Significance
Our study advances the understanding of how plants make cellulose, a critical constituent of the plant cell wall and the most abundant organic polymer in the terrestrial biosphere. Cellulose is synthesized at the plasma membrane by cellulose synthase (CESA) complexes. Here we highlight the importance of regulated CESA endocytosis in cellulose biosynthesis in Arabidopsis. Furthermore, we show that the TWD40-2 protein, a putative member of the TPLATE complex, functions in clathrin-mediated endocytosis (CME) in ways that are both distinct from and cooperative with the functionally conserved CME adaptor protein 2 (AP2) complex. Because the TPLATE complex of plants is not conserved in animals, our characterization of TWD40-2 offers the opportunity to provide insight into the evolution of eukaryotic trafficking machinery.
Keywords: cellulose synthase, endocytosis, clathrin, plasma membrane, trafficking
Abstract
Cellulose biosynthesis is performed exclusively by plasma membrane-localized cellulose synthases (CESAs). Therefore, the trafficking of CESAs to and from the plasma membrane is an important mechanism for regulating cellulose biosynthesis. CESAs were recently identified as cargo proteins of the classic adaptor protein 2 (AP2) complex of the clathrin-mediated endocytosis (CME) pathway. The AP2 complex of the CME pathway is conserved in yeast, animals, and plants, and has been well-characterized in many systems. In contrast, the recently discovered TPLATE complex (TPC), which is proposed to function as a CME adaptor complex, is only conserved in plants and a few other eukaryotes. In this study, we discovered that the TWD40-2 protein, a putative member of the TPC, is also important for the endocytosis of CESAs. Genetic analysis between TWD40-2 and AP2M of the AP2 complex revealed that the roles of TWD40-2 in CME are both distinct from and cooperative with the AP2 complex. Loss of efficient CME in twd40-2-3 resulted in the unregulated overaccumulation of CESAs at the plasma membrane. In seedlings of twd40-2-3 and other CME-deficient mutants, a direct correlation was revealed between endocytic deficiency and cellulose content deficiency, highlighting the importance of controlled CESA endocytosis in regulating cellulose biosynthesis.
Cellulose is synthesized at the plasma membrane (PM) by large cellulose synthase (CESA) complexes (CSCs) that directly extrude the nascent cellulose polymers into the cell wall (1–3). Fluorescent protein fusions of CESA proteins (FP-CESAs) have been instrumental in advancing our understanding of cellulose biosynthesis (4, 5). The delivery of CSCs to the PM has been shown to coincide with the position of cortical microtubules (CMTs) that run along the cytoplasmic surface of the PM (6, 7). At the PM, CSCs are joined by cellulose synthase interactive (CSI1 and CSI3) proteins during cellulose biosynthesis that link the PM-localized CSCs to the CMTs and guide CSC motility, which is believed to be driven by cellulose biosynthesis, laterally in the plane of the PM in linear trajectories along CMTs (8–10). Plant cells contribute a great deal of energy not only to the biosynthesis of cellulose but also to the synthesis, assembly, distribution, and organization of CSCs (3). The prerequisite that CSCs must be localized to the PM to synthesize cellulose and that CSCs are both delivered to and travel along CMTs highlights the importance of coordinated CSC trafficking in the regulation of cellulose biosynthesis.
The organized and particulate distribution of FP-CESAs at the PM makes CESA an ideal model cargo protein for the investigation of trafficking pathways and mechanisms in Arabidopsis (3). In fact, we recently identified CESA as a bona fide cargo protein of the clathrin-mediated endocytosis (CME) pathway (11). In that study, we showed that the medium subunit of the heterotetrameric CME adaptor protein 2 (AP2) complex, AP2M (previously referred to as μ2), directly interacts with CESAs to mediate CESA endocytosis and that inefficient CESA endocytosis in ap2m-1 (previously referred to as μ2-1) caused an increased density of FP-CESAs at the PM (11). Together, that study and others functionally characterized the existence of the AP2 complex in plants (11–15), which, in a wide variety of eukaryotes, acts as a central core in CME by docking to the PM and recruiting cargo proteins, clathrin cages, and other accessory proteins to the sites of CME (16). Recently, a series of coimmunoprecipitation experiments identified a new putative CME adaptor complex in plants, the TPLATE complex (TPC) (17). Subsequently, TPC components were shown to be partially or fully conserved in a few other eukaryotes, and have been speculated to represent the remnants of an evolutionarily ancient trafficking complex that existed in the last eukaryotic common ancestor (18). In Arabidopsis, many TPC mutations are lethal, but live-cell imaging and artificial knockdown of two TPC components, TML and TPLATE, have exposed a functional relationship between the TPC, AP2, and CME (17).
In the current study, a screen for mutants that exhibit short etiolated hypocotyls, a feature that is sometimes associated with cellulose deficiency, identified SALK_112497, which was named twd40-2-3 because it is allelic to two mutants of TWD40-2, a putative member of the TPC. The previously identified twd40-2-1 and twd40-2-2 alleles have a male sterility phenotype that prevented functional characterization of TWD40-2 (17). Currently, the only evidence connecting TWD40-2 to the TPC or to CME is its ability to coimmunoprecipitate with TPC components (17). Therefore, the specific function of TWD40-2 remains uncharacterized, and it remains unknown whether the association between TWD40-2 and the TPC is permanent or transient. Homozygous twd40-2-3 mutants are viable and propagable, which has enabled us to use functional genetics to investigate the role of TWD40-2 in endocytosis and to learn how defects in endocytosis interfere with cellulose biosynthesis through disrupting CESA trafficking.
Results
twd40-2-3 Was Isolated from a Short Etiolated Hypocotyl Screen and Shows a Genetic Interaction with ap2m-1.
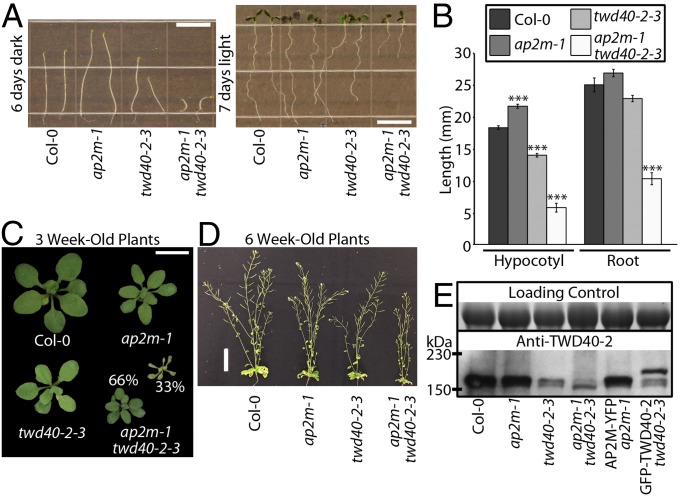
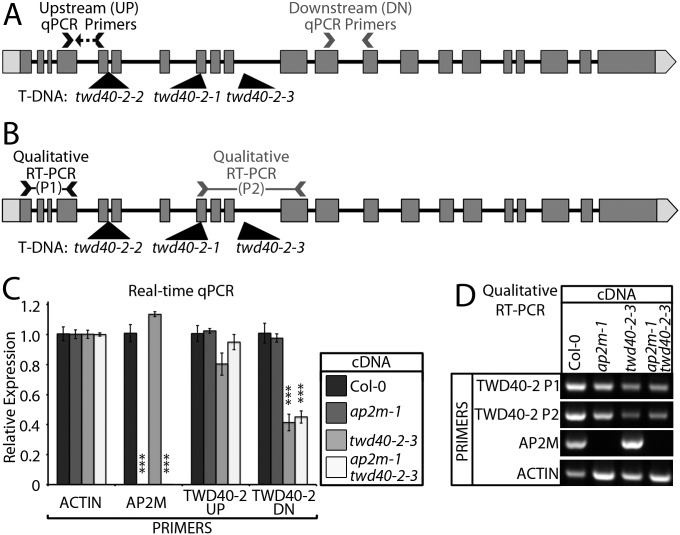
A large pool of T-DNA insertion lines was screened for mutants that exhibited short etiolated hypocotyls. twd40-2-3 not only displayed short etiolated hypocotyls but also had short roots in light-grown seedlings and dwarfed adult plants (Fig. 1 A–D). Although twd40-2-3 is propagable, twd40-2-1 and twd40-2-2 were previously shown to be lethal due to a male sterility phenotype (17). To discern the nature of twd40-2-3, anti–TWD40-2 antibodies were raised against heterologously expressed TWD40-2 and used for Western blot analysis. Anti–TWD40-2 recognized a protein with an electrophoretic mobility that was slightly larger than the predicted molecular mass of TWD40-2 of ∼150 kDa (Fig. 1E). TWD40-2 protein expression was specifically knocked down, but not nullified, in twd40-2-3, which may explain the viability of twd40-2-3 mutants (Fig. 1E). To understand the transcriptional influence of twd40-2-3, real-time quantitative (q)PCR analysis was performed with primer pairs upstream (UP) and downstream (DN) of the T-DNA insertion site (Fig. S1 A and C). In twd40-2-3, UP primers indicated normal levels of TWD40-2 expression but DN primers indicated an approximate 60% reduction in TWD40-2 expression (Fig. S1C), suggesting that incomplete TWD40-2 transcripts exist in twd40-2-3. Furthermore, RT-PCR with a primer set that flanks the T-DNA insertion site (P2) amplified a knocked-down level of TWD40-2 transcripts in twd40-2-3, which suggests that the T-DNA can be spliced out of some TWD40-2 transcripts in twd40-2-3 (Fig. S1 B and D).
