicular GABA release.
GABAAα1 receptor expression on BC dendrites in VIAAT KO retina also increased relative to control at P16 (Fig. 3F and SI Appendix, Fig. S4). At P16, dendritic expression of GABAAα1 in the KO retina increased above normal values for the CBCs and RBCs, but remained elevated in the mature retina only at CBC but not RBC dendrites (P30, Fig. 3F). Thus, the RBC dendritic compartment has the capacity to reset its GABAAα1 expression to wild-type levels by maturity.
To confirm that alterations in GABAAα1 receptor expression reflect functional changes, we recorded GABA-evoked responses from adult retinal BCs by puffing GABA onto their axon terminals or dendrites (Fig. 4). Indeed, puffing GABA onto axons of RBCs in the KO elicited a much-reduced response compared with cells in control retina (Fig. 4A). Because RBC terminals have both GABAA and GABAC receptors, we isolated the GABAA component by blocking GABAC receptors with TPMPA. The GABAA-evoked response was significantly reduced at RBC terminals in VIAAT KO retinas (Fig. 4A), corroborating the immunolabeling results. Consistent with the GABAC immunostaining, the GABAC component of the RBC terminal was also significantly reduced in the VIAAT KO (mean peak amplitudes: control = 100 ± 11 pA, KO = 48 ± 9 pA, P = 0.007). GABA application at RBC dendrites generated equivalent responses in KO and control retinas, also consistent with the immunostaining (Fig. 4B, Left). TPMPA did not reduce the dendritic GABA-evoked PSC amplitude in RBCs in the KO retina (Fig. 4B, Right), suggesting that RBC dendritic receptors are predominantly GABAA receptors. In keeping with these observations, puffing GABA onto dendrites of isolated mouse RBCs elicits GABAA- and not GABAC-mediated currents (25). In contrast to RBC responses, OFF CBC dendritic responses to puffed GABA were enhanced in the VIAAT KO retina compared with control (Fig. 4C, Left). The OFF CBC dendritic response in KO retina is mediated primarily by GABAA receptors (Fig. 4C, Right).
Fig. 4.

Bipolar cell axonal and dendritic alterations of GABA receptor expression in VIAAT KO retina correspond to functional changes. (A) GABA application onto axon terminals of adult rod bipolar cells (BCs) in VIAAT knockout (KO) and littermate control (Ctrl) retinas before (GABAA + GABAC) and after (GABAA) application of the GABAC receptor antagonist, TPMPA. Examples of the evoked currents (cells held at 0 mV) are displayed above the population peak response amplitude plots. (B) Outward currents evoked upon puffing GABA onto rod BC dendrites. (Left) GABA puff responses of Ctrl and KO rod BCs. (Right) GABA-evoked responses in a subset of rod BCs in the KO retina were measured before and after TPMPA application. (C) Responses from adult OFF cone bipolar cells (sampled across type 1 and type 2 BCs) evoked by puffing GABA onto their dendrites. (Left) OFF BCs in KO retina compared with Ctrl. (Right) For a subset of OFF BCs in the KO retina, GABA-evoked dendritic responses were measured before and after TPMPA application. All error bars indicate SEM. Number of cells is shown in parentheses; for all recordings, n > 3 animals. **P < 0.01; ***P < 0.001.
Collectively, our immunolabeling and electrophysiological experiments demonstrate that GABAC receptors at ON BC terminals and GABAAα1 receptors at ON and OFF BC axons are sensitive to loss of vesicular transmitter release. In addition, GABAAα1 receptors at BC dendrites increase in CBCs but not RBCs when vesicular release of GABA is impaired, suggesting rod/cone pathway-specific alterations in the outer retina.
Compartment (axon versus dendrite) specific alterations of GABAAα1 expression on BCs raised an important question: Does loss of VIAAT in both major inhibitory presynaptic cell classes, amacrine and horizontal interneurons, contribute to the GABAAα1 expression changes? To answer this question, we examined GABAAα1 immunoreactivity in retinas in which VIAAT was selectively eliminated from horizontal cells (HC VIAAT KO, Fig. 5). The pan-VIAAT KO line, generated by crossing with the αPax6-Cre line, showed the expected overall reduction of GABAAα1 immunoreactivity in the IPL and elevation of the signal in the OPL relative to control (Fig. 5A). In the HC VIAAT KO, however, GABAAα1 labeling in the IPL and OPL appeared normal (Fig. 5 B and C). Thus, GABAAα1 expression in the IPL and OPL depends on vesicular GABA release from amacrine cells but not horizontal cells. Our results, however, cannot exclude the possibility that nonvesicular release of GABA from horizontal cells could contribute to GABAAα1 regulation.
