Significance
The broadly neutralizing anti–HIV-1 monoclonal antibody (mAb) PG9 requires multiple posttranslational modifications to exhibit its full biological activity, including proper N-glycosylation and tyrosine sulfation. We now describe a technology that permits the controlled synthesis of these modifications in Nicotiana benthamiana. This technology allowed us to show that sulfated PG9 neutralizes HIV-1 with much higher potency than unsulfated antibody. We also found that glycooptimized mAb versions made in plants are superior to PG9 produced in mammalian cells with respect to mediating antibody-dependent cellular cytotoxicity, an important mAb effector function. Because effector functions are of key importance for antibody-mediated immune control of HIV-1 infections, our results can instruct the development of improved immunotherapeutics for the treatment of AIDS patients.
Keywords: antibody, biopharmaceutical, glycosylation, plant, sulfation
Abstract
Broadly neutralizing anti–HIV-1 monoclonal antibodies, such as PG9, and its derivative RSH hold great promise in AIDS therapy and prevention. An important feature related to the exceptional efficacy of PG9 and RSH is the presence of sulfated tyrosine residues in their antigen-binding regions. To maximize antibody functionalities, we have now produced glycan-optimized, fucose-free versions of PG9 and RSH in Nicotiana benthamiana. Both antibodies were efficiently sulfated in planta on coexpression of an engineered human tyrosylprotein sulfotransferase, resulting in antigen-binding and virus neutralization activities equivalent to PG9 synthesized by mammalian cells (CHOPG9). Based on the controlled production of both sulfated and nonsulfated variants in plants, we could unequivocally prove that tyrosine sulfation is critical for the potency of PG9 and RSH. Moreover, the fucose-free antibodies generated in N. benthamiana are capable of inducing antibody-dependent cellular cytotoxicity, an activity not observed for CHOPG9. Thus, tailoring of the antigen-binding site combined with glycan modulation and sulfoengineering yielded plant-produced anti–HIV-1 antibodies with effector functions superior to PG9 made in CHO cells.
Monoclonal antibodies (mAbs) offer great promise for AIDS treatment (1). In particular, the recent discovery of broadly neutralizing anti–HIV-1 mAbs (bNAbs) with extraordinary potency as exemplified by the antibodies PG9, PG16 (2), or those of the PGT series (3) creates hope for effective therapy by passive antibody transfer. PG9 and its close relative PG16 neutralize ∼80% of HIV-1 isolates across all clades (2, 4). The recognized epitopes are within the hypervariable and heavily glycosylated V1/V2 loops of the viral envelope glycoprotein gp120 and preferentially displayed in its trimeric state (2). Both mAbs use their unusually long complementarity-determining region (CDR) H3 domains (4–6) to penetrate the glycan shield of the virus and make contact with the underlying protein backbone (7). In addition, PG9 and PG16 recognize two highly conserved gp120 N-glycans attached to Asn160 and Asn156/173, which flank the peptide epitope (7–9). Remarkably, the glycan-binding properties of the two antibodies could be combined by modification of the PG9 light chain with RL94SHL95A as found in PG16. This PG9 variant (here termed RSH) has a superior neutralization capacity and broader coverage of HIV-1 isolates than either wild-type PG9 or PG16, which makes it an excellent choice for additional drug development studies (7).
Proper N-glycosylation is important for aspects of mAb functionality, because the oligosaccharides attached to Asn297 of the crystallizable fragment (Fc) are known to strongly affect binding to cellular Fc receptors and thus, in vivo functionalities (10). In particular, core α1,6-fucosylation, typically found on mAbs produced in mammalian cell lines, has been shown to hinder antibody-dependent, cell-mediated cytotoxicity (ADCC) and antibody-dependent, cell-mediated virus inactivation (11), key effector functions in the context of anti–HIV-1 immune responses (12–14). Hence, considerable efforts have been undertaken to establish mAb production systems generating human-type N-glycans lacking this modification. Plants, particularly Nicotiana benthamiana, are well-suited for glycan engineering processes. The advantages of plant-based expression platforms include a high extent of glycan homogeneity, the flexibility with which glycosylation can be modulated, high production speed, and ease of large-scale production (15). The superior efficiency of glycocengineered mAbs produced in plants has recently been highlighted by ZMapp, an mAb mixture developed for the treatment of Ebola patients (16). Similarly, improved effector potency has been observed for plant-made anti–HIV-1 bNAb 2G12 (17), rendering glycoengineered plants an interesting production system for mAbs.
Another posttranslational modification, namely tyrosine sulfation of selected residues in the CDR H3 region of PG9 and PG16, has recently been shown to be critical for high-affinity interactions with their antigen (4, 6). In humans, tyrosine sulfation is carried out by two closely related type II transmembrane proteins: tyrosylprotein sulfotransferase 1 (TPST1) and TPST2 (reviewed in refs. 18 and 19). Although plants contain TPSTs, these proteins are phylogenetically unrelated to the human enzymes and could, therefore, exhibit different enzymatic properties (18, 20). Previous attempts to produce bioactive PG9 and PG16 in N. benthamiana have failed, possibly because of deficient tyrosine sulfation (21). Hence, it is currently uncertain whether plant-based expression platforms are naturally capable of sulfating tyrosine residues in recombinant proteins.
Here, we aimed to maximize the potency of bNAbs against HIV-1 using a plant-based expression system. For this goal, PG9 and RSH were expressed in a xylosyltransferase (XT)- and fucosyltransferase (FT)-deficient N. benthamiana mutant (ΔXT/FT) supporting the synthesis of glycan-optimized, fucose-free mAbs (15). Whereas tyrosine sulfation of PG9 by endogenous plant enzymes was barely detectable, this additional posttranslational modification was efficiently introduced by coexpression of human TPST1 (hsTPST1) modified with a plant Golgi-targeting sequence. When sulfated, plant-derived PG9 had essentially the same antigen-binding and virus neutralization properties as its counterpart produced in CHO cells. Importantly, ADCC activity was displayed by fucose-free, plant-produced mAbs but not by CHO-derived PG9. Furthermore, the controlled production of both sulfated and unmodified PG9 in the same expression system enabled us to establish the impact of tyrosine sulfation on the functionality of this important bNAb.
Results
Coexpression of TPST1 Enables the in Planta Production of Sulfated PG9.
We expressed PG9 and its derivative RSH in ΔXT/FT N. benthamiana plants that have been glycoengineered to remove the plant-typical N-glycan residues β1,2-xylose and core α1,3-fucose (ΔXFPG9 and ΔXFRSH) (22, 23). RSH differs from PG9 by three amino acids in the light chain (PG9: T94RR95A; RSH: R94SH95A). MS analysis of CDR H3 peptides did not provide evidence for sulfation of PG9 and RSH by endogenous plant enzymes, whereas a high degree (82%) of CHO-derived PG9 (CHOPG9) was sulfated (Table 1). Although a functional TPST has been described in Arabidopsis thaliana (20), we could not retrieve a TPST candidate from the N. benthamiana draft genome (24). Because previous reports showed that inefficient tyrosine sulfation of PG9 by HEK293 cells can be rescued by TPST overexpression (6), PG9 was coexpressed with hsTPST1 in ΔXT/FT plants. To mediate proper targeting to sub-Golgi compartments, three constructs carrying different cytoplasmic tail, transmembrane domain, and stem (CTS) regions were tested. Expression of hsTPST1 combined with its native CTS region (pFullhsTPST1) led to 15–20% sulfated ΔXFPG9 (Table S1). Interestingly, replacement of the CTS region with the corresponding domain of glycosylation enzymes known to be targeted to the medial/trans region of the plant Golgi (pFut11hsTPST1 and pRSThsTPST1) led to the production of ΔXFPG9Sulf and ΔXFRSHSulf with substantially higher levels of sulfation, almost reaching the extent of tyrosine sulfation observed for CHOPG9. Using pRSThsTPST1, up to 57% of plant-produced mAbs were monosulfated or disulfated (Table 1 and Tables S1 and S2).
Table 1.
Tyrosine sulfation of plant-produced PG9 and RSH
| Sulfates | CHOPG9 | ΔXFPG9 | ΔXFPG9Sulf | ΔXFPG9SulfSia | ΔXFRSH | ΔXFRSHSulf | ΔXFRSHSulfSia |
| 0 | 19 | >98 | 49 | 43 | >98 | 53 | 49 |
| 1 | 60 | <1 | 33 | 34 | <1 | 31 | 34 |
| 2 | 21 | <1 | 18 | 23 | <1 | 17 | 17 |
| 1 or 2 | 82 | <2 | 51 | 57 | <2 | 47 | 51 |
Relative amounts of unsulfated, singly sulfated, and doubly sulfated PG9 and RSH when coexpressed with pRSTTPST1 in plants. The sulfation status of CHOPG9 is shown for comparison.
Table S1.