Fig. 1.
twd40-2-3 is a knockdown allele that exhibits dwarf phenotypes and shows a genetic interaction with ap2m-1. (A and B) The length of etiolated seedlings and light-grown seedling roots was measured. (Scale bars, 10 mm.) Error bars are SEM. ***P < 0.0001 (10 ≤ n ≤ 13 for each genotype). (C and D) Col-0, ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 plants are shown at 3 and 6 wk old. Thirty-three percent of ap2m-1 twd40-2-3 mutants died at 3 wk. [Scale bars, 1 cm (C) and 5 cm (D).] (E) Western blot analysis of TWD40-2 expression in total protein extracts from Col-0, ap2m-1, twd40-2-3, ap2m-1 twd40-2-3, AP2M-YFP ap2m-1, and GFP-TWD40-2 twd40-2-3 seedlings. TWD40-2 protein levels are reduced in seedlings harboring twd40-2-3. GFP-TWD40-2 twd40-2-3 shows both knocked-down endogenous TWD40-2 and mobility-shifted GFP-TWD40-2. The loading control is a Coomassie gel.
Fig. S1.
Influence of ap2m-1 and twd40-2–3 on AP2M and TWD40-2 transcription levels. (A) The gene model of TWD40-2 shows the positions of the qPCR primer pairs (UP and DN) and the positions of the T-DNA insertions of twd40-2-1, twd40-2-2, and twd40-2-3. (B) The gene model of TWD40-2 shows the positions of the RT-PCR primer pairs (P1 and P2) and the positions of the T-DNA insertions of twd40-2-1, twd40-2-2, and twd40-2-3. (C) The ΔΔCt method was used to perform a qPCR analysis that shows the relative mRNA levels of AP2M and TWD40-2 at the UP and DN primer loci. ACT2 was used as the reference gene. Error bars are SEM. ***P < 0.0001 (n = 5 or 6 for each cDNA–primer pair combination). (D) RT-PCR analysis of RNA from Col-0, ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 seedlings was performed using upstream (P1) and flanking (P2) primers of the twd40-2-3 insertion site, AP2M-specific primers, and ACT2-specific primers as a control.
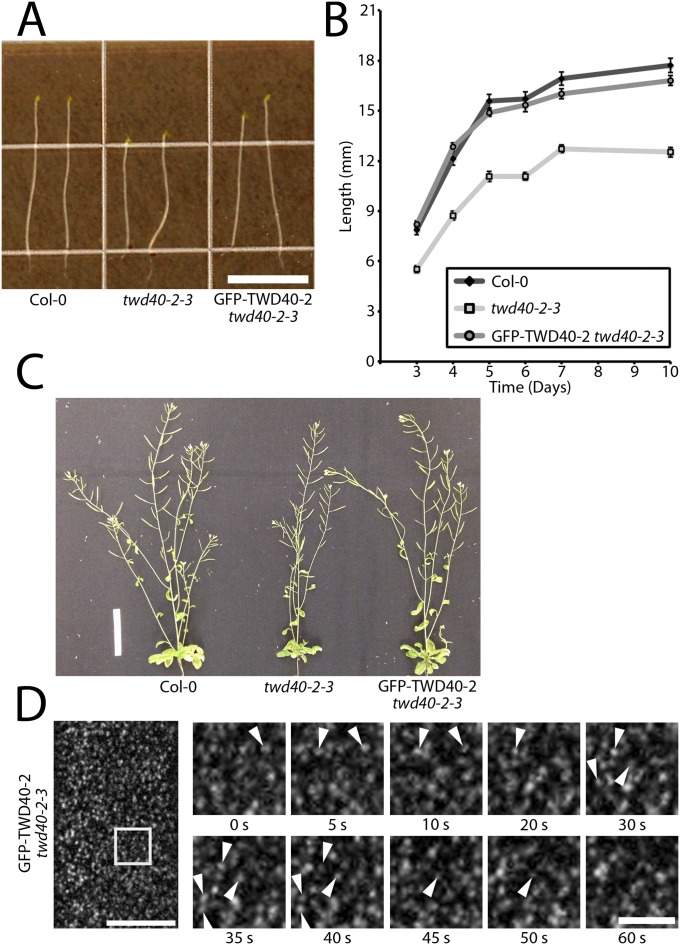
To gain insight into the function of TWD40-2, a construct containing GFP-TWD40-2 driven by its native promoter was transformed into twd40-2-3, which rescued the growth defects of twd40-2-3 (Fig. S2 A–C). As expected, Western blot analysis of GFP-TWD40-2 twd40-2-3 seedlings showed knocked-down expression of endogenous TWD40-2 and a mobility-shifted protein of increased molecular mass corresponding to GFP-TWD40-2 (Fig. 1E). In epidermal cells of etiolated hypocotyls, GFP-TWD40-2 localized exclusively to discrete foci at the PM that exhibited appearing and disappearing behavior that was reminiscent of previously observed components of the CME pathway including clathrin light chain (CLC), AP2M, the sigma subunit of the AP2 complex (AP2S), and dynamin related proteins (DRPs) (11, 13, 19), suggesting that TWD40-2 might also be involved in endocytosis (Fig. S2D).
Fig. S2.
GFP-TWD40-2 rescues the growth phenotype of twd40-2-3 and has localization and behavior that are reminiscent of clathrin-mediated endocytosis components. (A) Representative images of 6-d-old etiolated seedlings of Col-0, twd40-2-3, and GFP-TWD40-2 twd40-2-3. (Scale bar, 10 mm.) (B) The growth curves of Col-0, twd40-2-3, and GFP-TWD40-2 twd40-2-3 etiolated hypocotyls show that twd40-2-3 is significantly shorter than both Col-0 and GFP-TWD40-2 twd40-2-3 at all time points (n = 35 seedlings) (P < 0.0001). GFP-TWD40-2 twd40-2-3 is statistically indistinguishable from Col-0 seedlings at all time points (n = 35 seedlings) (P > 0.07). Error bars are SEM. (C) Representative images of 6-wk-old Col-0, twd40-2-3, and GFP-TWD40-2 twd40-2-3 plants. (Scale bar, 5 cm.) (D) A still image shows GFP-TWD40-2 foci localized to the PM of 3-d-old etiolated hypocotyls, and a series of time-lapse images shows the appearing and disappearing behavior of GFP-TWD40-2 particles. The arrowheads accentuate representative particles that exhibit appearing and disappearing behavior. [Scale bars, 10 μm (Left) and 3 μm (Right).]
To better understand the role of TWD40-2 in endocytosis and the functional relationship between TWD40-2 and the AP2 complex, twd40-2-3 was crossed with ap2m-1 (11). The double mutant, ap2m-1 twd40-2-3, exhibited enhanced dwarfism in etiolated hypocotyls, light-grown roots, and adult plants compared with twd40-2-3, and one-third of ap2m-1 twd40-2-3 plants died ∼3 wk after germination (Fig. 1 A–C). The variation of phenotype was probably due to variation in the expression of TWD40-2 and the gametophytic penetrance of twd40-2-3 in the ap2m-1 mutant background. In agreement with previous studies (11), qPCR and qualitative RT-PCR analysis confirmed that ap2m-1 is a null allele (Fig. S1 C and D). The expression of TWD40-2 was not significantly altered in a compensatory manner by ap2m-1, and AP2M transcription levels were not significantly altered in twd40-2-3 (Fig. S1C). The severe growth defects of ap2m-1 twd40-2-3 etiolated hypocotyls and light-grown roots represent a synergistic genetic interaction between AP2M and TWD40-2, because although twd40-2-3 showed intermediately shortened hypocotyls and roots, ap2m-1 single mutants displayed mildly elongated hypocotyls and roots. Together, the difference in phenotype between ap2m-1 and twd40-2-3 coupled with the synergistic phenotype displayed in ap2m-1 twd40-2-3 seedlings suggests that AP2M and TWD40-2 probably undertake distinct yet cooperative roles in the process of clathrin-mediated endocytosis.
ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 Have Unique and Varying Influences on Clathrin-Mediated Endocytosis.
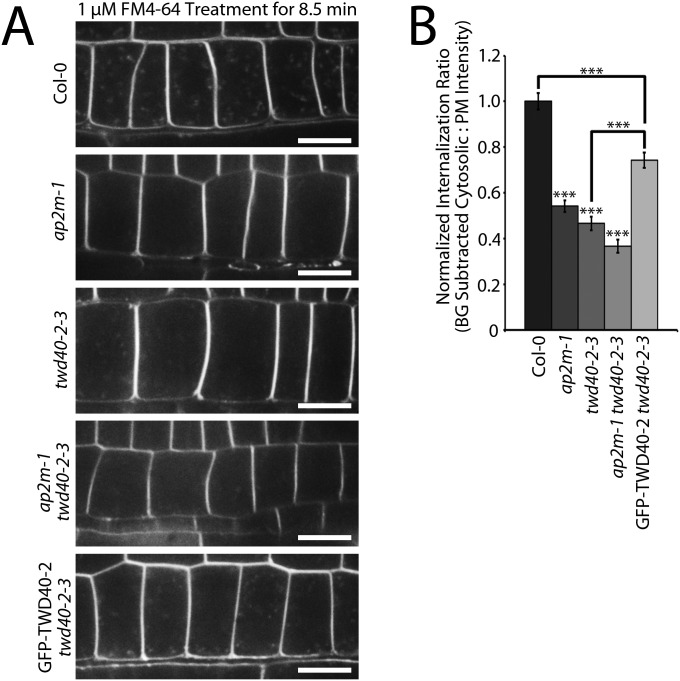
To better understand the differences in the phenotypes of ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 as well as the differences in the function of AP2M and TWD40-2 in endocytosis, an array of endocytosis efficiency experiments was performed in each of the genetic backgrounds. FM4-64 is a lipophilic fluorescent dye that is commonly used to label the plasma membrane and subsequently trace the route of endocytosed material in plant cells (20). After 8.5 min of exposure to 1 μM FM4-64, root meristematic epidermal cells of wild-type seedlings showed a strong labeling of the plasma membrane and exhibited labeling of many bright intracellular puncta that were representative of endocytosed material (Fig. S3). Under the same conditions, the endocytosis of FM4-64 in ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 cells was significantly reduced compared with wild type (Fig. S3). The FM4-64 internalization defect of twd40-2-3 was partially rescued by the expression of GFP-TWD40-2 (Fig. S3). In addition to the stark differences between the general endocytosis efficiency of wild-type and mutant seedlings exposed in the FM4-64 internalization experiments, a gradient of increasing endocytosis defect severity became apparent between ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 (Fig. S3B).