Fig. 5.
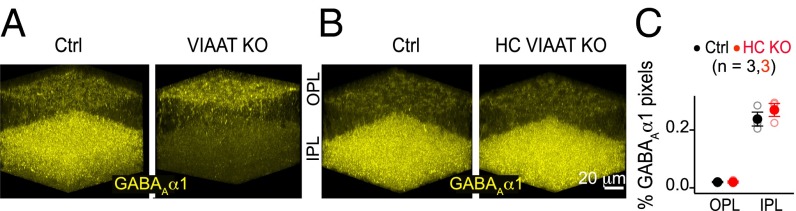
Bipolar cell GABAA receptor alterations in VIAAT deficient retina are due to impaired vesicular GABA release from amacrine but not horizontal cells. (A) α1-subunit-containing GABAA (GABAAα1) receptor immunolabeling in outer (OPL) and inner (IPL) plexiform layers of adult VIAAT knockout (KO) and littermate control (Ctrl) retinas (n = 10 KO–Ctrl pairs). (B) GABAAα1 immunoreactivity when VIAAT is selectively deleted in horizontal cells (HCs) using Cx57-Cre mice (HC VIAAT KO; n = 3 KO–Ctrl pairs). (C) Quantification of the % of pixels in the GABAAα1 receptor channel above background in OPL and IPL of Ctrl and HC VIAAT KO retinas. Number of animals is shown in parentheses.
Differential Regulation of Axonal and Dendritic GABAAα1 Subunits May Depend on Coassembly with GABAAγ2 Subunit.
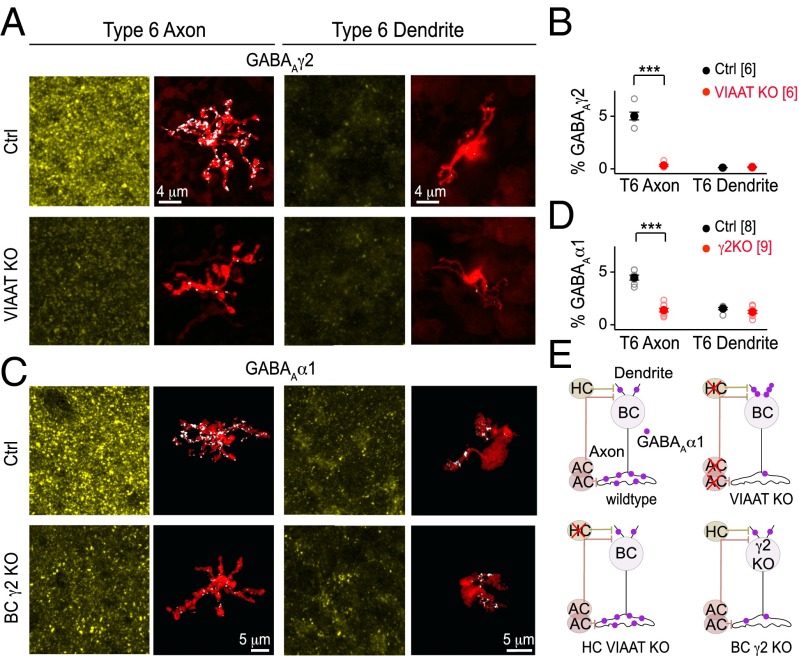
GABAAα1 expression on BC axons and dendrites may be regulated differentially if GABAA receptors in these separate compartments have different subunit compositions. Alternatively, GABAA receptors on the axons and dendrites could have the same subunit composition, but their regulation by activity could depend on their subcellular location. To distinguish between these possibilities, we immunolabeled adult wild-type retina for the γ2 subunit of the GABAA receptor (GABAAγ2) known to be abundant in the retina (15), and localized at the majority of CNS GABAergic synapses (26). We found that type 6 CBC axon terminals robustly express GABAAγ2, but surprisingly, their dendrites are deficient in this subunit (SI Appendix, Fig. S5A). In fact there was very little GABAAγ2 immunoreactivity in the OPL (SI Appendix, Fig. S5A). Using cross-correlation analysis, we confirmed that GABAAα1 and γ2 immunolabeling overlapped highly at type 6 CBC axon terminals (SI Appendix, Fig. S5 B and C).