Tyrosine sulfation of PG9 and RSH
| mAb | TPST1 construct | OD600 | 0S (%) | 1S (%) | 2S (%) |
| ΔXFPG9Sulf | pFullhsTPST1 | 0.01 | 91.5 ± 7.0 | 8.2 ± 6.4 | 0.3 ± 0.7 |
| ΔXFPG9Sulf | pFullhsTPST1 | 0.05 | 83.4 ± 13.9 | 16 ± 13.1 | 0.6 ± 0.9 |
| ΔXFPG9Sulf | pFullhsTPST1 | 0.2 | 83.7 ± 8.8 | 15.6 ± 8.2 | 0.7 ± 0.9 |
| ΔXFPG9Sulf | pFullhsTPST1 | 0.8 | 82.8 ± 11.7 | 16.8 ± 11 | 0.4 ± 0.7 |
| ΔXFPG9Sulf | pFut11hsTPST1 | 0.01 | 73.7 ± 5.8 | 17.8 ± 3.3 | 8.6 ± 2.6 |
| ΔXFPG9Sulf | pFut11hsTPST1 | 0.05 | 57.4 ± 0.8 | 32.1 ± 2.3 | 10.6 ± 3.1 |
| ΔXFPG9Sulf | pFut11hsTPST1 | 0.2 | 59.6 ± 5.8 | 28.1 ± 5.4 | 12.3 ± 5.4 |
| ΔXFPG9Sulf | pFut11hsTPST1 | 0.8 | 58.7 ± 8.7 | 32.7 ± 10.8 | 8.6 ± 2.1 |
| ΔXFPG9Sulf | pRSThsTPST1 | 0.01 | 69.5 ± 8.9 | 20.6 ± 6.9 | 9.9 ± 2.3 |
| ΔXFPG9Sulf | pRSThsTPST1 | 0.05 | 53.9 ± 6.1 | 30.8 ± 2.3 | 15.3 ± 3.8 |
| ΔXFPG9Sulf | pRSThsTPST1 | 0.2 | 57.2 ± 10.4 | 29.3 ± 7.4 | 13.5 ± 5.1 |
| ΔXFPG9Sulf | pRSThsTPST1 | 0.8 | 53.7 ± 11.1 | 36.8 ± 11.8 | 9.5 ± 2.7 |
| ΔXFRSHSulf | pRSThsTPST1 | 0.2 | 57.4 ± 7.6 | 29.6 ± 5.2 | 13.1 ± 3.6 |
| CHOPG9 | 18.5 ± 1.5 | 60.4 ± 3.9 | 21.1 ± 5.4 |
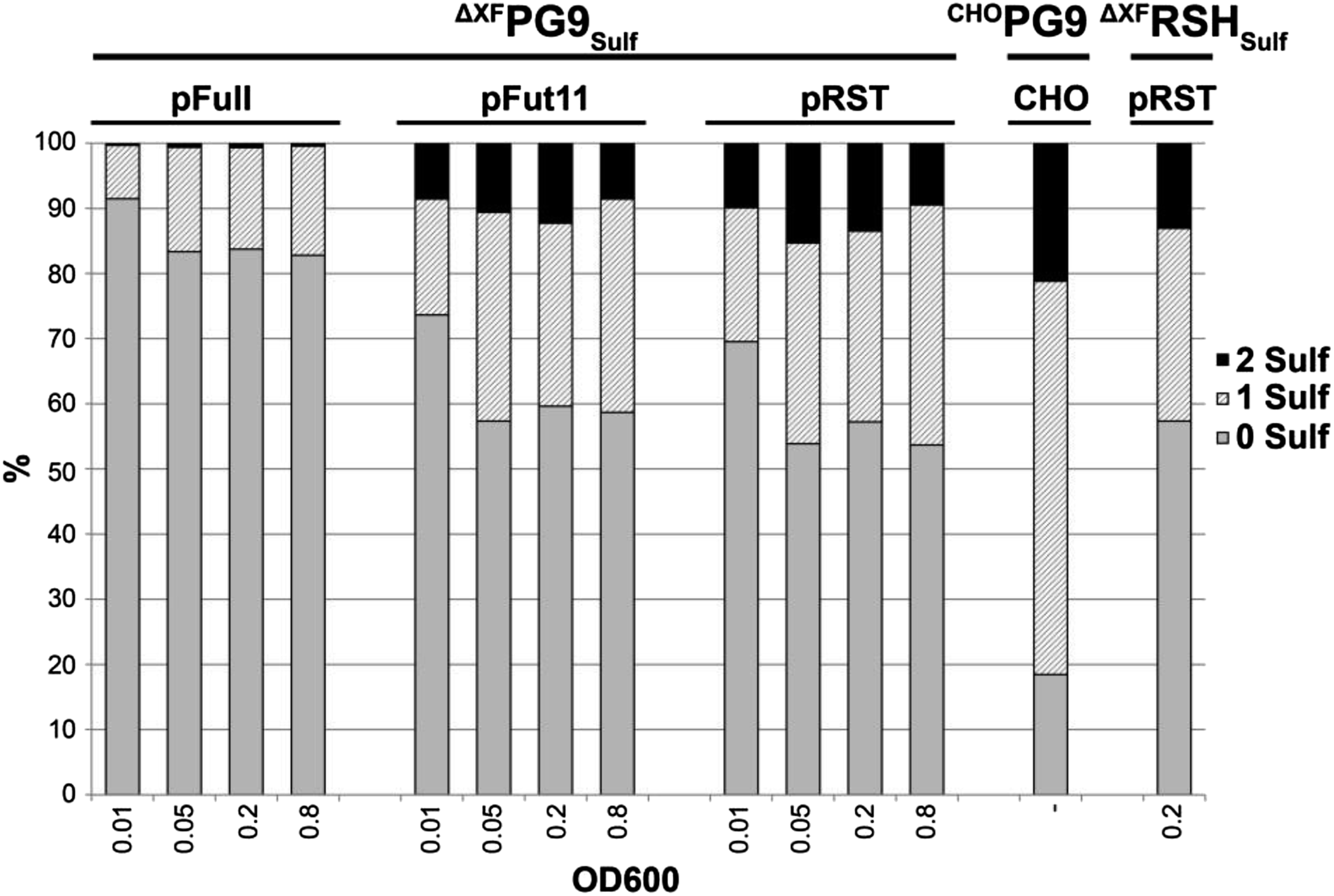
Relative amounts of unsulfated (0S), monosulfated (1S), and disulfated (2S) PG9 and RSH when coexpressed with different hsTPST1 constructs in plants. The sulfation status of CHOPG9 is shown for comparison. Data are presented as means ± SD of two to nine analyses.
Table S2.
N-glycosylation of PG9 and RSH expressed in plants and CHO cells
| Glycan | ΔXFPG9 (%) | ΔXFPG9Sulf (%) | ΔXFPG9SulfSia (%) | ΔXFRSH (%) | ΔXFRSHSulf (%) | ΔXFRSHSulfSia (%) | CHOPG9 (%) |
| UGnF* | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 |
| MGn* | 1.8 | 2.1 | 1.3 | 1.5 | 2.1 | 1.3 | 0.5 |
| MGnF* | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.8 |
| GnGn* | 45.0 | 50.9 | 17.5 | 41.1 | 56.1 | 18.1 | 3.0 |
| GnGnX* | 1.3 | 1.9 | 0.5 | 0.8 | 1.0 | 0.2 | 0.0 |
| GnGnF* | 0.8 | 1.4 | 0.1 | 0.5 | 0.5 | 0.2 | 69.8 |
| MA* | 0.1 | 0.2 | 0.9 | 0.1 | 0.0 | 1.4 | 0.0 |
| GnA* | 0.6 | 0.3 | 9.8 | 1.2 | 1.1 | 12.3 | 0.3 |
| GnAF* | 0.1 | 0.1 | 0.2 | 0.2 | 0.0 | 0.5 | 17.7 |
| AA* | 0.0 | 0.1 | 17.6 | 0.0 | 0.1 | 24.4 | 0.0 |
| AAF* | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 | 0.7 | 2.5 |
| MNa* | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.9 | 0.0 |
| GnNa* | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.3 | 0.0 |
| ANa* | 0.0 | 0.0 | 2.9 | 0.0 | 0.0 | 6.4 | 0.0 |
| ANaF* | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.7 | 0.3 |
| NaNa* | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.6 | 0.0 |
| NaNaF* | 0.0 | 0.0 | 1.2 | 0.0 | 0.0 | 2.7 | 0.0 |
| Man5† | 1.0 | 0.2 | 0.9 | 0.5 | 0.3 | 0.2 | 0.5 |
| Man6† | 1.8 | 1.3 | 1.7 | 1.6 | 1.4 | 1.0 | 0.0 |
| Man7† | 2.8 | 1.4 | 2.8 | 2.2 | 2.2 | 2.7 | 0.1 |
| Man8† | 18.7 | 8.8 | 14.0 | 12.9 | 13.1 | 11.5 | 0.0 |
| Man9† | 26.0 | 31.4 | 25.6 | 37.3 | 22.0 | 13.0 | 0.0 |
| Gn‡ | 48.9 | 56.2 | 19.4 | 44.0 | 59.8 | 19.9 | 78.5 |
| Gal‡ | 0.8 | 0.7 | 29.6 | 1.5 | 1.2 | 39.2 | 20.6 |
| Sia‡ | 0.0 | 0.0 | 6.1 | 0.0 | 0.0 | 12.5 | 0.3 |
| Man5-9‡ | 50.3 | 43.1 | 44.9 | 54.5 | 39.0 | 28.4 | 0.6 |
| Fuc§ | 0.9 | 1.5 | 3.2 | 0.7 | 0.5 | 4.8 | 95.6 |
N-glycan acronyms are based on the ProGlycAn nomenclature (www.proglycan.com).