Fig. S3.
ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 exhibit defects in endocytosis of FM4-64. (A) Five-day-old seedlings of Col-0, ap2m-1, twd40-2-3, ap2m-1 twd40-2-3, and GFP-TWD40-2 twd40-2-3 were grown in a 16-h/8-h light cycle. After 8.5 min of 1 μM FM4-64 treatment, images were gathered at the cross-sectional plane of epidermal cells of the meristematic zone of the root that showed the best intracellular FM4-64 puncta distribution. (Scale bars, 10 μm.) (B) FM4-64 internalization was quantified based on the ratio between the difference of the average cytosolic fluorescence intensity and the mean cytosolic background intensity (background subtracted cytosolic) and the mean plasma membrane intensity. The average internalization ratio of each line was then normalized against the average internalization ratio of Col-0. Error bars are SEM. ***P < 0.0001 (n = 32 cells, eight seedlings for each genotype).
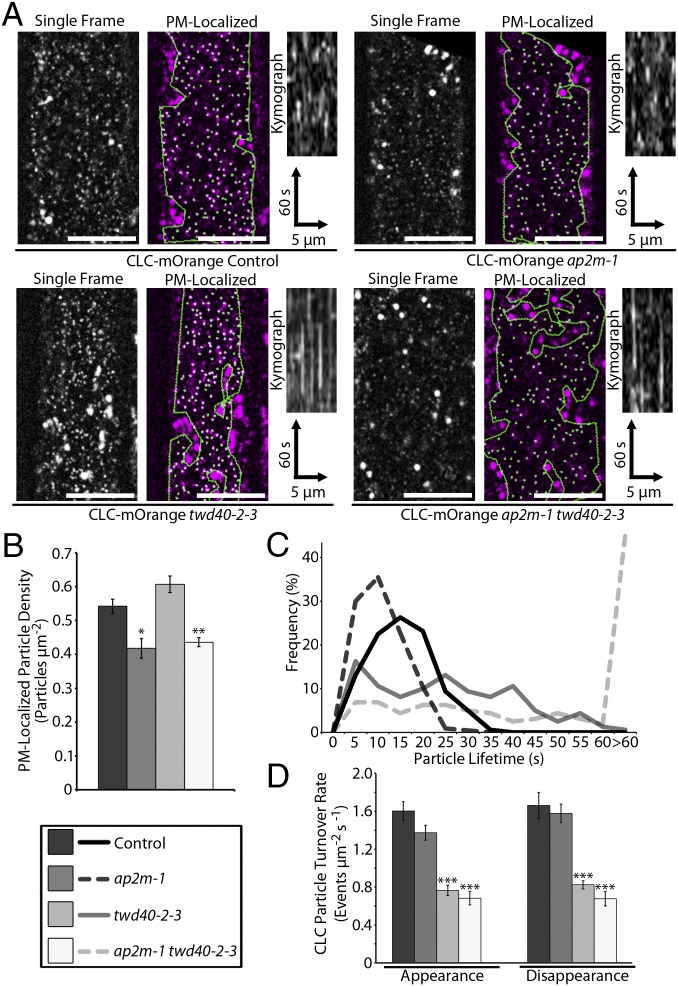
Although the FM4-64 internalization assay is suitable for monitoring general endocytosis deficiencies, it is unable to probe how each mutation mechanistically influences CME. To directly observe CME events, a previously reported CLC-mOrange line was crossed with ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 (19). Two populations of CLC are visible in spinning-disk confocal images of CLC-mOrange. CLC-mOrange localized to globular intracellular structures that have been previously identified as trans-Golgi network/early endosome (TGN/EE) compartments and to small, diffraction-limited particles at the PM that exhibit appearing and disappearing behavior, which is representative of the formation of clathrin-coated pits (CCPs) and scission of clathrin-coated vesicles (CCVs), respectively (Movie S1) (19, 21). Analysis of CLC-mOrange particles in this study was limited to regions of interest that exclusively contained PM-localized CLC-mOrange particles as determined by the size and spatiotemporal behavior of the particles. The density of PM-localized CLC particles was significantly decreased in both ap2m-1 and ap2m-1 twd40-2-3 lines and insignificantly increased in twd40-2-3 (Fig. 2 A and B). The resident lifetimes of PM-localized CLC-mOrange particles were slightly shortened in ap2m-1 and drastically lengthened by twd40-2-3 and ap2m-1 twd40-2-3 (Fig. 2 A and C and Movie S1). Clearly, ap2m-1 and twd40-2-3 differentially influenced CLC behavior and ap2m-1 twd40-2-3 shared characteristic effects of both ap2m-1 and twd40-2-3, suggesting that AP2M and TWD40-2 each plays important, cooperative, and distinct roles in CME.
Fig. 2.
PM-localized CLC-mOrange particles are differentially affected by ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3. (A) A representative image of CLC-mOrange is displayed with and without automated particle detection of PM CLC particles in control, ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 etiolated hypocotyls. (Scale bars, 10 μm.) Kymographs display particle dynamics over a 2-min time course. (B) Quantification of PM CLC particle densities. Error bars are SEM. *P < 0.01, **P < 0.001 (n = 10 for each genotype). (C) A histogram of PM CLC particle lifetimes (n = 160 particles from eight seedlings for each genotype). (D) The average CLC particle turnover rate is displayed as the rate of appearance and disappearance events per μm2 per second. Error bars are SEM. ***P < 0.0001 (n = 10 for each genotype).
Because ap2m-1 and twd40-2-3 influenced the behavior of CLC in different ways, the turnover rate of PM-localized CLC-mOrange particles within a specified area was used as a unified metric for the overall efficiency of CME and compared among the mutants. Both twd40-2-3 and ap2m-1 twd40-2-3 exhibited significantly reduced rates of CLC-mOrange turnover (Fig. 2D). In contrast, despite the reduced density of CLC particles in ap2m-1, the reduction in turnover rates in ap2m-1 was statistically insignificant due to the shortened lifetimes of CLC-mOrange particles in ap2m-1 (Fig. 2D).
An Increasing Severity of Endocytic Deficiency Correlates with Reduced Cellulose Biosynthesis by Influencing CESA Distribution and Dynamics.
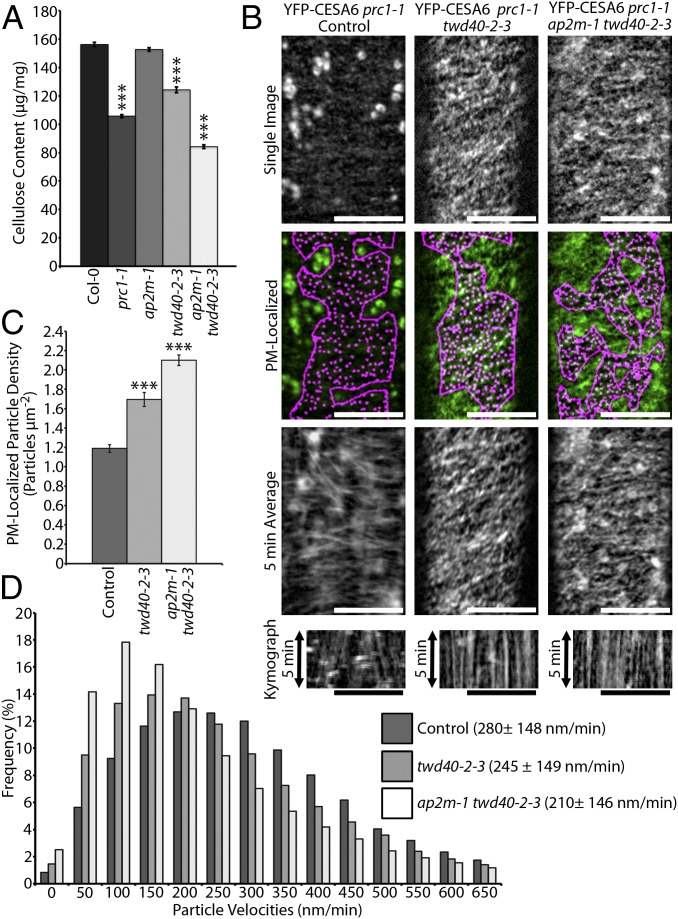
To investigate whether CME efficiency is important for maintaining efficient cellulose biosynthesis, the crystalline cellulose content was measured in each CME mutant background. Wild-type (Col-0) and cesa6prc1-1 seedlings were used as controls for normal and deficient cellulose content, respectively (Fig. 3A). Although the cellulose content of ap2m-1 seedlings was not significantly different from Col-0, twd40-2-3 and ap2m-1 twd40-2-3 each showed significant cellulose deficiencies of increasing severity (Fig. 3A), suggesting that CME defects can lead to inefficient cellulose biosynthesis.
Fig. 3.
Endocytic deficiencies of twd40-2-3 and ap2m-1 twd40-2-3 negatively influence cellulose biosynthesis by altering CESA distribution and dynamics. (A) The graph compares the crystalline cellulose content of 5-d-old etiolated seedlings of Col-0, cesa6prc1-1, ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3. Error bars are SEM. ***P < 0.0001 (n = 10 replicates per genotype). (B) A representative single-frame image of YFP-CESA6 is displayed in control (cesa6prc1-1), twd40-2-3, and ap2m-1 twd40-2-3 with and without automated particle detection of PM YFP-CESA6 particles. Corresponding time-averaged projections and kymographs show the trajectories and movement of PM YFP-CESA6 particles, respectively. (Scale bars, 10 μm.) (C) The average PM YFP-CESA6 particle density is quantified. Error bars are SEM. ***P < 0.0001 (n ≥ 20 cells for each line). (D) The distribution and average of YFP-CESA6 velocities in each genetic background are displayed (n ≥ 19 cells for each line). Averages are displayed ± SD.