These observations raise the possibility that GABAA receptors on BC axon and dendrites may not have the same subunit composition. This also makes the prediction that loss of axonal GABAAα1 in the VIAAT KO retina should be accompanied by a loss of axonal GABAAγ2. Indeed, we found that axonal GABAAγ2 in type 6 CBCs was reduced in VIAAT KO retina compared with control but dendritic GABAAγ2 expression was not altered (Fig. 6 A and B). We focused on type 6 CBCs because their morphology remains unaltered in the VIAAT KO (Fig. 2). To further examine whether GABAAγ2 coassembles with axonal but not dendritic GABAAα1, we genetically deleted GABAAγ2 specifically in ON BCs by crossing GABAAγ2 conditional KO mice (27) with a transgenic line we created in which Cre-recombinase is expressed by ON BCs shortly after their differentiation (Grm6–Cre mice; Fig. 6C, SI Appendix, Fig. S6 and SI Materials and Methods; ref. 28). As expected, we found that a loss of GABAAγ2 in type 6 BCs caused a reduction in axonal but not dendritic GABAAα1 receptors (Fig. 6 C and D). Together, these observations demonstrate that axonal but not dendritic GABAAα1 receptors on BCs coassemble with the γ2 subunit, and lend support to the possibility that GABAA receptors with distinct compositions, rather than the same receptors at separate cell compartments, are differentially regulated by vesicular GABA release.
Fig. 6.
Bipolar cell axonal and dendritic GABAA receptors have different subunit compositions. (A) γ2-subunit-containing GABAA (GABAAγ2) receptor immunolabeling (yellow) in VIAAT knockout (KO) and littermate control (Ctrl) retinas in the same field as the labeled bipolar cell (BC). GABAAγ2 receptors (white) on a type 6 cone BC (red). (B) GABAAγ2% occupancy on the axonal and dendritic arbors of type 6 BCs in VIAAT KO and Ctrl. (C) GABAAα1 receptor immunolabeling (yellow) in Ctrl retinas and retinas with ON BC-specific GABAAγ2 deletion [BC γ2 KO: GABAAγ2 conditional knockout (cKO)/Grm6-Cre mice]. Examples of GABAAα1 receptors (white) on type 6 BC axons and dendrites (red). (D) Quantification of GABAAα1 receptor occupancy (%) on type 6 BCs in γ2 KO and Ctrl. Error bars represent SEM. Number of cells is shown in parentheses; all genotypes, n > 3 animals. ***P < 0.001. (E) Schematic summarizing compartment-specific changes in the expression of BC axonal and dendritic GABAAα1 receptors in pan-VIAAT KO (both horizontal cell (HC) and amacrine cell (AC) affected), HC-specific VIAAT KO (HC VIAAT KO), and BC-specific γ2 KO.
Discussion
Our observations corroborate previous findings showing that inhibitory neurotransmission is not essential for synapse formation but is necessary for synaptic maturation (8–11, 29). However, our findings here add significantly to our current understanding of how neurotransmission influences the in vivo maturation of inhibitory circuits. We found that alterations in inhibitory receptor expression are not simply a general and uniform response to an overall loss of inhibitory drive in the network. Instead, (i) inhibitory transmitter release plays a central role in dictating the levels of GABA but not glycine receptor expression on retinal BCs, and (ii) activity-dependent regulation of GABA receptor expression is cell-compartment (axon versus dendrite) and input-type specific (summarized in Fig. 6E).
Activity-Dependent Regulation of Inhibitory Receptor Expression Varies with Presynaptic Input Type.