Man5-9, oligomannosidic N-glycans with five to nine mannose residues.
Gn/Gal/Sia, sum of complex N-glycans with terminal GlcNAc/galactose/sialic acid residues; Man5-9, sum of oligomannosidic N-glycans with five to nine mannose residues.
Fuc, sum of core-fucosylated N-glycans.
Tyrosine Sulfation of PG9 Produced in Plants and CHO Cells Occurs at the Same Positions.
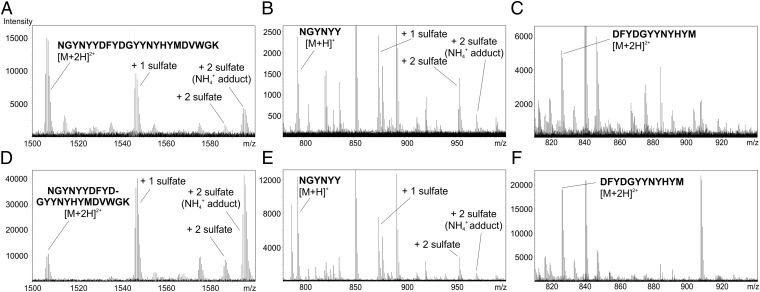
Sulfation of PG9 at specific tyrosine residues is believed to be important for high-affinity antigen binding. Thus, we set out to establish which tyrosines are sulfated in hsTPST1-expressing plants. The tryptic CDR H3 peptide used for analyzing the PG9 sulfation status by liquid chromatography-electrospray ionization (LC-ESI) MS (N100CGYNYYDFYDGYYNYHYMDVWGK105) contains several tyrosine residues that are potential TPST targets. Additional digestion by AspN cleaved the peptide into three parts. In CHOPG9 as well as sulfated PG9 and RSH produced in plants, only one of three fragments was found to be singly or doubly sulfated (N100CGYNYY100H), whereas the other two (D100IFYDGYYNYHYM100T and D101WGK105) were not modified (Fig. 1). This finding indicates that the sulfate groups are attached to Y100E, Y100G, and/or Y100H independent of the expression platform used for PG9 production. It has been shown previously by X-ray crystallography that Y100G and Y100H of PG9 produced in mammalian cells can be sulfated (6). Hence, it is likely that hsTPST1 also modifies the same tyrosine residues in planta.
Fig. 1.
ΔXFPG9Sulf and CHOPG9 are singly and doubly sulfated in the region N100CGYNYY100H. The sulfation sites of (A) ΔXFPG9Sulf and (D) CHOPG9 were mapped by liquid chromatography-electrospray ionization MS to the tryptic PG9 peptide N100CGYNYYDFYDGYYNYHYMDVWGK105. Additional digestion with AspN revealed nonsulfated, singly sulfated, and doubly sulfated variants of the peptide N100CGYNYY100H [(B) ΔXFPG9Sulf and (E) CHOPG9]. No sulfated residues were found on the other tyrosine-containing AspN fragment: D100IFYDGYYNYHYM100T [(C) ΔXFPG9 and (F) CHOPG9].
PG9 Carries Human-Type N-Glycans When Expressed in Glycoengineered Plants.
Because N-glycans are known to modulate binding to cellular Fc receptors (10), several glycoforms of PG9 and RSH were produced. MS N-glycan analysis of ΔXFPG9, ΔXFPG9Sulf, ΔXFRSH, and ΔXFRSHSulf revealed the presence of a predominant complex N-glycan species, G0 (GnGn) (Table 2 and Table S2). This glycoform accounted for roughly 45–50% of all N-glycan species. On coexpression of PG9 and RSH with mammalian genes necessary for terminal galactosylation and sialylation in planta (25) resulting in the synthesis of ΔXFPG9SulfSia and ΔXFRSHSulfSia, the N-glycosylation profiles shifted to 30–40% galactosylated and 6–12% sialylated oligosaccharides, with G0 reduced to 15–20% (Table 2). Importantly, core α1,3-fucose and β1,2-xylose residues were hardly detectable in plant-produced mAbs (below 5%). By contrast, CHOPG9 carried mainly α1,6-fucosylated N-glycans (more than 95%), with G0F6 (GnGnF6) being the most prevalent structure (70%). Roughly 20% of CHOPG9 was galactosylated, and less than 1% was sialylated (Table 2).
Table 2.
N-glycosylation of PG9 and RSH expressed in plants and CHO cells
| Glycans | ΔXFPG9 | ΔXFPG9Sulf | ΔXFPG9SulfSia | ΔXFRSH | ΔXFRSHSulf | ΔXFRSHSulfSia | CHOPG9 |
| Gn | 50 | 56 | 19 | 44 | 60 | 20 | 79 |
| Gal | 1 | 1 | 30 | 2 | 1 | 39 | 21 |
| Sia | 0 | 0 | 6 | 0 | 0 | 13 | 0 |
| Man5-9 | 50 | 43 | 45 | 55 | 39 | 28 | 1 |
| Fuc | 1 | 2 | 3 | 1 | 1 | 5 | 96 |
N-glycan acronyms are based on the ProGlycAn nomenclature (www.proglycan.com). Fuc, sum of core-fucosylated N-glycans; Gn/Gal/Sia, sum of complex N-glycans with terminal GlcNAc/galactose/sialic acid residues; Man5-9, sum of oligomannosidic N-glycans with five to nine mannose residues.
Antigen Binding by ΔXFPG9 Is Enhanced by Tyrosine Sulfation.
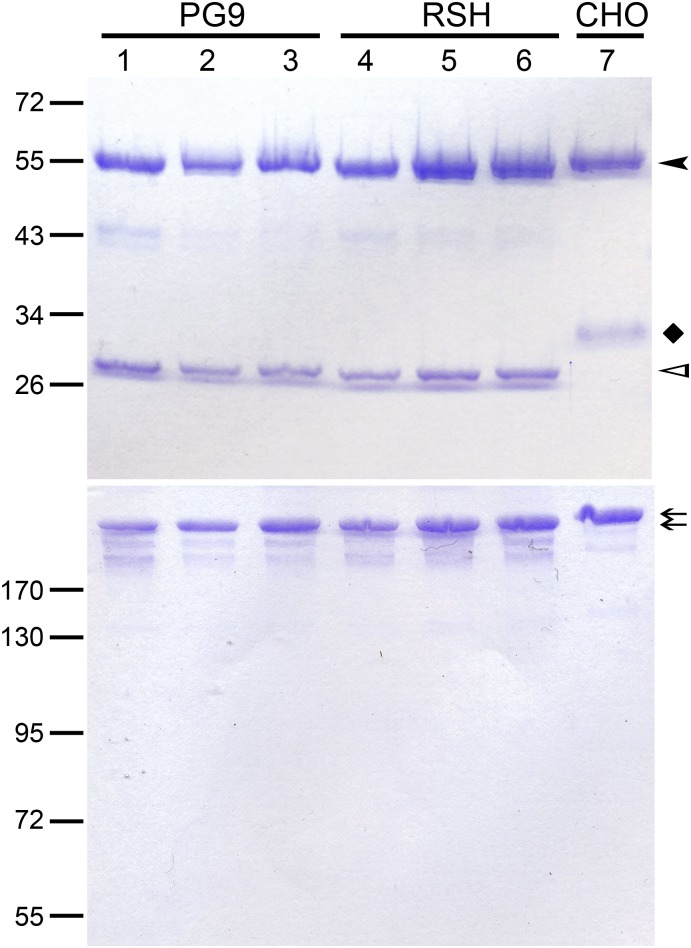
All six PG9 variants produced in plants (ΔXFPG9, ΔXFPG9Sulf, ΔXFPG9SulfSia, ΔXFRSH, ΔXFRSHSulf, and ΔXFRSHSulfSia) could be purified from leaf extracts in good yields. When analyzed by SDS/PAGE under reducing conditions, the heavy and light chains of the plant-made mAbs showed the expected migration pattern, with the light chains displaying higher electrophoretic mobilities than those of CHOPG9 (Fig. S1). This difference is due to the removal of a functionally unnecessary N-glycosylation site in the light chain of the latter antibody (4), which could accelerate its clearance from the circulation (21). Under nonreducing conditions, plant- and CHO-derived PG9 and RSH variants yielded single bands migrating at the same position (Fig. S1).