To gain insight into the manner by which endocytic defects lead to cellulose biosynthesis defects, a YFP-CESA6 line that complements cesa6prc1-1 was crossed into the twd40-2-3 and ap2m-1 twd40-2-3 genetic backgrounds. As previously observed, YFP-CESA6 in the control line localized to large globular Golgi bodies and to discrete, diffraction-limited particles at the PM (Fig. 3B and Movie S2) (5). The ap2m-1 mutation was previously shown to cause an increase in the particle density of PM-localized YFP-CESA6 but did not significantly influence the dynamics of PM-localized YFP-CESA6 particles (11). An increase in PM-localized YFP-CESA6 particle density was also caused by twd40-2-3 and ap2m-1 twd40-2-3, as indicated by the quantification of particle densities measured by automated particle detection within regions of interest that exclusively contained PM-localized YFP-CESA6 particles (Fig. 3 B and C). Interestingly, PM-localized YFP-CESA6 particle velocities were negatively influenced by twd40-2-3 and ap2m-1 twd40-2-3 compared with the control line, which correlates with the cellulose deficiency phenotypes of twd40-2-3 and ap2m-1 twd40-2-3 (Fig. 3 A, B, and D and Movie S2). The double mutant ap2m-1 twd40-2-3 caused a more severe increase in particle density and a more severe decrease in CSC velocity than twd40-2-3 alone. Approximately one-third of the YFP-CESA6 ap2m-1 twd40-2-3 prc1-1 seedlings exhibited an abnormal YFP-CESA6 distribution in some epidermal cells. These abnormal cells had attributes that included a diffuse PM-localized YFP-CESA6 population that was extremely sensitive to photobleaching, faint PM-localized YFP-CESA6 particles, some MASC/SmaCC-like YFP-CESA6 compartments, and severely slowed or stalled cytoplasmic streaming (Movie S2). These abnormal cells were not included in the analysis of YFP-CESA6 density or dynamics.
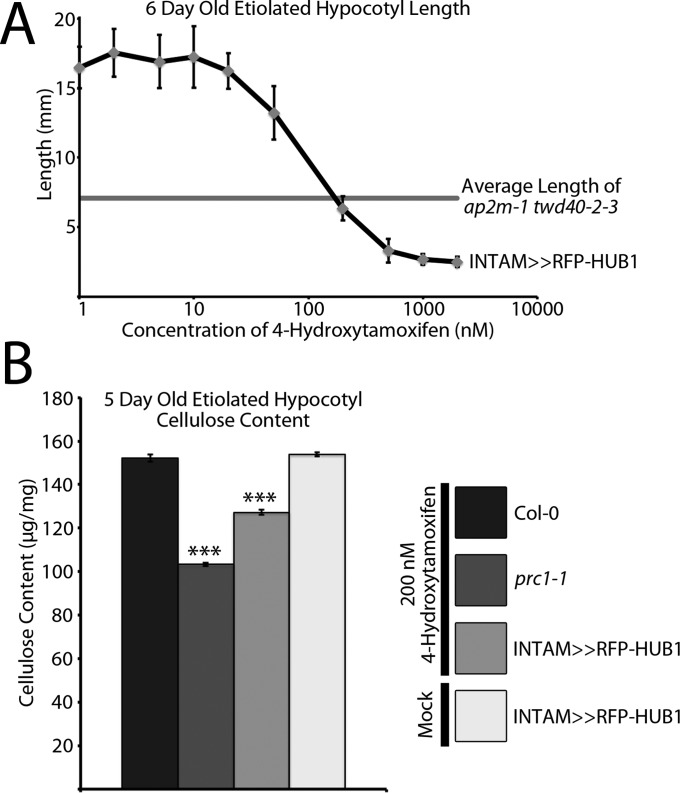
The negative influence of twd40-2-3 and ap2m-1 twd40-2-3 on endocytosis, CESA function, and cellulose content revealed the importance of efficient CESA trafficking in the process of cellulose biosynthesis. To further corroborate the connection between intracellular trafficking and cellulose biosynthesis, we used an alternative means of CME disruption. Chemical induction of HUB1, a dominant-negative clathrin heavy chain (CHC) truncation, was previously shown to inhibit CME and arrest root elongation (22). In the HUB1 inducible line, INTAM>>RFP-HUB1, etiolated hypocotyl elongation inhibition was dependent on the concentration of the inducer, 4-hydroxytamoxifen (TAM) (Fig. S4A). HUB1 induction by 200 nM TAM caused a reduction of etiolated hypocotyl growth that was comparable to that of ap2m-1 twd40-2-3 grown in normal conditions (Fig. S4A). Under HUB1 induction with 200 nM TAM, INTAM>>RFP-HUB1 seedlings exhibited a significant cellulose content deficiency, further emphasizing the influence of CME on cellulose biosynthesis (Fig. S4B). In controls, 200 nM TAM did not alter the cellulose content of Col-0 or cesa6prc1-1 seedlings, and INTAM>>RFP-HUB1 etiolated hypocotyls grown in the absence of TAM had a normal cellulose content (Fig. S4B).
Fig. S4.
CME deficiency caused by HUB1 induction causes cellulose content deficiency. (A) Six-day-old etiolated seedlings of INTAM>>RFP-HUB1 exhibited a concentration-dependent reduction in growth when exposed to the inducer 4-hydroxytamoxifen. Induction with 200 nM TAM most closely mimicked the growth phenotype of ap2m-1 twd40-2-3. Error bars are SD (n = 15 seedlings at each concentration). (B) The crystalline cellulose content of 5-d-old etiolated seedlings is compared among Col-0, cesa6prc1-1, and INTAM>>RFP-HUB1 grown in the presence of 200 nM TAM and INTAM>>RFP-HUB1 grown in the absence of TAM. Error bars are SEM. ***P < 0.0001 (n = 10 replicates from each condition).
TWD40-2 and AP2M Play Distinct but Associated Roles in CME.
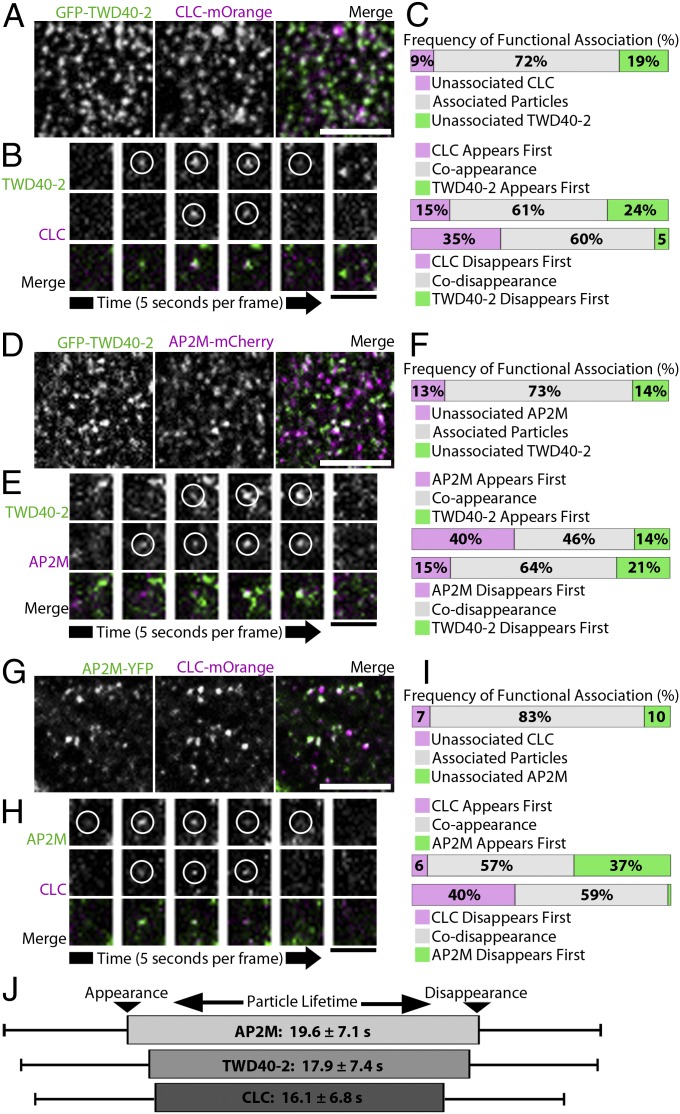
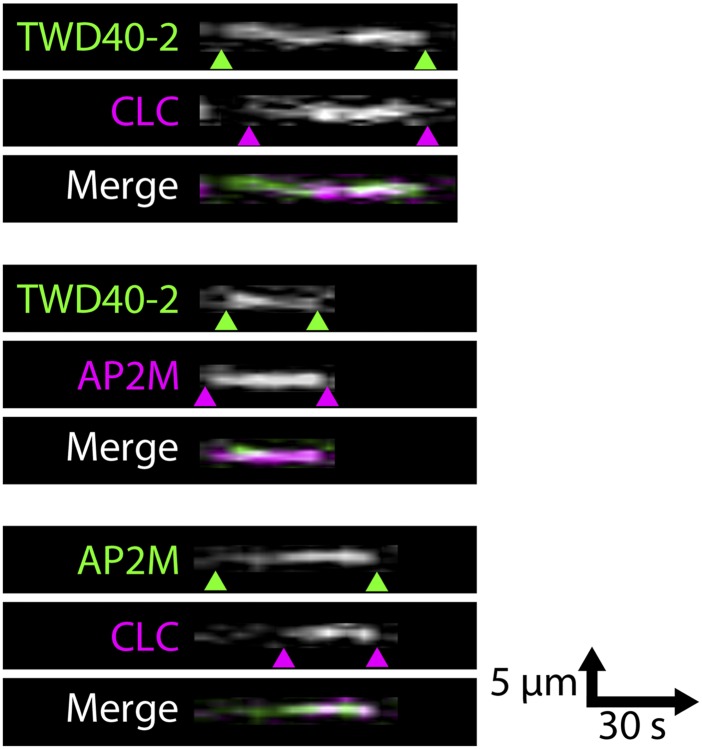
Although evidence suggests that AP2M and TWD40-2 are both involved in CME, ap2m-1 and twd40-2-3 mutants vary in the manner and magnitude by which they influence CME, endocytic cargo such as CESA, and cellular processes such as cellulose biosynthesis. A series of cell biology experiments was carried out to functionally position TWD40-2 within the CME process. To ascertain the spatiotemporal relationship among TWD40-2, AP2M, and CLC, we generated three dual-labeled lines, GFP-TWD40-2 CLC-mOrange, GFP-TWD40-2 AP2M-mCherry, and AP2M-YFP CLC-mOrange. In single-frame images, many colocalized particles were observed in each dual-labeled line (Fig. 4 A, D, and G); however, a particle often appeared or disappeared at a slightly different time point than its corresponding colocalized particle (Fig. 4 B, E, and H and Fig. S5). Therefore, colocalization analyses of single-frame images fail to represent the frequency of functional association between particles. Instead, each individual particle of interest was tracked temporally to assess whether a corresponding colocalized particle existed at any time during the lifetime of the particle of interest. Two particles were deemed to be functionally associated if they were spatiotemporally colocalized for at least two frames (for greater than 5 s) of live-cell imaging and only if colocalization was initiated by the appearance of a particle and terminated by the disappearance of a particle and not by the lateral diffusion of either particle. High frequencies of functional association were observed in each dual-labeled line, confirming that TWD40-2 cooperates with AP2M and predominantly participates in CME (Fig. 4 C, F, and I).