The diversity of inhibitory connections onto retinal BCs enabled us to investigate the dependence of postsynaptic expression of inhibitory receptors on presynaptic input type. We found that glycine receptor expression on OFF CBC terminals is unchanged in VIAAT KOs even though GABAA receptors on the same axons are reduced. Spinal cord cultures from VIAAT KO animals also show no alteration in the density of glycine receptors (8). Even among GABAergic connections, the effects of neurotransmission on GABAergic synapse development and maturation have been found to vary with input types (30–32). After visual deprivation, transmission from basket interneurons onto dendrites of Layer 4 pyramidal cortical neurons in the visual cortex is reduced whereas input from regular spiking nonpyramidal interneuron is increased (31). Our study further uncovered distinct requirements for vesicular GABA release in regulating the maturation of synaptic contact from separate presynaptic GABAergic cell types on the axon and dendrite of an individual neuron. Comparing pan-VIAAT and HC-VIAAT KO retinas, we discovered that vesicular GABA release from amacrine but not horizontal cells regulates BC GABA receptors. Moreover, even among amacrine cells, expression of GABAA receptors at amacrine synapses within the IPL versus the OPL are regulated in distinct ways. In the VIAAT KO, GABAAα1 at synapses onto BC axons is down-regulated whereas its expression on the dendrites is up-regulated. Furthermore, different ionotropic GABA receptor types (GABAA and GABAC) clustered at nonoverlapping sites (33, 34) but opposite the same presynaptic A17 amacrine cell bouton (35) are similarly down-regulated in the VIAAT KO. Together, these observations imply that activity-dependent regulation of inhibitory receptors is input type-specific.
Activity Differentially Controls α1-Subunit Containing GABAA Receptor Expression on Bipolar Cell Axons and Dendrites.
A differential effect on axonal and dendritic GABAA receptors has been observed in hippocampal cell cultures in which chronic depolarization increased the GABAA receptor mobility in the axon initial segment but not in dendrites (36). We found that in vivo GABAAα1 expression at input and output compartments of individual BCs are altered differently in the absence of GABA-mediated transmission. In VIAAT KO retina, cone bipolar axonal GABAAα1 is lost whereas dendritic GABAAα1 is up-regulated. The homeostatic increase of dendritic GABAAα1 receptors in the absence of GABA release is reminiscent of the increase in glutamate receptors found in other brain regions when excitatory transmission is suppressed or blocked (37, 38). Homeostatic regulation of dendritic GABAAα1 receptor levels in BCs, however, is pathway specific: Cone but not rod BCs maintain elevated GABAAα1 levels in VIAAT KO retina at maturity. Differential effects on transmitter receptors on rod versus cone bipolar dendrites have also been observed in visually deprived mice where metabotropic glutamate receptors on cone but not rod BC dendrites are up-regulated (39).
What factors could be responsible for the distinct activity-dependent changes of receptor expression in BC axons and dendrites? Although contact with different amacrine subtypes could be responsible for the opposite outcomes, it is also possible that “postsynaptic” factors play a role. We found that GABAA receptors on the axons but not dendrites of BCs are composed of GABAAγ2 subunit. Thus, differences in receptor subunit composition may explain why axonal and dendritic GABAA receptors of the same BC are regulated in opposite directions by GABA release. Our observations also raise the possibility that GABAA receptors with α1γ2 subunit composition could be highly susceptible to perturbed neurotransmission. The γ2 subunit is necessary for synaptic localization of GABAA receptors (3). Thus, α1γ2 receptors may be sensitive to loss of GABA release because they are clustered directly opposite transmitter release sites, whereas receptors further away may be less susceptible. GABAA receptors on BC axons are localized at synapses (11), but the location of BC dendritic GABAA receptors relative to GABA release sites in the OPL is not yet known. It is also possible that regardless of their location, α1γ2-subunit-containing GABAA receptors at all synapses require presynaptic GABA release for their maintenance. If so, one may find that such receptors on other neurons or at other cellular compartments exhibit the same form of regulation by GABAergic transmission.
A final factor to consider is that the effects of GABA release on synaptic development or maintenance could be “dose dependent.” For example, reducing GABA synthesis by deleting one of the GABA synthetic enzymes, GAD1, from basket interneurons decreases synapse size and density, as well as axonal arbor size (9). In contrast, complete blockade of inhibitory transmission (VIAAT KO) yields opposite effects comprising overproliferation of small synapses and overgrowth of the axonal arbor (10). We also found that in retinas lacking GAD1, GABAAα1 but not GABAC receptor expression on RBC terminals is diminished (11). However, our current observations show a loss of both receptor types in VIAAT KO retina. The disparate observations from GAD1 and VIAAT retinal KOs may be explained by GABAC receptors having a higher affinity for GABA compared with GABAAα1 (40). Lower levels of GABA in the GAD1 mutant could thus be sufficient to maintain GABAC receptors, but already trigger loss of GABAAα1 receptors.