Fig. S1.
Comparison of plant-produced PG9 and RSH with CHOPG9. Coomassie Brilliant Blue staining of purified PG9 and RSH after separation by SDS/PAGE under (Upper) reducing and (Lower) nonreducing conditions. Heavy chain (HC) bands are indicated by a black arrowhead. A black diamond indicates the light chain (LC) band of CHOPG9, which is N-glycosylated and therefore, has a higher molecular mass than the LC of the plant-produced mAbs (white arrowhead). Arrows indicate fully assembled IgG molecules separated under nonreducing conditions. Lane 1, ΔXFPG9; lane 2, ΔXFPG9Sulf; lane 3, ΔXFPG9SulfSia; lane 4, ΔXFRSH; lane 5, ΔXFRSHSulf; lane 6, ΔXFRSHSulfSia; lane 7, CHOPG9.
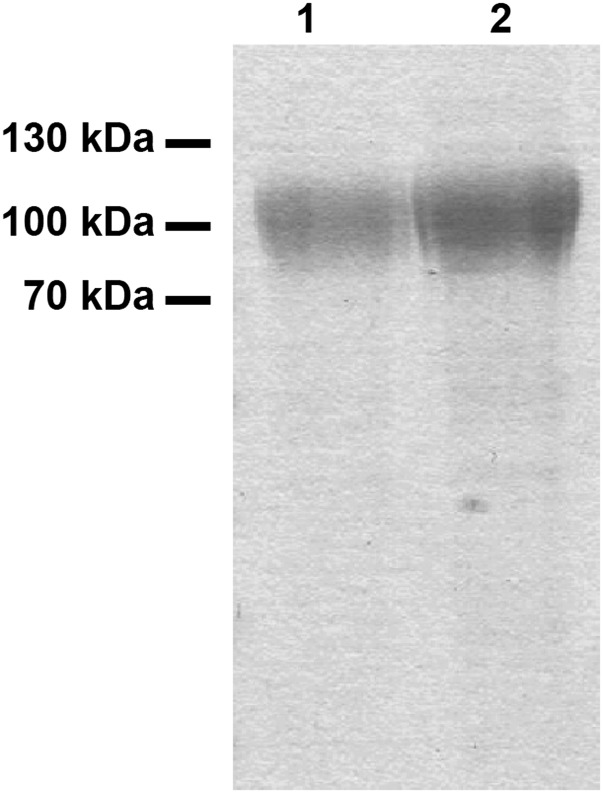
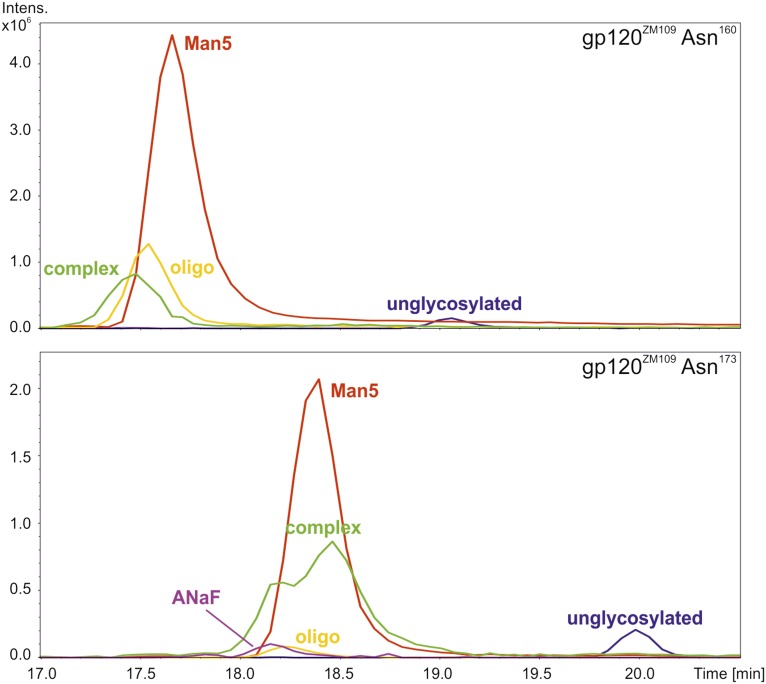
PG9 has been described to bind with high affinity to trimeric envelope glycoproteins of a wide variety of HIV-1 isolates, and also to gp120 monomers of selected HIV-1 strains, including ZM109 (2, 6). To investigate the antigen-binding properties of plant-derived PG9 and RSH variants compared with CHOPG9, we expressed gp120ZM109 containing a C-terminal hexahistidine tag in FreeStyle 293 (FS293) cells and purified it to apparent homogeneity. SDS/PAGE revealed a diffuse band as expected for a heavily glycosylated protein (Fig. S2). N-glycosylation of two gp120 asparagines (Asn160 and Asn173) has been shown to be important for PG9 binding (2, 6). Glycosylation analysis by MS revealed mainly Man5 structures on either of these N-glycosylation sites (Fig. S3 and Table S3). Importantly, PG9 is known to prefer such N-glycans on Asn160, while tolerating them on Asn173. Only minor amounts of other N-glycans were detected on either site, showing that FS293-derived gp120ZM109 meets the prerequisites for a high-affinity PG9 ligand.
Fig. S2.
SDS/PAGE analysis of purified HIV-1 gp120ZM109; 2.6 (lane 1) and 5.2 µg (lane 2) purified gp120ZM109 was separated by SDS/PAGE. Visualization by Coomassie Brilliant Blue staining revealed diffuse bands at 100–110 kDa. The fuzzy appearance of the bands is caused by the high extent of glycosylation.
Fig. S3.
HIV-1 gp120ZM109 carries mainly Man5 glycans on Asn160 and Asn173. Glycans attached to (Upper) Asn160 and (Lower) Asn173 were analyzed by liquid chromatography–MS. Both peptides carried mainly oligomannosidic glycans, with Man5 being the major species. Additionally, minor amounts of other oligomannosidic forms (oligo) as well as nonsialylated complex glycans (complex) were detected [GnGnF(bi), GnGnF, MGnF, GnAF(bi), MAF, GnAF, AAF, and AA]. On Asn173, small amounts of a sialylated glycan (ANaF) were found.
Table S3.
gp120ZM109 carries mainly Man5 glycans on Asn160 and Asn173
| Glycan | gp120ZM109-Asn160 (%) | gp120ZM109-Asn173 (%) |
| Man5* | 68.4 | 54.1 |
| Man6* | 9.8 | 3.2 |
| Man7* | 6.2 | 1.7 |
| Man8* | 2.1 | 1.0 |
| MGnF† | 2.2 | 3.8 |
| GnGnF† | 2.5 | 5.6 |
| MAF† | 2.4 | 2.3 |
| GnAF† | 1.7 | 3.4 |
| AA† | 0.6 | 2.3 |
| AAF† | 1.1 | 3.7 |
| GnGnF(bi)† | 1.8 | 9.7 |
| GnAF(bi)† | 0.9 | 6.1 |
| ANaF† | 0.3 | 3.2 |
| Man5-9‡ | 86.5 | 60 |
| Gn‡ | 4.7 | 9.4 |
| Gal‡ | 8.5 | 27.5 |
| Sia‡ | 0.3 | 3.2 |
Glycans attached to Asn160 and Asn173 were analyzed by liquid chromatography–MS. Both peptides carried mainly oligomannosidic glycans, with Man5 being the major species. Additionally, minor amounts of other oligomannosidic forms as well as nonsialylated complex glycans were detected [MGnF, GnGnF, MAF, GnAF, AA, AAF, GnGnF(bi), and GnAF(bi)]. Small amounts of a sialylated glycan (ANaF) were detected as well.
Man5-8, oligomannosidic N-glycans with five to eight mannose residues.
N-glycan acronyms are based on the ProGlycAn nomenclature (www.proglycan.com).
Man5-9, sum of oligomannosidic N-glycans with five to nine mannose residues; Gn/Gal/Sia, sum of complex N-glycans with terminal GlcNAc/galactose/sialic acid residues.
Binding of the different PG9 and RSH variants to monomeric gp120ZM109 and trimeric gp140BG505.SOSIP.664 (26) was tested by ELISA (Table 3). Although unsulfated ΔXFPG9 and ΔXFRSH had substantially lower affinity to either antigen than CHOPG9, sulfation increased their affinities 10–16 times for trimeric gp140BG505.SOSIP.664 and 2–5 times for monomeric gp120ZM109. Thus, sulfated PG9 produced in plants displays a similar affinity to its antigens as CHOPG9. RSH showed up to threefold better binding than PG9 to either gp120 or gp140. The different glycoforms of each mAb showed comparable EC50 values.