Fig. 4.
TWD40-2, AP2M, and CLC are functionally associated. (A, D, and G) Single-frame images of GFP-TWD40-2/CLC-mOrange, GFP-TWD40-2/AP2M-mCherry, and AP2M-YFP/CLC-mOrange show colocalized particles. (Scale bars, 5 μm.) (B, E, and H) Representative time-lapse images show that functionally associated particles often appear or disappear at different time points during endocytic events. (Scale bars, 1 μm.) (C, F, and I) The frequency of functional association between particles was analyzed spatiotemporally over the entire lifetime of each particle. For each pair of functionally associated particles, the order of appearance and disappearance of the two particles was recorded (n = 100 observations, five seedlings for each line). (J) A schematic diagram shows the most likely sequence of appearance and disappearance of AP2M, TWD40-2, and CLC particles scaled to the average particle lifetimes of each protein. Error bars and ± values are SD (n > 350 particles, 26 seedlings for each protein).
Fig. S5.
TWD40-2, AP2M, and CLC particles often appear and/or disappear at slightly different time points. A series of kymographs depicts the temporal relationship among plasma membrane-localized TWD40-2, AP2M, and CLC particles that is described in Fig. 4. Green and magenta arrowheads denote the appearance and disappearance events of one pair of functionally associated particles in each dual-labeled line, respectively.
Furthermore, the dynamic relationship between each pair of functionally associated particles was recorded to determine whether a bias exists for the temporal order of appearance or disappearance among TWD40-2, AP2M, and CLC (Fig. 4 C, F, and I). AP2M showed a strong bias to appear before or at the same time as either TWD40-2 or CLC. Also, CLC showed a strong bias to disappear before or at the same time as either TWD40-2 or AP2M. Average particle lifetimes of each component were measured to be 19.6 ± 7.1 s for AP2M, 17.9 ± 7.4 s for TWD40-2, and 16.1 ± 6.8 s for CLC (Fig. 4J). These observations were used to develop a schematic diagram of CME that depicts the average particle lifetimes of each component and the most probable order of recruitment and dissociation of each component during CME (Fig. 4J). In this model, AP2M recruitment precedes that of TWD40-2 and CLC, whereas CLC dissociation precedes that of TWD40-2 and AP2M.
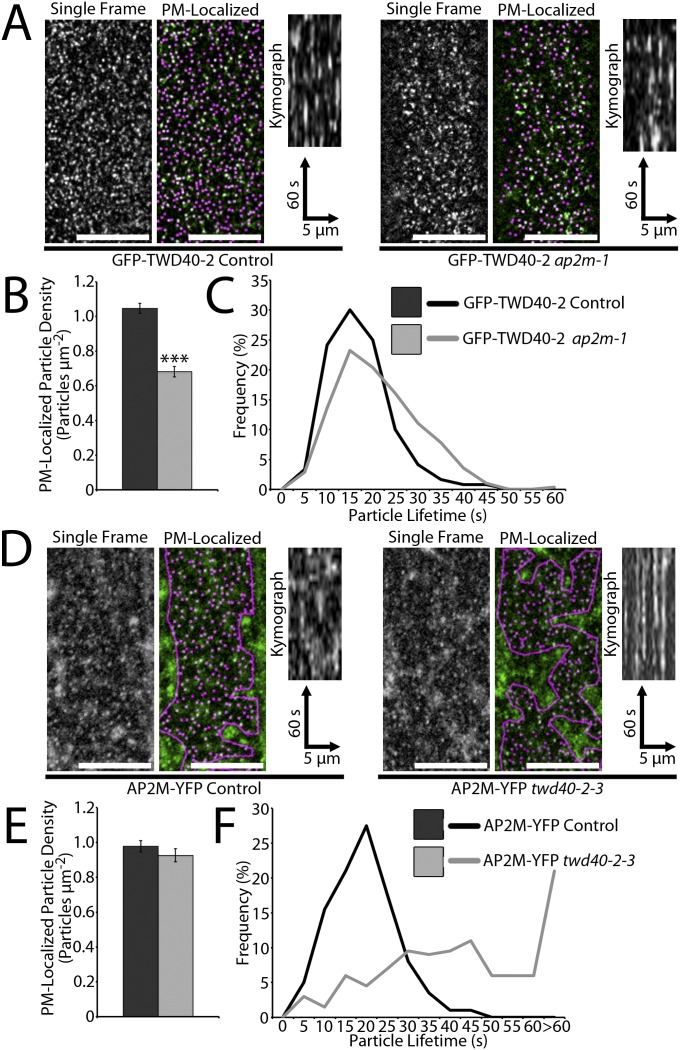
If AP2M and TWD40-2 are truly cooperating in CME, ap2m-1 would influence TWD40-2 and twd40-2-3 would influence AP2M. GFP-TWD40-2 particle density was significantly decreased in ap2m-1, similar to how ap2m-1 influenced CLC-mOrange density (Fig. S6 A and B and Movie S3). However, unlike CLC-mOrange, which exhibited shortened particle lifetimes in ap2m-1, the lifetimes of GFP-TWD40-2 particles at the PM were relatively unchanged or lengthened in ap2m-1 (Fig. S6 A and C and Movie S3). The converse experiment was also performed in which the effect of twd40-2-3 on AP2M-YFP was analyzed. twd40-2-3 did not affect the density of PM-localized AP2M-YFP particles, but did cause a severe increase in the lifetimes of AP2M-YFP particles (Fig. S6 D–F and Movie S3). The influence of twd40-2-3 on AP2M was similar to its influence on CLC, but AP2M exhibited a more severe increase in particle lifetimes than CLC. These observations show that TWD40-2 and AP2M are codependent on each other and assume different roles in endocytosis.
Fig. S6.
Normal functions of AP2M and TWD40-2 are codependent. (A) Representative images of PM-localized GFP-TWD40-2 particles with and without automated particle detection are compared in control and ap2m-1 etiolated hypocotyls. (Scale bars, 10 μm.) Kymographs display representative particle dynamics over a 2-min time course. (B) The average GFP-TWD40-2 particle density is compared between control and ap2m-1 lines. Error bars are SEM. ***P < 0.0001 (n = 14 and 20, respectively). (C) The distribution of GFP-TWD40-2 particle lifetimes is compared between control and ap2m-1 lines (n = 120 particles, 6 seedlings and 280 particles, 14 seedlings, respectively). (D) Representative images of PM-localized AP2M-YFP particles with and without automated particle detection within a region of interest that excludes intracellular AP2M-YFP signal are compared in control and twd40-2-3 etiolated hypocotyls. (Scale bars, 10 μm.) Kymographs display representative particle dynamics over a 2-min time course. (E) The average AP2M-YFP particle density is compared between control and twd40-2-3 lines. Error bars are SEM (n = 14 and 20, respectively). (F) The distribution of AP2M-YFP particle lifetimes is compared between control and twd40-2-3 lines (n = 200 particles, 9 seedlings and 200 particles, 10 seedlings, respectively).