In summary, our study has distinguished the role of vesicular release of inhibitory transmitters per se from the effects produced by overall changes in network activity on the postsynaptic expression of inhibitory receptors in vivo. This regulation is specific to presynaptic input type, and varies with receptor subunit composition, cellular compartment, and the levels of transmitter release. Although complex, such diverse roles for inhibitory transmitter release provide a rich platform from which pre- and postsynaptic mechanisms can be selected to control the maturation and maintenance of distinct inhibitory connections within the network.
Materials and Methods
Transgenic Mouse Lines.
All animal experiments were carried out in accordance with the Institutional Animal Care and Use Committee at the University of Washington and University of California, Los Angeles. Vsx1-cerulean, Grm6-tdTomato, αPax6-Cre/VIAAT KO, Cx57-Cre/VIAAT KO, and Ai9/ GABAAγ2 conditional KO/Grm6-Cre mouse lines were used in this study. Detailed information on the transgenic lines is provided in SI Appendix, SI Materials and Methods.
Immunolabeling.
Retinas were isolated in cold oxygenated mouse artificial cerebrospinal fluid and fixed in 4% (wt/vol) paraformaldehyde. Primary antibodies were directed against: VIAAT, GFP, Synaptotagmin-2, GlyRα1-subunit, GABAAα1-subunit, GABAAα3-subunit, GABAAγ2-subunit, GABACρ-subunit, RFP, DsRed, PKC, and cone arrestin. Detailed information on immunohistochemistry is provided in SI Appendix, SI Materials and Methods.
Image Acquisition and Analysis.
Samples were imaged using an Olympus FV 1000 laser scanning confocal microscope and images were processed using MetaMorph (Molecular Devices) and Amira (FEI Visualization Sciences Group) software. Detailed information on image analysis routines is provided in SI Appendix, SI Materials and Methods.
Functional Recordings and Data Analysis.
Retinal slices (200 µm thick) were prepared from dark-adapted VIAAT KO and control mice as described (41). Detailed information on functional recordings and analyses is provided in SI Appendix, SI Materials and Methods.
Statistics.
As the data passed the normality test, for control–KO comparisons, a two-tailed unpaired T test was used. For comparing cone contact numbers, Wilcoxon–Mann–Whitney rank sum test was used. *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary Material
Acknowledgments
We thank J. M. Fritschy, R. Enz, H. Wässle and S. Haverkamp for their generous gifts of GABA receptor antibodies, and B. Luscher for GABAγ2lox mice. We also thank R. Lewis for assistance with generating Grm6-Cre mice and C. Gamlin and P. Mardoum for helpful comments on the manuscript. This work was supported by Knights Templar Eye Foundation (career starter grant to M.H.), Human Frontier Science Foundation (long-term fellowship to R.S.), VA Career Scientist Award (to N.B.), the Howard Hughes Medical Institute (F.R.), and NIH Grants EY10699 (to R.O.L.W.), EY11850 (to F.R.), Vision Core Grant EY01730 (to M. Neitz), and EY15573 (to N.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510483112/-/DCSupplemental.