Table 3.
Sulfation enhances antigen binding of plant-derived PG9 and RSH
| Antibody | EC50 (ng/mL) | |
| gp120 | gp140 | |
| CHOPG9 | 89 ± 2 | 290 ± 85 |
| ΔXFPG9 | 421 ± 42 | 4,870 ± 1105 |
| ΔXFPG9Sulf | 92 ± 9 | 490 ± 170 |
| ΔXFPG9SulfSia | 101 ± 7 | 310 ± 30 |
| ΔXFRSH | 179 ± 49 | 2,230 ± 115 |
| ΔXFRSHSulf | 82 ± 19 | 180 ± 5 |
| ΔXFRSHSulfSia | 73 ± 13 | 180 ± 25 |
Binding of PG9 and RSH to immobilized monomeric gp120ZM109 or trimeric gp140BG505.SOSIP.664 was measured by ELISA. Data are presented as means ± SEM of two or three independent experiments.
The avidity of the antigen–antibody interaction was also determined by biolayer interferometry (Table 4). ΔXFPG9Sulf, ΔXFRSHSulf, and CHOPG9 showed roughly the same affinity for gp120ZM109 (Kd values of 525, 605, and 756 nM, respectively), whereas unsulfated ΔXFRSH exhibited a roughly fourfold lower affinity (Kd value of 2,510 nM). The affinity of unsulfated PG9 for gp120ZM109 was too low to be accurately determined under the experimental conditions used (Kd > 3 µM). Overall, these results confirm that tyrosine sulfation increases the affinity of both antibodies.
Table 4.
Affinities of PG9 and RSH for gp120ZM109
| Antibody | Kd (nM) |
| CHOPG9 | 756 ± 163 |
| ΔXFPG9Sulf | 525 ± 75 |
| ΔXFRSHSulf | 605 ± 138 |
| ΔXFRSH | 2,510 ± 39 |
| ΔXFPG9 | >3,000 |
Biolayer interferometry data are presented as means ± SEM of two (ΔXFRSH) or four to six individual determinations. The binding of ΔXFPG9 to gp120ZM109 was too weak for accurate determination of Kd under the experimental conditions used.
Increased Virus Neutralization by Sulfated PG9 and RSH Variants.
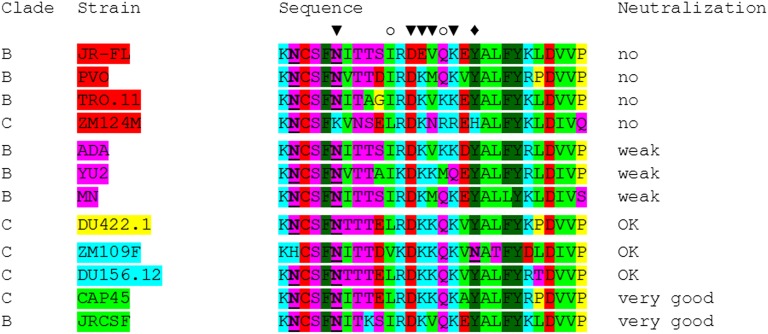
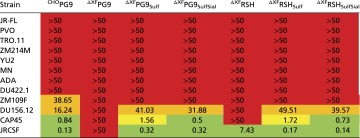
Neutralization efficiencies of the antibodies were tested on a panel of HIV-1 clade B and clade C pseudoviruses (Table 5 and Table S4). The viruses were chosen based on previously published data regarding their susceptibility to PG9 and RSH produced in mammalian cells (2, 6, 7) ranging from well-neutralized to resistant isolates. As expected, a number of pseudoviruses was not neutralized under the tested conditions (JRFL, ZM214M, PVO, and TRO.11), whereas neutralization of others was intermediate (ADA, YU2, and MN), good (DU156.12, DU422.1, and ZM109), or very efficient (JRCSF and CAP45). Interestingly, PG9 variants displayed pronounced differences with respect to their neutralization efficiencies. In accordance with the results of the antigen-binding assays, tyrosine sulfation strongly enhanced neutralization of highly sensitive isolates (50-fold and more; e.g., JRCSF, DU156.12, ZM109, CAP45, and DU422.1), whereas only a modest improvement was observed for more resistant strains (up to 1.5-fold; ADA, YU2, and MN). These data provide unprecedented quantitative evidence for the pivotal role of CDR H3 sulfotyrosines in effective HIV-1 neutralization by PG9 as previously proposed based on the tertiary structure of the PG9/gp120 complex (6). In general, the varying sensitivities of the tested HIV-1 strains to PG9 and RSH were in good agreement with the presence or absence of PG9-interacting residues in their gp120 V2 sequences (Fig. S4). The increase in neutralization efficiency observed for RSH is in good agreement with results obtained in antigen-binding assays. Notably, glycoengineering of plant-derived PG9 and RSH did not affect virus neutralization.
Table 5.
Neutralization efficiencies of PG9 and RSH against a panel of pseudoviruses
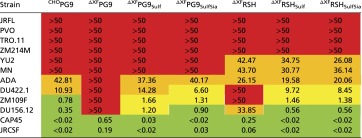 |
IC50 values (in micrograms per milliliter) are color-coded (green, <1 µg/mL; orange, 10–50 µg/mL; red, >50 µg/mL; yellow, 1–10 µg/mL).
Table S4.
Neutralization efficiencies of PG9 and RSH against a panel of pseudoviruses
 |
IC90 values (in micrograms per milliliter) are color-coded (green, <1 µg/mL; orange, 10–50 µg/mL; red, >50 µg/mL; yellow, 1–10 µg/mL).
Fig. S4.
Envelope glycoprotein sequences of the HIV-1 isolates used for neutralization assays. Sequence alignment of the PG9 epitopes (gp120 amino acids 155–183) of 12 pseudoviruses used for neutralization tests. Amino acids are color-coded according to their physicochemical properties: blue, basic; purple, polar/uncharged; red, acidic; green, hydrophobic; gray, aromatic; yellow, G/P. Glycosylated asparagine residues are underlined. Amino acids (and attached glycans) of ZM109F or CAP45 interacting with PG9 are marked (▼, both interacting; ♦, only ZM109F interacting; ○, only CAP45 interacting). The sulfated tyrosine residue H100G of PG9 forms direct interactions with amino acid 168 when it is a lysine or arginine; however, it is repelled when this residue is a glutamic acid as in strain JRFL.
Plant-Derived PG9 and RSH Are Capable of Mediating Antibody-Dependent Cellular Cytotoxicity.
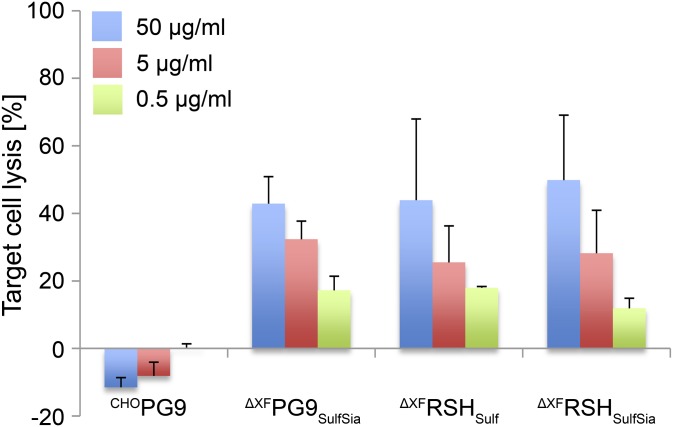
Finally, the capability of PG9 and RSH to elicit ADCC was evaluated. This effector function is suggested to have an important role in the control of HIV-1 viral load and infection (12–14), although its significance in virus clearance in vivo remains controversial, because not all bNAbs against HIV-1 mediate ADCC (27). The latter also applies to CHOPG9, which did not support ADCC activity of human peripheral blood mononuclear cells against target cells inoculated with a PG9-sensitive strain of HIV-1 (JRCSF). In contrast, plant-derived PG9 and RSH induced a potent ADCC response in a concentration-dependent manner (Fig. 2). This difference can be explained by the absence of core fucose residues in the N-glycans of the plant-produced mAbs, which confers an up to 100-fold increase in ADCC potency (11).
Fig. 2.
Plant-derived PG9 and RSH variants are capable of inducing ADCC. ADCC assays were performed in the presence of the indicated PG9 or RSH concentrations. Data were corrected for the extent of nonspecific cell lysis observed in the presence of a control antibody and expressed as means ± SEM of three independent experiments (each performed with effector cells isolated from a different donor).