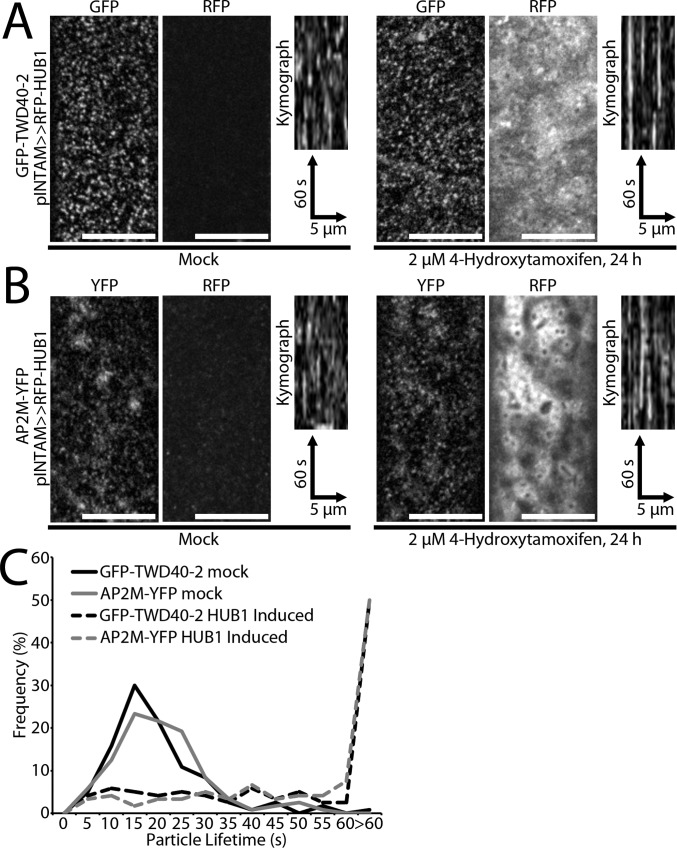
Knowing that ap2m-1 and twd40-2-3 affect CME in different ways, we wondered whether inhibition of CME would likewise differentially influence AP2M and TWD40-2. The INTAM>>RFP-HUB1 line was crossed with GFP-TWD40-2 and AP2M-YFP, and CME inhibition was carried out by 24-h RFP-HUB1 induction with 2 μM TAM. To minimize the effect of induction-level variation, only seedlings with strong RFP-HUB1 induction were selected for analysis and compared against DMSO mock-treated seedlings from the same line that had no detectable RFP-HUB1 expression. Both AP2M-YFP and GFP-TWD40-2 were still recruited to discrete particles at the PM in the presence of strong RFP-HUB1 induction (Fig. S7 A and B). However, the lifetimes of these AP2M-YFP and GFP-TWD40-2 particles were extremely extended (Fig. S7C and Movie S4). These observations suggest that AP2M and TWD40-2 are early CME components that are recruited to the PM independent of a functional clathrin coat. However, the removal of AP2M and TWD40-2 from the PM is dependent on functional clathrin coats.
Fig. S7.
Induction of HUB1 does not inhibit the recruitment of AP2M-YFP or GFP-TWD40-2 particles to the PM but severely increases the particle lifetimes. (A and B) Representative images show GFP-TWD40-2 or AP2M-YFP particles at the PM of 4-d-old etiolated hypocotyls after 24 h of mock treatment or 2 μM TAM treatment. RFP images show no RFP-HUB1 expression in mock-treated seedlings and strong RFP-HUB1 induction in 2 μM TAM-treated seedlings. (Scale bars, 10 μm.) Kymographs display the dynamics of GFP-TWD40-2 or AP2M-YFP particles. (C) The distribution of GFP-TWD40-2 or AP2M-YFP particle lifetimes is compared between mock and RFP-HUB1–induced seedlings (n = 120 particles, six seedlings for each condition).
Discussion
The Cooperative and Distinct Functions of TWD40-2, the AP2 Complex, and the TPC in Clathrin-Mediated Endocytosis.
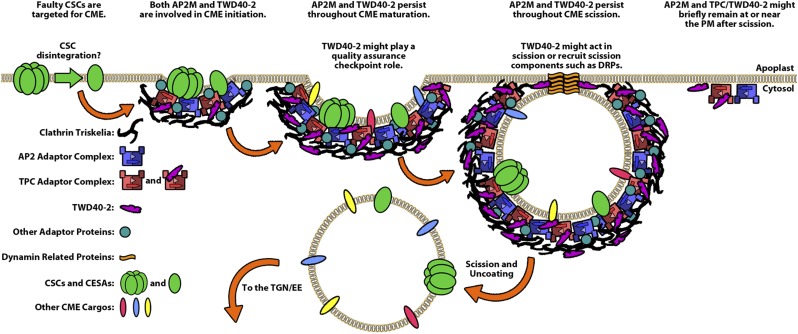
The relatively mild phenotypes of AP2 mutants caused us to speculate that other AP complexes or proteins were redundant with AP2 in CME in Arabidopsis (11). As it turns out, the TPC could be the culprit for AP2 redundancy (17). Several lines of evidence suggest that AP2M and TWD40-2 cooperate in the process of CME. In animal cells, which lack homologs of the TPC and TWD40-2, the AP2 complex plays a crucial role in recruiting clathrin throughout the CME process (16). Accordingly, AP2 knockdown in animal cells caused a reduction in the density and an increase in the lifetimes of PM CLC particles due to reduced clathrin recruitment during CME initiation and maturation, respectively (23). In plants, although ap2m-1 and twd40-2-3 each impaired CME and influenced PM CLC particles independently, only the double mutant, ap2m-1 twd40-2-3, both reduced the density and increased the lifetimes of PM CLC particles, suggesting that AP2 and TWD40-2/TPC cooperate in plants to fulfill the function of the AP2 complex in animals. Further showing AP2M and TWD40-2 cooperation, both AP2M and TWD40-2 appeared early and persisted throughout CME in spatiotemporal analyses, suggesting that both proteins likely facilitate CME initiation, maturation, and scission (Fig. S8). The functions of AP2M and TWD40-2 were shown to be dependent on one another because ap2m-1 altered GFP-TWD40-2 particles and twd40-2-3 altered AP2M-YFP particles. AP2M and TWD40-2 were also similarly affected by HUB1 induction, under which AP2M and TWD40-2 particles were each recruited to stalled particles at the plasma membrane, suggesting that functional clathrin is required for the disengagement but not the recruitment of AP2M and TWD40-2 at the PM.
Fig. S8.
Schematic model shows the cooperation between AP2 and TWD40-2/TPC during clathrin-mediated endocytosis and describes potential unique functions of TWD40-2. Our study suggests that faulty CSCs are likely targeted for endocytosis because defective CME leads to the accumulation of enzymatically inefficient CSCs at the plasma membrane. It is unknown whether CSCs disintegrate into smaller complexes before endocytosis. CSCs and CESAs are displayed as representative CME cargos, but many other CME cargo proteins also exist in Arabidopsis. Our study shows that both AP2M and TWD40-2 persist throughout CME initiation, maturation, and scission and that the behavior of each component is dependent on the other. Our study also shows that genetic disruptions in AP2M and TWD40-2 differentially affect the CME process, suggesting that TWD40-2 has certain functions that are distinct from AP2. The model proposes that TWD40-2 might play unique roles during maturation or scission. Future studies can focus on deciphering specific functions of TWD40-2 and whether TWD40-2 is a permanent or transient member of the TPC.
Several opposing characteristics of AP2M and TWD40-2 suggest that they play some distinct roles in CME. The contrast between the mildly lengthened etiolated hypocotyls of ap2m-1 and dwarfed etiolated hypocotyls of twd40-2-3 exemplifies the differences in AP2 and TWD40-2 mutant phenotypes. We hypothesize that these different growth phenotypes can be attributed to differences in the magnitude of CME deficiency in ap2m-1 and twd40-2-3, which was reflected in the varying CLC-mOrange particle turnover rates of each mutant. For instance, cellular processes that are sensitive to trafficking perturbations may be affected to a smaller extent in ap2m-1, which has a mild CME deficiency, compared with twd40-2-3, which has a more severe CME deficiency, thereby leading to the different growth phenotypes.
The differences in the manner and magnitude of ap2m-1 and twd40-2-3 influences on CME components may help in hypothesizing the distinct attributes of AP2M and TWD40-2. Evidence suggests that CME initiation might be assumed by both AP2M and TWD40-2. The early recruitment of AP2M during CME and the reduced densities of CLC and TWD40-2 particles in ap2m-1 implicate AP2M in CME initiation. In ap2m-1, TWD40-2/TPC may be able to assume the responsibilities of AP2 but operate at a reduced efficiency. Appropriately, GFP-TWD40-2 particles were still recruited to dynamic PM-localized particles in ap2m-1, but the density of TWD40-2 particles was reduced and the lifetimes of TWD40-2 particles were slightly extended. The involvement of TWD40-2 in CME initiation is supported by the significant reductions in the rate of appearance of CLC particles per μm2 observed in twd40-2-3. The severely extended lifetimes of both CLC and AP2M particles in twd40-2-3 suggest that TWD40-2 might play a distinct role in CME maturation or scission that cannot be assumed by AP2 (Fig. S8). A possible unique role of TWD40-2 in CME scission could involve the recruitment of dynamin-related proteins by TWD40-2 (Fig. S8). Alternatively, TWD40-2 could play a unique quality assurance “checkpoint” role during CME maturation that triggers faulty CME events to be aborted (Fig. S8). In other organisms, abortive CME events result in abbreviated CME particle lifetimes (24–26). In our study, ap2m-1 had an elevated percentage of CLC particles with short lifetimes, which might be indicative of CME abortive events. In contrast, the extended CLC and AP2M lifetimes of twd40-2-3 and ap2m-1 twd40-2-3 could represent faulty CME events that are unable to abort. If ap2m-1 has an increased incidence of abortive CME events, it is possible that the actual CME efficiency of ap2m-1 is lower than our CLC particle turnover measurements suggest, which might be supported by the prominent FM4-64 internalization defect in ap2m-1.
Even if the AP2 complex, TWD40-2, and the TPC are all mechanistically similar to one another, different ap2m-1 and twd40-2-3 phenotypes could result from differences in cargo specificity or differences in the physical dimensions or integrity of CCVs with varying ratios of AP2 complexes, TWD40-2 proteins, and TPCs. Another interesting hypothesis is that TWD40-2 could act as a coat protein. The predicted structure of TWD40-2 has two β-propeller motifs followed by an extended α-solenoid region, which is a feature of the β′-COP subunit of the coat protein complex 1 (COPI) and is similar to many other vesicle coat proteins (18). Our data neither support nor refute the possibility of TWD40-2 acting as a coat protein in CME, but this proposition and the remaining mystique surrounding the TPC are representative of the excitement and opportunity that exist in the study of plant endocytosis.
Cellulose Biosynthesis Relies on Efficient Endocytosis of CESA.