References
- 1.Jadi M, Polsky A, Schiller J, Mel BW. Location-dependent effects of inhibition on local spiking in pyramidal neuron dendrites. PLOS Comput Biol. 2012;8(6):e1002550. doi: 10.1371/journal.pcbi.1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16(4):815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AM, Jovanovic JN. Mechanisms underlying synapse-specific clustering of GABA(A) receptors. Eur J Neurosci. 2010;31(12):2193–2203. doi: 10.1111/j.1460-9568.2010.07252.x. [DOI] [PubMed] [Google Scholar]
- 4.Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007;582(Pt 2):569–582. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wässle H, et al. Glycinergic transmission in the Mammalian retina. Front Mol Neurosci. 2009;2:6. doi: 10.3389/neuro.02.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: Implications for structure-function relations and synaptic transmission. J Physiol. 1995;489(Pt 2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagné C, et al. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417(2):177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- 8.Wojcik SM, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50(4):575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyaya B, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54(6):889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, et al. GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. J Neurosci. 2012;32(1):331–343. doi: 10.1523/JNEUROSCI.3189-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schubert T, Hoon M, Euler T, Lukasiewicz PD, Wong RO. Developmental regulation and activity-dependent maintenance of GABAergic presynaptic inhibition onto rod bipolar cell axonal terminals. Neuron. 2013;78(1):124–137. doi: 10.1016/j.neuron.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlachos A, Reddy-Alla S, Papadopoulos T, Deller T, Betz H. Homeostatic regulation of gephyrin scaffolds and synaptic strength at mature hippocampal GABAergic postsynapses. Cereb Cortex. 2013;23(11):2700–2711. doi: 10.1093/cercor/bhs260. [DOI] [PubMed] [Google Scholar]
- 13.Hoon M, Okawa H, Della Santina L, Wong RO. Functional architecture of the retina: Development and disease. Prog Retin Eye Res. 2014;42:44–84. doi: 10.1016/j.preteyeres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2(4):240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 15.Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38(10):1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- 16.Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460(7258):1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun MH, Wässle H. GABA-like immunoreactivity in the cat retina: Electron microscopy. J Comp Neurol. 1989;279(1):55–67. doi: 10.1002/cne.902790106. [DOI] [PubMed] [Google Scholar]
- 18.Fisher LJ. Interplexiform cell of the mouse retina: A Golgi demonstration. Invest Ophthalmol Vis Sci. 1979;18(5):521–523. [PubMed] [Google Scholar]
- 19.Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci. 2011;28(1):95–108. doi: 10.1017/S0952523810000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sassoè-Pognetto M, Wässle H, Grünert U. Glycinergic synapses in the rod pathway of the rat retina: Cone bipolar cells express the alpha 1 subunit of the glycine receptor. J Neurosci. 1994;14(8):5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cueva JG, et al. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J Comp Neurol. 2002;445(3):227–237. doi: 10.1002/cne.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquardt T, et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105(1):43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 23.Belhage B, Hansen GH, Elster L, Schousboe A. Effects of gamma-aminobutyric acid (GABA) on synaptogenesis and synaptic function. Perspect Dev Neurobiol. 1998;5(2-3):235–246. [PubMed] [Google Scholar]
- 24.Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28(6):278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Varela C, Igartua I, De la Rosa EJ, De la Villa P. Functional modifications in rod bipolar cells in a mouse model of retinitis pigmentosa. Vision Res. 2003;43(8):879–885. doi: 10.1016/s0042-6989(02)00493-5. [DOI] [PubMed] [Google Scholar]
- 26.Möhler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326(2):505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 27.Schweizer C, et al. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24(2):442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 28.Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9(1):85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- 29.Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoè-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the alpha1 subunit in Purkinje cells. J Neurosci. 2006;26(12):3245–3255. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartley AF, Huang ZJ, Huber KM, Gibson JR. Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits. J Neurophysiol. 2008;100(4):1983–1994. doi: 10.1152/jn.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7(12):1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- 32.Bloodgood BL, Sharma N, Browne HA, Trepman AZ, Greenberg ME. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature. 2013;503(7474):121–125. doi: 10.1038/nature12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fletcher EL, Koulen P, Wässle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol. 1998;396(3):351–365. doi: 10.1002/(sici)1096-9861(19980706)396:3<351::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Koulen P, Brandstätter JH, Enz R, Bormann J, Wässle H. Synaptic clustering of GABA(C) receptor rho-subunits in the rat retina. Eur J Neurosci. 1998;10(1):115–127. doi: 10.1046/j.1460-9568.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 35.Chávez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci. 2010;30(6):2330–2339. doi: 10.1523/JNEUROSCI.5574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muir J, Kittler JT. Plasticity of GABAA receptor diffusion dynamics at the axon initial segment. Front Cell Neurosci. 2014;8:151. doi: 10.3389/fncel.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13(5):560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 39.Dunn FA, Della Santina L, Parker ED, Wong RO. Sensory experience shapes the development of the visual system’s first synapse. Neuron. 2013;80(5):1159–1166. doi: 10.1016/j.neuron.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur J Pharmacol. 1994;288(1):97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 41.Field GD, Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron. 2002;35(4):733–747. doi: 10.1016/s0896-6273(02)00822-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.