Discussion
In this study, we aimed to optimize the potency of anti–HIV-1 bNAb PG9 by combining CDR engineering with the modulation of different posttranslational modifications (i.e., glycosylation and tyrosine sulfation). Sulfation of specific tyrosine residues of PG9 and its CDR-engineered variant RSH is considered important for high-affinity binding to antigens. Although some plants have a TPST and can sulfate phytohormones (20), PG9 expressed in N. benthamiana did not contain detectable amounts of sulfotyrosines, indicating that mammalian-type sulfation does not occur naturally in N. benthamiana leaves. Overexpression of the hsTPSTs hsTPST1 and hsTPST2 (18) increased sulfation of recombinantly produced proteins in mammalian cells (6, 28). However, expression of full-length hsTPST1 in N. benthamiana did not yield efficient levels of PG9 tyrosine sulfation. We and others have shown previously that engineering of posttranslational modifications in plants by overexpression of human enzymes necessitates targeting of the respective enzyme to its correct subcellular location (29). Indeed, on replacing the authentic CTS sequence of hsTPST1 with a plant CTS region known to target proteins to late Golgi compartments, a marked increase in sulfation efficiency was observed. This improvement suggests that the native CTS of hsTPST1 is not capable of mediating the efficient delivery of the enzyme to its proper intracellular destination in plant cells.
The crystal structure of PG9 in complex with its antigen has revealed that Y100G and Y100H of the PG9 heavy chain can be sulfated (4, 6). The functional relevance of this posttranslational modification has been largely inferred from structural studies and a comprehensive mutational assessment of the antibody’s CDR H3 region. Replacement of Y100G and Y100H with alanine or phenylalanine, respectively, resulted in substantially weaker antigen binding and HIV-1 neutralization. However, the functionality of PG9 was also reduced by mutagenesis of other CDR H3 tyrosines (4). We could map the sulfation sites of CHOPG9 and plant-produced PG9/RSH by MS to a short CDR H3 peptide containing three tyrosine residues (Y100E, Y100G, and Y100H). Sulfation of plant-produced PG9 and RSH enhanced antigen binding and virus neutralization, indicating that, in plants as well, Y100G and Y100H are the sulfated residues. Although the impact of tyrosine sulfation on neutralization efficiency was previously only assessed for singly and doubly sulfated PG9 (4), we now compared sulfated and unsulfated PG9/RSH and observed a far more pronounced difference in antiviral potency. Taken together, these results show that singly sulfated PG9 binds and neutralizes HIV-1 better than nonsulfated antibody and that the doubly sulfated mAb displays even further enhanced binding. Interestingly, the effect of tyrosine sulfation on antigen binding was more pronounced for trimeric gp140 than for monomeric gp120.
A dependence on sulfated tyrosines was also observed for other anti–HIV-1 mAbs binding to the V1/V2 loops or the coreceptor binding site (30). This finding indicates that tyrosine sulfation is a critical posttranslational modification common to many of these antibodies (5, 30). It is of note that tyrosine sulfation also affects the antigenicity of gp120 itself. It has been reported that gp120 from CD4+ T cell-produced virions is more extensively sulfated than cell line-produced gp120 (28) and that V2 needs to be sulfated to stabilize V3. Thus, efficient tyrosine sulfation of gp120 vaccine candidates might be crucial for eliciting a sustained antibody response. Other biotherapeutic candidates also depend on sulfation for optimal activity (31, 32). Hence, the procedures developed for human-type protein sulfation in N. benthamiana further widen the scope of this powerful expression system.
We also intended to improve the in vivo efficacies of PG9 and RSH by means of glycoengineering. In IgGs, elimination of core fucose from the conserved Fc glycans strongly increases Fc-mediated effector functions, like ADCC or antibody-dependent, cell-mediated virus inactivation (11, 17, 33). These effector functions seem to play an important role in HIV-1 control by the immune system (12–14). For this reason, we produced mAbs decorated with fucose-free, human-type N-glycans (22). Such structures are considered “glycan-optimized,” and they are the basis for next generation antibodies in cancer therapies (34) and other indications (16). As expected, PG9 and RSH produced in ΔXT/FT plants contained, in all cases, less than 5% fucosylated glycans. By contrast, CHOPG9 was highly fucosylated (>95%), which is typical for CHO-produced mAbs. The elimination of core fucose residues clearly improves the potential of the plant-derived antibodies to mediate ADCC. However, it should be noted that the significance of ADCC in HIV-1 clearance by bNAbs in vivo is still a controversial issue. Although a nonfucosylated version of the anti–HIV-1 mAb b12 was more effective in ;inducing the ADCC-dependent killing of virus-infected cells in vitro, it did not improve protection against vaginal challenge with a hybrid simian–human immunodeficiency virus in macaques (35). Nevertheless, it is now well-established that Fc effector functions are of pivotal importance for the therapeutic activity of bNAbs against HIV-1 (36, 37).
It cannot be excluded that even the low residual levels of nonmammalian glycans (less than 5%) present on plant-derived PG9 and RSH could lead to adverse immune reactions on regular treatment of humans with therapeutically relevant doses of these bNAbs (10–30 mg/kg) (38). However, biweekly infusions of Gaucher disease patients with up to 2 mg/kg human glucocerebrosidase produced in carrot cells have proven clinically safe over a period of 9–12 mo, with none of the treated individuals developing neutralizing antibodies to the recombinant enzyme (39, 40). Noteworthy, the nonmammalian glycan content of plant-made glucocerebrosidase (41, 42) is per mass unit about 100 times higher than that of PG9 and RSH produced in ΔXT/FT N. benthamiana plants. This observation suggests that human recipients could tolerate even long-term therapy with these bNAbs. Notwithstanding, efforts should be undertaken to completely eliminate undesirable N-glycan species from N. benthamiana and other plant-based expression platforms (for instance, by means of genome editing) (43).
In addition to core fucosylation, it has been also reported that Fc sialylation can modulate the biological activities of antibodies (44, 45). Although this modification has been shown to impede the binding of IgG to FcγRII and FcγRIII (44, 46), recent structural evidence suggests that sialylated Fc glycans could tighten the interaction with FcγRI (47). Because it has not been tested before whether sialylation of bNAbs influences their effector functions, we have produced terminally sialylated PG9 and RSH variants in N. benthamiana by coexpression of genes from the mammalian galactosylation and sialylation pathway (25). Plant-produced PG9 and RSH were 30–40% galactosylated and 6–12% sialylated, similar to human serum IgG (48) and clearly higher than for CHOPG9. Interestingly, none of the tested functionalities of PG9 and RSH (antigen binding, virus neutralization, and ADCC) were affected by the extent of galactosylation and sialylation. However, an in vivo contribution of these N-glycan moieties to serum half-life or other pharmacokinetically important properties cannot be ruled out.
Materials and Methods
Experimental procedures are detailed in SI Materials and Methods. These outlines include cloning of expression constructs, recombinant protein purification and biochemical characterization, glycosylation and sulfation analysis by MS, ELISA, and biolayer interferometry. The protocols used for virus neutralization and ADCC assays are also provided.
SI Materials and Methods
PG9 and RSH Cloning.
The vectors used for PG9 expression have been described previously (23). Briefly, the signal peptide of barley α-amylase was cloned into MagnIcon vectors pICH26033 and pICH31160 (provided by Viktor Klimyuk, Icon Genetics, Halle, Germany) to give rise to pICHα26033 and pICHα31160. cDNA encoding the PG9 VH and CH1 domains (6) (Protein Data Bank ID code 3U36) was codon-optimized for Nicotiana benthamiana, and a BsaI site added. The fragment was then PCR-amplified with primers PG9VH-SOE-F1 (5′-GTACGGTCTCAAGGTCAGAGG-3′) and IgG1Hinge-SOE-R1 (5′-CTGGAGCTGGACATGGTG-3′), fused to the Fc fragment generated with IgG1Hinge-SOE-F1 (5′-CACCATGTCCAGCTCCAG-3′) and RxCH3-SOE-R1 (5′-GCTAGGTCTCAAAGCTCACTTTCCAGGAGAAAGAGAAAG-3′) by splicing-by-overlap-extension PCR, and then, cloned into the BsaI sites of pICHα31160. PG9LC and PG9LC-RSH cDNAs (both lacking the signal peptide and encoding an N23Q mutation) were synthesized with codons optimized for N. benthamiana and flanking BsaI sites. PG9LC and PG9LC-RSH were then inserted into pICHα26033. All vectors were then transformed into the Agrobacterium tumefaciens strain GV3101pMP90.
Cloning of TPST Constructs.
Three different hsTPST1 constructs (49) (GenBank accession no. AK313098.1) were cloned into binary vector pPT2 (50). pFullhsTPST1 contains the authentic CTS region. In pFut11hsTPST1, Met1-Ser39 of hsTPST1 is replaced by the CTS of Arabidopsis thaliana α1,3-fucosyltransferase 11 (Met1-Val68, AEE76217.1); in pRSThsTPST, it is replaced by the CTS region of rat α2,6-sialyltransferase (Met1-Gly54, M18769.1). All constructs were transformed into A. tumefaciens strain UIA143pMP90.