We hypothesize that CSC endocytosis acts as a regulatory mechanism to maintain an organized distribution of productive CSCs at the PM. The directed delivery of CSCs along CMTs during exocytosis is evidence of organized CSC trafficking to the PM (6, 7). It seems likely that the endocytosis of CSCs is equally organized and regulated. CSCs with deficiencies in cellulose synthesis caused either by disengagement from the CMTs or by other means may be actively targeted for endocytosis. Once endocytosed, CSCs may either be recycled to the PM or directed for degradation, depending on the integrity of the CSC. We hypothesize that the severe endocytic deficiencies of twd40-2-3 or ap2m-1 twd40-2-3 lead to the excessive accumulation of these faulty CSCs at the PM. Consistent with this hypothesis, twd40-2-3 and ap2m-1 twd40-2-3 exhibited a reduced cellulose content, an increased density of PM CSCs, and a reduced velocity of PM CSCs which is indicative of the enzymatic inefficiency of faulty CSCs. In ap2m-1, which has a milder endocytic deficiency, CSC overaccumulation still occurs, but low levels of endocytosis may still adequately remove many faulty CSCs from the PM, resulting in higher PM CSC density without significantly influencing PM CSC velocities. The inverse relationship between the density of PM-localized CSCs and cellulose content and the direct relationship between CSC velocities and cellulose content in ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 suggest that the quality of PM-localized CSCs is more influential for cellulose biosynthesis than the sheer abundance of PM-localized CSCs, and that CME can influence CSC quality in addition to CSC quantity at the PM.
In this study, CESA and cellulose biosynthesis represent just a single cargo protein and a single cellular process that is influenced by regulated endocytosis. A broader understanding of both the mechanisms and cargo specificities of plant CME machinery components is sure to underscore the importance of regulated CME in many other processes in the future. Plants offer the opportunity to characterize the functions of rarely conserved TPC machinery that may provide insight into the evolution of trafficking machinery of all eukaryotes.
Materials and Methods
Plant Materials.
SALK_112497 (twd40-2-3) was obtained from the Arabidopsis Biological Resource Center (ABRC) at Ohio State University. The ap2m-1 (previously referred to as μ2-1) and AP2M-YFP (previously referred to as μ2-YFP) lines were previously described (11). The YFP-CESA6, CLC-mOrange, and INTAM>>RFP-HUB1 lines were also previously described (5, 19, 22). The GFP-TWD40-2 twd40-2-3 line was constructed with a 2-kb native TWD40-2 promoter region using the primers in Table S1.
Table S1.
Sequences of the primers used in this study
| Primer | Sequence, 5′-3′ |
| twd40-2-3 genotyping | |
| RP | TGTGCTTTCCCTATCACCATC |
| LP | TAGGGGACTATGACATGCTCG |
| BP LBb1.3 | ATTTTGCCGATTTCGGAAC |
| GFP-TWD40-2 cloning | |
| SacI prom F | CGAGCTCTGTACATGGATTGTCAGGC |
| SpeI prom R | ACTAGTCTTCGATCTCTCTCTCTCTCTC |
| TWD40-2 F | ATGTTGCGGGCGAGAGCATTTCG |
| TWD40-2 R | TTAGCCATTCAAGAAATCAAAATCTATTG |
| Qualitative RT-PCR | |
| P1 F | ATGTTGCGGGCGAGAGCATTTCG |
| P1 R | CTTGCTTTGGCACATCACGACC |
| P2 F | GGTGGGGTGGTGGCTGTTG |
| P2 R | CTCACAGAAAGAACTGAGTATG |
| ACT2 F | ATGGCTGAGGCTGATGATATTCAACC |
| ACT2R | CTCTCTGTAAGGATCTTCATGAGG |
| AP2M F | CCTGTGCGTCAGATTGGTGGCTGCTCC |
| AP2M R | GAATCTGGTCAGGTTCACACACTGGTG |
| Real-time qPCR | |
| ACT2 F | TGGTCGTACAACCGGTATTGTG |
| ACT2 R | GCTGTTGTGGTGAACATGTAACC |
| AP2M F | TTGTTGGATGAGATTATGGACTTTG |
| AP2M R | CCAACAGCACCTGTAACTTGTAATGT |
| TWD40-2 UP F | TCCCCTGCTCCATCGACTAA |
| TWD40-2 UP R | CGAAAGAAACTCCATGCAGAGA |
| TWD40-2 DN F | TGCAGCTTCATCGGCTAGTG |
| TWD40-2 DN R | CTTCGGGATGTGCGATAACC |
BP, T-DNA border primer; F, forward; LP, left genomic primer; R, reverse; RP, right genomic primer.
See SI Materials and Methods for details on the construction, genotyping, and expression analysis of the transgenic and mutant lines.
Live-Cell Imaging and Analysis.
With the exception of two experiments (FM4-64 internalization assay and imaging under HUB1 induction), all images were obtained of epidermal cells 1–2 mm below the apical hook of 3-d-old etiolated hypocotyls.
See SI Materials and Methods for details on dye treatment, induction conditions, and image analysis.
Cellulose Content Analysis.
The crystalline cellulose content was measured by the Updegraff method (27).
See SI Materials and Methods for details.
SI Materials and Methods
Plant Materials and Growth Conditions.
SALK_112497 (twd40-2-3) was obtained from the Arabidopsis Biological Resource Center (ABRC) at Ohio State University and genotyped using the primers specified in Table S1. The ap2m-1 (previously referred to as μ2-1) and AP2M-YFP (previously referred to as μ2-YFP) lines were previously described (11). The YFP-CESA6, CLC-mOrange, and INTAM>>RFP-HUB1 lines were also previously described (5, 19, 22). The pTWD40-2::GFP-TWD40-2 line was constructed by first modifying the pH7WGF2 vector (28). The 35S promoter was removed from pH7WGF2 by SacI and SpeI digestion and replaced by the 2-kb promoter region of TWD40-2, which was amplified from genomic DNA using the primers specified in Table S1. The coding sequence of TWD40-2 was PCR-amplified from a cDNA preparation that was made from Col-0 seedling RNA, inserted into the pCR8/GW/TOPO vector, sequenced, and transferred to the modified pH7WGF2 described above via Gateway cloning (Life Technologies). pTWD40-2::GFP-TWD40-2 was transformed into twd40-2-3 by Agrobacterium-mediated transformation using the floral dip method. Several independent lines exhibited similar rescue of twd40-2-3 and similar fluorescence signal to the line used for experiments in this study, which was considered to be representative of TWD40-2.
All seeds were sterilized using 30% (vol/vol) bleach, thoroughly washed, and cold-treated at 4 °C for a minimum of 3 d. Dark-grown seedlings were grown on vertical half-strength Murashige and Skoog (MS) plates without sucrose at 21 °C in sterile conditions in the dark. Light-grown seedlings were grown on vertical half-strength MS plates with 1% sucrose at 21 °C in sterile conditions on a 16-h light/8-h dark cycle. Potted plants were grown at 22 °C with a 16-h light/8-h dark cycle.
Real-Time qPCR, RT-PCR, and Western Blot Analysis.
RNA was purified from 50 mg of 11-d-old light-grown seedlings of Col-0, ap2m-1, twd40-2-3, and ap2m-1 twd40-2-3 using the RiboPure Kit (Ambion). The reverse-transcriptase cDNA synthesis reaction was carried out by the Transcriptor High Fidelity cDNA Synthesis Kit (Roche). Minus reverse-transcriptase controls were devoid of amplification, ensuring no interference from DNA contamination in both qPCR and RT-PCR experiments. For qPCR analysis, TWD40-2, AP2M, and ACTIN2 primers (Table S1) were selected using Primer Express software (Life Technologies), and 40-cycle reactions were carried out on an Applied Biosystems 7300 sequence detection system with Applied Biosystems SYBR Green PCR Mix (Life Technologies). All reactions displayed high specificity as determined by dissociation curves containing a single species. High amplification efficiencies were measured for all primer sets by analyzing serial dilutions of RNA from two independent RNA isolations. qPCR analysis was performed on two biological replicates with five or six technical replicates for each cDNA–primer pair combination, and quantification was performed via the ΔΔCt method (29). For RT-PCR analysis, cDNA from each genotype was subjected to PCR using primer pairs (Table S1) that were specific to the coding sequence of TWD40-2, AP2M, and ACTIN2 for 18 temperature cycles and analyzed by gel electrophoresis.
A total protein extraction was performed on Col-0, ap2m-1, twd40-2-3, ap2m-1 twd40-2-3, AP2M-YFP ap2m-1, and GFP-TWD40-2 twd40-2-3. For each line, 1 g of 7-d-old light-grown seedlings was flash-frozen and ground in liquid nitrogen. On ice, 500 μL of extraction buffer [25 mM Tris⋅HCl, pH 7.4, 15 mM MgCl2, 5 mM EDTA, 150 mM NaCl, 0.5% Nonidet P-40, 1 mM DTT, 1 mM PMSF, 5% (vol/vol) ethylene glycol] was added to extract the total protein. Equal volumes of total protein extraction were then mixed with Laemmli buffer, boiled for 5 min, and subjected to SDS/PAGE. For Western blot analysis, protein was transferred from SDS/PAGE gels to nitrocellulose membrane using electrophoresis overnight at 4 °C. The membrane was blocked with 5% (wt/vol) nonfat milk in TBST buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 0.05% Tween 20) for 1.5 h, followed by three rinses with TBST buffer. One hundred and fifty microliters of serum from rabbits that had undergone 8 wk of injections with heterologous TWD40-2 protein (Cocalico Biologicals) was diluted in 15 mL of TBST buffer, incubated with the membrane for 2 h, and followed by four washes with TBST. HRP-linked anti-rabbit IgG secondary antibody from donkey (2.5 μL; GE Healthcare) was diluted in 15 mL of TBST, incubated with the membrane for 1.5 h, and followed by four TBST washes. Signal was detected on film by chemiluminescence using SuperSignal West Femto substrate (Thermo Scientific). Coomassie-stained SDS/PAGE gels were used as a loading control to ensure equal loading of total protein from each line in the Western blots.
Live-Cell Imaging and Analysis.