Cloning, Expression, and Purification of gp120ZM109.
The codon-optimized coding sequence for gp120 of HIV-1 strain ZM109 (gp120ZM109) was appended with a C-terminal hexahistidine tag and inserted into the HindIII/NotI sites of pCEP4 (Life Technologies). Transient expression in FreeStyle293F cells (Life Technologies) was performed following the instructions of the manufacturer. Culture supernatants were subjected to affinity chromatography using Ni2+-charged Chelating Sepharose (GE Healthcare) as reported previously (51), omitting the addition of phosphatase and protease inhibitors. Fractions eluted with 250 mM imidazole were dialyzed against PBS containing 0.02% (wt/vol) NaN3 and then, concentrated by ultrafiltration.
In Planta Expression of PG9 and RSH.
N. benthamiana ΔXT/FT plants (age of 4–5 wk) were used for coinfiltration with agrobacteria as described previously (22, 52). Briefly, liquid cultures of agrobacterial strains carrying pPG9HC, pPG9LC, pPG9LC-RSH, pFullhsTPST1, pFut11hsTPST1, and pRSThsTPST1 were grown overnight, pelleted, and resuspended in infiltration buffer (25 mM MES, pH 5.5, 25 mM MgSO4, 0.1 mM acetosyringone). Mixtures of bacteria containing pPG9HC and pPG9LC (or pPG9LC-RSH) were infiltrated with or without different TPST constructs into ΔXT/FT leaves. For in planta galactosylation and sialylation, an additional six genes had to be infiltrated (25). OD600 for infiltration was 0.01 for each of the IgG vectors, up to 0.8 for the TPST constructs, and 0.05 for the vectors required for galactosylation and sialylation. Plants were harvested 3–6 d postinfiltration.
mAb Purification.
mAb extraction and purification were carried out as described (23). Briefly, infiltrated leaf material was homogenized under liquid nitrogen, and proteins were extracted in 45 mM Tris⋅HCl (pH 7.4) containing 1.5 M NaCl, 40 mM ascorbic acid, and 1 mM EDTA. The supernatant was clarified by a series of centrifugation and filtration steps. mAbs were then purified by protein A affinity chromatography.
SDS/PAGE and Western Blotting.
mAb samples were separated under reducing or nonreducing conditions [12% and 8% (wt/vol) polyacrylamide gels, respectively] followed by Coomassie Brilliant Blue staining or immunoblotting. mAb heavy and light chains were detected with anti-human γ-chain–peroxidase conjugate (A8775; Sigma-Aldrich) or anti-human λ-light chain–peroxidase conjugate (A5175; Sigma-Aldrich) and visualized with a chemiluminescence detection kit (Bio-Rad).
Glycosylation Analysis of mAbs and gp120.
The N-glycosylation profiles of mAbs (Asn297) and gp120ZM109 (Asn160 and Asn173) were determined by liquid chromatography-electrospray ionization as described recently (48, 53). Briefly, samples were separated by reducing SDS/PAGE, stained with Coomassie Brilliant Blue, and then, excised from the gel. On S-alkylation with iodoacetamide and tryptic or tryptic/chymotryptic digestion (gp120ZM109 Asn173), fragments were eluted with 50% acetonitrile and separated on a reversed-phase column (150 × 0.32 mm BioBasic-18; Thermo Scientific) with a gradient of 1–80% (vol/vol) acetonitrile. Glycopeptides were analyzed with a Q-TOF Ultima Global Mass Spectrometer (Waters). Annotation of glycoforms was done according to the ProGlycAn nomenclature (www.proglycan.com).
Sulfation Analysis of PG9 and RSH.
Tryptic peptides were prepared as above, digested with AspN where appropriate, and then, separated on a Dionex Ultimate 3000 HPLC System using a Thermo BioBasic C18 Separation Column (5-µm particle size; 150 × 0.32 mm) with a gradient from 95% (vol/vol) solvent A (65 mM ammonium formate) and 5% solvent B (acetonitrile) to 75% (vol/vol) B in 50 min at a flow rate of 6 µL/min. Peptides were analyzed on a maXis 4G ETD QTOF Mass Spectrometer (Bruker Daltonik) equipped with the standard electrospray ionization source in the positive ion mode (switching to MS/MS mode for eluting peaks). MS/MS scans of dominant precursor peaks were acquired and manually analyzed with DataAnalysis software, version 4.0 (Bruker Daltonik).
ELISA.
mAbs were quantified by a sandwich ELISA using anti-human γ-chain (I3391; Sigma-Aldrich) as coating antibody and anti-human λ-light chain-peroxidase conjugate (A5175; Sigma-Aldrich) as detection antibody; 3,3′,5,5′-tetramethylbenzidine (TMB) solution (T0440; Sigma-Aldrich) was used as peroxidase substrate. mAb binding to gp120 was also tested by ELISA; 96-well plates were coated with 1 µg/mL gp120ZM109, and mAb sample concentrations ranged from 10 ng/mL to 4 µg/mL. Anti-human IgG (Fcγ-specific)-peroxidase conjugate (62–8420; Invitrogen) and TMB substrate solution (T0440; Sigma-Aldrich) were used as detection reagents. For binding to gp140BG505.SOSIP.664 (26), 96-well plates were coated with 2 µg/mL (100 ng per well) sheep anti-gp120 antibody (D7324; Aalto) and incubated overnight at 4 °C. After washing and blocking with 4% nonfat dry milk in phosphate-buffered saline containing 0.05% (wt/vol) Tween 20 for 1 h at 37 °C, plates were further incubated with 50 ng per well gp140BG505.SOSIP.664 for 2 h at 37 °C. Plates were then washed and subsequently incubated with serially diluted (1:4) antibody samples (starting at 10 µg/mL). After 1 h at 37 °C, the plates were washed, and bound antibody was detected with anti-human IgG + IgA + IgM-peroxidase conjugate (109035064; Jackson ImmunoResearch). Finally, plates were washed and developed with TMB as substrate as above. EC50 values were calculated using GraphPad Prism 5.0 software for Windows (GraphPad Software).
Biolayer Interferometry.
mAbs were bound at 20 µg/mL to Dip and Read Protein A Biosensor Sticks (fortéBio). Antigen-binding kinetics were then determined with 6.25–50 µg/mL gp120ZM109. Controls were run without mAbs or gp120ZM109. All measurements were conducted at 30 °C. Results were analyzed with Octet Data Analysis Software 6.4 with single-reference well subtractions. The kinetic constants were computed for each curve separately assuming that dissociation does not reach the preassociation baseline. All estimates with R2 above 0.85 were considered for calculation of Kd.
Virus Neutralization Assays.
Pseudotyped virions were generated as described previously (54). In brief, 5 × 105 HEK293T cells (ATCC) were cotransfected with 4 µg HIV-1 gp160-deleted backbone plasmid pSG3ΔEnv (NIH AIDS Reagent Program) and 2 µg respective complementation plasmid using polyethyleneimine (18 µg) as a transfection reagent. Cell culture supernatants were harvested 48 h after transfection, cleared by centrifugation at 4,000 × g for 10 min, and then, used for single-round infectivity assays as described elsewhere (55). Briefly, pseudotyped virus was added at a 1:1 volume ratio to serially diluted (1:3) mAbs (starting at 40 µg/mL) and incubated at 37 °C. After 1 h, TZM-bl reporter cells (NIH AIDS Reagent Program) were added (1:1 by volume) at 1 × 104 cells per well, supplemented with 10 µg/mL DEAE-dextran, and then, incubated for another 48 h at 37 °C. The cells were then lysed and developed with luciferase assay reagent according to the manufacturer’s instructions (Promega). Relative light units were measured using a microplate luminometer (Synergy 2 Luminescence Microplate Reader; Biotek). All experiments were performed at least in duplicate. The extent of virus neutralization in the presence of antibody was determined as IC50 or IC90 compared with samples treated without mAb.
ADCC Assays.
Peripheral mononuclear cells were isolated from human whole blood obtained from healthy donors using Ficoll-Paque Premium (GE Healthcare). Target cells were prepared by spin-inoculating CEM.NKr-CCR5 cells (NIH AIDS Reagent Program) with HIV-1 strain JRCSF (400 ng p24 per 106 cells) for 2 h at 2,000 × g and 10 °C. Opsonization of the cells (1 × 104 per well) was then performed by incubation with serially diluted mAbs (starting at 50 µg/mL) for 1 h at 4 °C. Isolated peripheral mononuclear cells were then added at an effector to target ratio of 50:1 and incubated for 4 h at 37 °C. Cell death was determined with a CytoTox-Glo Cytotoxicity Assay Kit according to the manufacturer’s instructions (Promega). Data were corrected for spontaneous effector and target cell lysis and then expressed as the percentage of detergent-lysed controls.
Acknowledgments
We thank Michaela Bogner (Department of Applied Genetics and Cell Biology, University of Natural Resources and Life Sciences) for technical support, Dietmar Katinger (Polymun Scientific) for PG9 produced in CHO cells, and John P. Moore and Albert Cupo (Weill-Cornell Medical College) for BG505 SOSIP.664 gp140. MagnIcon vectors were provided by Viktor Klimyuk (Icon Genetics). This work was supported by Austrian Research Promotion Agency Laura Bassi Centres of Expertise Grant 822757 and Austrian Science Fund Grants L575-B13 and W1224-B09.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509090112/-/DCSupplemental.
References
- 1.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker LM, et al. Protocol G Principal Investigators Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker LM, et al. Protocol G Principal Investigators Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pejchal R, et al. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc Natl Acad Sci USA. 2010;107(25):11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pancera M, et al. Crystal structure of PG16 and chimeric dissection with somatically related PG9: Structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J Virol. 2010;84(16):8098–8110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLellan JS, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pancera M, et al. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat Struct Mol Biol. 2013;20(7):804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin MN, et al. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat Chem Biol. 2013;9(8):521–526. doi: 10.1038/nchembio.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julien JP, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci USA. 2013;110(11):4351–4356. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys. 2012;526(2):159–166. doi: 10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 12.Lewis GK. Qualitative and quantitative variables that affect the potency of Fc- mediated effector function in vitro and in vivo: Considerations for passive immunization using non-neutralizing antibodies. Curr HIV Res. 2013;11(5):354–364. doi: 10.2174/1570162x113116660060. [DOI] [PubMed] [Google Scholar]
- 13.Holl V, Peressin M, Moog C. Antibody-mediated Fcγ receptor-based mechanisms of HIV inhibition: Recent findings and new vaccination strategies. Viruses. 2009;1(3):1265–1294. doi: 10.3390/v1031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baum LL, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157(5):2168–2173. [PubMed] [Google Scholar]
- 15.Strasser R, Altmann F, Steinkellner H. Controlled glycosylation of plant-produced recombinant proteins. Curr Opin Biotechnol. 2014;30:95–100. doi: 10.1016/j.copbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Qiu X, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forthal DN, et al. Fc-glycosylation influences Fcγ receptor binding and cell-mediated anti-HIV activity of monoclonal antibody 2G12. J Immunol. 2010;185(11):6876–6882. doi: 10.4049/jimmunol.1002600. [DOI] [PubMed] [Google Scholar]
- 18.Moore KL. Protein tyrosine sulfation: A critical posttranslation modification in plants and animals. Proc Natl Acad Sci USA. 2009;106(35):14741–14742. doi: 10.1073/pnas.0908376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone MJ, Chuang S, Hou X, Shoham M, Zhu JZ. Tyrosine sulfation: An increasingly recognised post-translational modification of secreted proteins. N Biotechnol. 2009;25(5):299–317. doi: 10.1016/j.nbt.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Komori R, Amano Y, Ogawa-Ohnishi M, Matsubayashi Y. Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc Natl Acad Sci USA. 2009;106(35):15067–15072. doi: 10.1073/pnas.0902801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg Y, et al. Pharmacokinetics and immunogenicity of broadly neutralizing HIV monoclonal antibodies in macaques. PLoS One. 2015;10(3):e0120451. doi: 10.1371/journal.pone.0120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strasser R, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J. 2008;6(4):392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 23.Niemer M, et al. The human anti-HIV antibodies 2F5, 2G12, and PG9 differ in their susceptibility to proteolytic degradation: Down-regulation of endogenous serine and cysteine proteinase activities could improve antibody production in plant-based expression platforms. Biotechnol J. 2014;9(4):493–500. doi: 10.1002/biot.201300207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bombarely A, et al. A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Mol Plant Microbe Interact. 2012;25(12):1523–1530. doi: 10.1094/MPMI-06-12-0148-TA. [DOI] [PubMed] [Google Scholar]
- 25.Castilho A, et al. In planta protein sialylation through overexpression of the respective mammalian pathway. J Biol Chem. 2010;285(21):15923–15930. doi: 10.1074/jbc.M109.088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders RW, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9(9):e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollara J, et al. Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr HIV Res. 2013;11(5):378–387. doi: 10.2174/1570162X113116660059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cimbro R, et al. Tyrosine sulfation in the second variable loop (V2) of HIV-1 gp120 stabilizes V2-V3 interaction and modulates neutralization sensitivity. Proc Natl Acad Sci USA. 2014;111(8):3152–3157. doi: 10.1073/pnas.1314718111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loos A, Steinkellner H. Plant glyco-biotechnology on the way to synthetic biology. Front Plant Sci. 2014;5:523. doi: 10.3389/fpls.2014.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CC, et al. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci USA. 2004;101(9):2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niehrs C, Huttner WB, Carvallo D, Degryse E. Conversion of recombinant hirudin to the natural form by in vitro tyrosine sulfation. Differential substrate specificities of leech and bovine tyrosylprotein sulfotransferases. J Biol Chem. 1990;265(16):9314–9318. [PubMed] [Google Scholar]
- 32.Leyte A, et al. Sulfation of Tyr1680 of human blood coagulation factor VIII is essential for the interaction of factor VIII with von Willebrand factor. J Biol Chem. 1991;266(2):740–746. [PubMed] [Google Scholar]
- 33.Zeitlin L, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci USA. 2011;108(51):20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reichert JM, Dhimolea E. The future of antibodies as cancer drugs. Drug Discov Today. 2012;17(17-18):954–963. doi: 10.1016/j.drudis.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Moldt B, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86(11):6189–6196. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 37.Bournazos S, et al. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimran A, et al. Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood. 2011;118(22):5767–5773. doi: 10.1182/blood-2011-07-366955. [DOI] [PubMed] [Google Scholar]
- 40.Zimran A, et al. Safety and efficacy of two dose levels of taliglucerase alfa in pediatric patients with Gaucher disease. Blood Cells Mol Dis. 2015;54(1):9–16. doi: 10.1016/j.bcmd.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Shaaltiel Y, et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J. 2007;5(5):579–590. doi: 10.1111/j.1467-7652.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 42.Tekoah Y, et al. Glycosylation and functionality of recombinant β-glucocerebrosidase from various production systems. Biosci Rep. 2013;33(5):771–781. doi: 10.1042/BSR20130081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nekrasov V, Staskawicz B, Weigel D, Jones JD, Kamoun S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31(8):691–693. doi: 10.1038/nbt.2655. [DOI] [PubMed] [Google Scholar]
- 44.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 45.Anthony RM, et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320(5874):373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105(50):19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu J, et al. Structure of FcγRI in complex with Fc reveals the importance of glycan recognition for high-affinity IgG binding. Proc Natl Acad Sci USA. 2015;112(3):833–838. doi: 10.1073/pnas.1418812112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics. 2008;8(14):2858–2871. doi: 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- 49.Ouyang Yb, Lane WS, Moore KL. Tyrosylprotein sulfotransferase: Purification and molecular cloning of an enzyme that catalyzes tyrosine O-sulfation, a common posttranslational modification of eukaryotic proteins. Proc Natl Acad Sci USA. 1998;95(6):2896–2901. doi: 10.1073/pnas.95.6.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strasser R, et al. Molecular basis of N-acetylglucosaminyltransferase I deficiency in Arabidopsis thaliana plants lacking complex N-glycans. Biochem J. 2005;387(Pt 2):385–391. doi: 10.1042/BJ20041686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schähs P, et al. Cellular repressor of E1A-stimulated genes is a bona fide lysosomal protein which undergoes proteolytic maturation during its biosynthesis. Exp Cell Res. 2008;314(16):3036–3047. doi: 10.1016/j.yexcr.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Strasser R, et al. A unique beta1,3-galactosyltransferase is indispensable for the biosynthesis of N-glycans containing Lewis a structures in Arabidopsis thaliana. Plant Cell. 2007;19(7):2278–2292. doi: 10.1105/tpc.107.052985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pabst M, Chang M, Stadlmann J, Altmann F. Glycan profiles of the 27 N-glycosylation sites of the HIV envelope protein CN54gp140. Biol Chem. 2012;393(8):719–730. doi: 10.1515/hsz-2012-0148. [DOI] [PubMed] [Google Scholar]
- 54.Gach JS, et al. A human antibody to the CD4 binding site of gp120 capable of highly potent but sporadic cross clade neutralization of primary HIV-1. PLoS One. 2013;8(8):e72054. doi: 10.1371/journal.pone.0072054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gach JS, et al. HIV-1 specific antibody titers and neutralization among chronically infected patients on long-term suppressive antiretroviral therapy (ART): A cross-sectional study. PLoS One. 2014;9(1):e85371. doi: 10.1371/journal.pone.0085371. [DOI] [PMC free article] [PubMed] [Google Scholar]