With the exception of two experiments (FM4-64 internalization assay and imaging under HUB1 induction), all images were obtained of epidermal cells of 3-d-old etiolated hypocotyls 0.5–2 mm below the apical hook. For imaging of YFP-CESA6, the cells chosen for imaging were all positioned two to four cells below the curved cells of the apical hook. The FM4-64 internalization assay was performed in epidermal cells of the meristematic zone of 5-d-old seedling roots grown under a 16-h light/8-h dark cycle. The seedlings were immersed in half-strength MS liquid with 1% sucrose and 1 μM FM4-64 for 3 min with gentle agitation on a rocker before slide preparation. Imaging of etiolated hypocotyls of RFP-HUB1–induced and mock-treated seedlings occurred on the fourth day because 24-h 4-hydroxytamoxifen (TAM) or mock treatment began after 3 d of growth. Half-strength MS liquid without sucrose was used to prepare microscope slides for the imaging of all dark-grown seedlings, and half-strength MS liquid with 1% sucrose and 1 μM FM4-64 was used to prepare microscope slides for the imaging of FM4-64–treated seedlings. All images were acquired by the imaging system described previously (11).
In cases where the labeled protein exhibited both PM and intracellular localization, automated particle detection analyses were limited to PM-localized particles by manually selecting regions of interest that excluded fluorescence from intracellular compartments. In all such cases, PM particles could be differentiated from intracellular signals by differences in morphology and temporal behavior in corresponding time-lapse images. The area of the regions of interest was measured in pixels in ImageJ (NIH) and converted to μm2. For particle-density analysis of CME components, ImageJ was used to enhance the contrast and subtract the background of images to better facilitate particle recognition before the selection of the region of interest. The parameters used in contrast enhancement and background subtraction were always identical between control and experimental lines within an experiment. When analyzing the particle density of YFP-CESA6, no contrast or background subtraction modifications were made, and the analysis was performed on raw images. Automated particle detection was performed on 8-bit images using the “process>find maxima” feature of ImageJ and using an identical noise threshold for all images, which best represented the distribution of particles for each image. For each region of interest, the particle density was calculated by dividing the number of detected particles within a region of interest by the area of the region of interest.
CME machinery particle lifetime measurements were measured manually by tracking particles of interest frame by frame in a series of time-lapse images and calculating the time elapsed between appearance and disappearance events. A randomly drawn line was used to create kymographs of CME particle dynamics. The functional association between CME components was measured by randomly choosing an initial particle of interest in one fluorescence channel and subsequently tracking the particle of interest and, if applicable, its associated particle in the other fluorescence channel simultaneously with the “sync windows” tool in ImageJ. Equal representation of unassociated particles from each channel was ensured by choosing an equal number of particles from each channel as the initial particle of interest in each analysis. For each pair of functionally associated particles, the order of appearance and disappearance was recorded to analyze the temporal sequence of recruitment and dissociation of each component.
The analyses of YFP-CESA6 velocities and FM4-64 internalization were performed as described previously (11).
Cellulose Content Analysis.
The crystalline cellulose content was measured by the Updegraff method (27). Etiolated seedlings were harvested into 40 mL of 80% (vol/vol) ethanol and incubated for 24 h at 65 °C. The ethanol was decanted and replaced with 40 mL of acetone and incubated at room temperature for 24 h. The acetone was decanted and the seedlings were allowed to dry in a fume hood for 4 d. Each batch of dried seedlings was ball-milled into a fine powder and divided into 10 technical replicates, each with a mass between 2 and 3 mg. One milliliter of acid reagent (8:2:1, acetic acid:water:nitric acid) was added to each replicate and boiled for 30 min. The remaining insoluble crystalline cellulose was pelleted by centrifugation at 17,000 × g. Cellulose pellets were washed with ddH2O followed by a wash with acetone and allowed to dry overnight. Dried cellulose pellets were dissolved in 1 mL 67% (vol/vol) sulfuric acid at room temperature for 6 h. For each replicate, 50 μL of dissolved cellulose was diluted by 450 μL ddH2O, treated with 1 mL 0.02% anthrone in concentrated sulfuric acid, and boiled for 5 min. Glucose concentrations of each replicate, which are indicative of the crystalline cellulose content of the original sample, were measured by colorimetric analysis at a wavelength of 620 nm against a standard curve of known glucose concentrations that were subjected to the same treatment. Cellulose content was expressed as microgram of crystalline cellulose measured per milligram of dried seedling powder material.
RFP-HUB1 Induction.
A 10 mM 4-hydroxytamoxifen stock was stored in DMSO at −20 °C. To test the inducer concentration-dependent growth phenotype of etiolated INTAM>>RFP-HUB1 seedlings, half-strength MS plates without sucrose were supplemented with various concentrations of TAM. Sterilized and cold-treated seeds were plated on TAM-containing plates and hypocotyl lengths were measured after 6 d in the dark at 21 °C. For cellulose content analysis, etiolated seedlings were grown on half-strength MS plates without sucrose with or without 200 nM TAM for 5 d and subjected to the cellulose content analysis described above.
For the 24-h RFP-HUB1 induction studies used for imaging, the 10 mM stock solution was diluted 5,000-fold to a working concentration of 2 μM in half-strength MS liquid without sucrose. The mock solution consisted of a 5,000-fold dilution of DMSO in half-strength MS liquid without sucrose. Three-day-old etiolated seedlings were transferred to TAM or mock solutions under extremely low light conditions and incubated at room temperature in the dark for 24 h before imaging. The microscope slides were prepared with the same TAM or mock solutions, and images were analyzed as described above.
Supplementary Material
Acknowledgments
We thank S. Bednarek for providing the CLC-mOrange line, J. Friml for the INTAM>>RFP-HUB1 line, and C. R. Somerville for the YFP-CESA6 line. We also thank Deborah Grove and the Penn State Genomics Core Facility for aiding us in designing and conducting the real-time qPCR experiments. This work was supported by the Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences, under Award DE-SC0001090.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509292112/-/DCSupplemental.
References
- 1.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6(11):850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 2.Somerville C. Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol. 2006;22:53–78. doi: 10.1146/annurev.cellbio.22.022206.160206. [DOI] [PubMed] [Google Scholar]
- 3.Bashline L, Li S, Gu Y. The trafficking of the cellulose synthase complex in higher plants. Ann Bot (Lond) 2014;114(6):1059–1067. doi: 10.1093/aob/mcu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Bashline L, Lei L, Gu Y. Cellulose synthesis and its regulation. Arabidopsis Book. 2014;12:e0169. doi: 10.1199/tab.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312(5779):1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- 6.Crowell EF, et al. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell. 2009;21(4):1141–1154. doi: 10.1105/tpc.108.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez R, Lindeboom JJ, Paredez AR, Emons AMC, Ehrhardt DW. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol. 2009;11(7):797–806. doi: 10.1038/ncb1886. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, et al. Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc Natl Acad Sci USA. 2010;107(29):12866–12871. doi: 10.1073/pnas.1007092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei L, Li S, Du J, Bashline L, Gu Y. Cellulose synthase INTERACTIVE3 regulates cellulose biosynthesis in both a microtubule-dependent and microtubule-independent manner in Arabidopsis. Plant Cell. 2013;25(12):4912–4923. doi: 10.1105/tpc.113.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Lei L, Somerville CR, Gu Y. Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc Natl Acad Sci USA. 2012;109(1):185–190. doi: 10.1073/pnas.1118560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bashline L, Li S, Anderson CT, Lei L, Gu Y. The endocytosis of cellulose synthase in Arabidopsis is dependent on μ2, a clathrin-mediated endocytosis adaptin. Plant Physiol. 2013;163(1):150–160. doi: 10.1104/pp.113.221234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Rubbo S, et al. The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell. 2013;25(8):2986–2997. doi: 10.1105/tpc.113.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan L, et al. Dynamic analysis of Arabidopsis AP2 σ subunit reveals a key role in clathrin-mediated endocytosis and plant development. Development. 2013;140(18):3826–3837. doi: 10.1242/dev.095711. [DOI] [PubMed] [Google Scholar]
- 14.Kim SY, et al. Adaptor protein complex 2-mediated endocytosis is crucial for male reproductive organ development in Arabidopsis. Plant Cell. 2013;25(8):2970–2985. doi: 10.1105/tpc.113.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaoka S, et al. Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell. 2013;25(8):2958–2969. doi: 10.1105/tpc.113.114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 17.Gadeyne A, et al. The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell. 2014;156(4):691–704. doi: 10.1016/j.cell.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 18.Hirst J, et al. Characterization of TSET, an ancient and widespread membrane trafficking complex. eLife. 2014;3:e02866. doi: 10.7554/eLife.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konopka CA, Backues SK, Bednarek SY. Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell. 2008;20(5):1363–1380. doi: 10.1105/tpc.108.059428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolte S, et al. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc. 2004;214(Pt 2):159–173. doi: 10.1111/j.0022-2720.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito E, et al. Dynamic behavior of clathrin in Arabidopsis thaliana unveiled by live imaging. Plant J. 2012;69(2):204–216. doi: 10.1111/j.1365-313X.2011.04782.x. [DOI] [PubMed] [Google Scholar]
- 22.Kitakura S, et al. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell. 2011;23(5):1920–1931. doi: 10.1105/tpc.111.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boucrot E, Saffarian S, Zhang R, Kirchhausen T. Roles of AP-2 in clathrin-mediated endocytosis. PLoS One. 2010;5(5):e10597. doi: 10.1371/journal.pone.0010597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguet F, Antonescu CN, Mettlen M, Schmid SL, Danuser G. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev Cell. 2013;26(3):279–291. doi: 10.1016/j.devcel.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cocucci E, Aguet F, Boulant S, Kirchhausen T. The first five seconds in the life of a clathrin-coated pit. Cell. 2012;150(3):495–507. doi: 10.1016/j.cell.2012.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loerke D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7(3):e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Updegraff DM. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- 28.Karimi M, Inze D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7(5):193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.