Significance
In activated platelets, a scramblase(s) scrambles phospholipids at plasma membranes and exposes phosphatidylserine (PtdSer) to the surface. PtdSer on activated platelets serves as a scaffold for the tenase and prothrombinase complexes for blood coagulation. Among five transmembrane protein 16 (TMEM16) members that support Ca2+-dependent phospholipid scrambling, TMEM16F is highly expressed in mouse platelets, and is indispensable for activation-induced PtdSer exposure and microparticle shedding. Thus, the rate of platelet-induced thrombin generation is severely reduced by the TMEM16F deficiency. The role of TMEM16F in PtdSer exposure on aggregated platelets is also shown in vivo with laser-induced thrombus formation. The phenotypes of TMEM16F-null mice resemble those of patients with Scott syndrome, a mild bleeding disorder, indicating that these mice provide a good model for this disease.
Keywords: platelets, phosphatidylserine, microvesicles, scramblase, calcium
Abstract
Phosphatidylserine (PtdSer) exposure on the surface of activated platelets requires the action of a phospholipid scramblase(s), and serves as a scaffold for the assembly of the tenase and prothrombinase complexes involved in blood coagulation. Here, we found that the activation of mouse platelets with thrombin/collagen or Ca2+ ionophore at 20 °C induces PtdSer exposure without compromising plasma membrane integrity. Among five transmembrane protein 16 (TMEM16) members that support Ca2+-dependent phospholipid scrambling, TMEM16F was the only one that showed high expression in mouse platelets. Platelets from platelet-specific TMEM16F-deficient mice exhibited defects in activation-induced PtdSer exposure and microparticle shedding, although α-granule and dense granule release remained intact. The rate of tissue factor-induced thrombin generation by TMEM16F-deficient platelets was severely reduced, whereas thrombin-induced clot retraction was unaffected. The imaging of laser-induced thrombus formation in whole animals showed that PtdSer exposure on aggregated platelets was TMEM16F-dependent in vivo. The phenotypes of the platelet-specific TMEM16F-null mice resemble those of patients with Scott syndrome, a mild bleeding disorder, indicating that these mice may provide a useful model for human Scott syndrome.
Phospholipids are asymmetrically distributed between the inner and outer leaflets of plasma membranes as a result of the activity of flippase(s), which specifically translocates phosphatidylserine (PtdSer) and phosphatidylethanolamine from the outer to the inner leaflet of plasma membranes (1). PtdSer is preferentially exposed on the cell surface during certain physiological processes. During apoptosis, cell-surface PtdSer functions as an “eat me” signal to induce engulfment by phagocytic cells, and, during platelet activation, it serves as a scaffold for the activation of clotting factors. Exposed PtdSer is also implicated in pathological processes and may promote the retention of Ca2+ oxalate in kidneys, leading to kidney stone formation (2).
PtdSer exposure is accomplished by the inactivation of flippase(s), along with the activation of scramblases (3). We recently identified two protein families (TMEM16 and Xkr) that support phospholipid scrambling (4–6). The TMEM16 family consists of 10 members with 10 transmembrane regions, and TMEM16C, 16D, 16F, 16G, and 16J support the Ca2+-dependent scrambling of phospholipids. Scott syndrome is a mild bleeding disorder caused by a defect in platelet procoagulant activity (7, 8). Platelets, red blood cells, and EBV-transformed B cells from patients with Scott syndrome exhibit defective PtdSer exposure following platelet activation or treatment with Ca2+ ionophore (9–11). We and others reported that patients with Scott syndrome carry null mutations in the TMEM16F gene (6, 12).
The fetal thymocyte cell lines established from TMEM16F−/− mouse embryos exhibit defective PtdSer exposure upon treatment with Ca2+ ionophore (5), reminiscent of the EBV-transformed B-cell lines from patients with Scott syndrome. In contrast, Yang et al. (13) reported that TMEM16F-deficient mouse platelets exhibit only a mild defect in Ca2+ionophore–induced PtdSer exposure and tissue factor-induced thrombin generation. Furthermore, in contrast to a human patient with Scott syndrome (14) and dogs with a similar hereditary syndrome (15), neither of which exhibits apparent bleeding-time defects, TMEM16F−/− mice exhibit a prolonged bleeding time—twice that of WT mice (13).
To address the apparent discrepancies between TMEM16F−/− mouse phenotypes and the clinical presentation of patients with Scott syndrome, and to examine the role of platelet-expressed TMEM16F in blood clotting, we generated a platelet-specific TMEM16F deletion in mice. Our in vitro and in vivo analyses of thrombus formation induced by TMEM16F-null platelets suggested a role for TMEM16F in activation-induced PtdSer exposure, and supported the model in which Ca2+-induced PtdSer exposure is involved in the generation of thrombin and fibrin, but not clot retraction (16, 17). Mice with the platelet-specific deletion of TMEM16F exhibited a phenotype similar to that of human patients with Scott syndrome, and may provide a useful model for this human disease.
Results
PtdSer Exposure on Activated Mouse Platelets.
The activation of platelets with thrombin, collagen, or Ca2+ ionophore induces PtdSer exposure (18). However, platelet activation often causes rupture (19), which complicates the determination of whether the PtdSer exposure is caused by phospholipid scrambling at the plasma membrane or the rupture of plasma membranes. Apoptotic stimuli cause apoptotic cell death and PtdSer exposure, but can also cause necrosis, particularly when the stimuli are strong and cells are exposed to them for long periods (20). To distinguish apoptosis-induced PtdSer exposure from that caused by necrosis, cells are usually stained with membrane-impermeable DNA-staining dyes together with PtdSer-staining reagents (21). However, such dyes cannot be used for staining platelets because they lack nuclei. Because phalloidin, which is also a membrane-impermeable reagent, binds to F-actin in the cytoplasm (22), we reasoned that phalloidin staining could be useful in identifying and excluding necrotic cells from the analysis of PtdSer-exposing platelets.
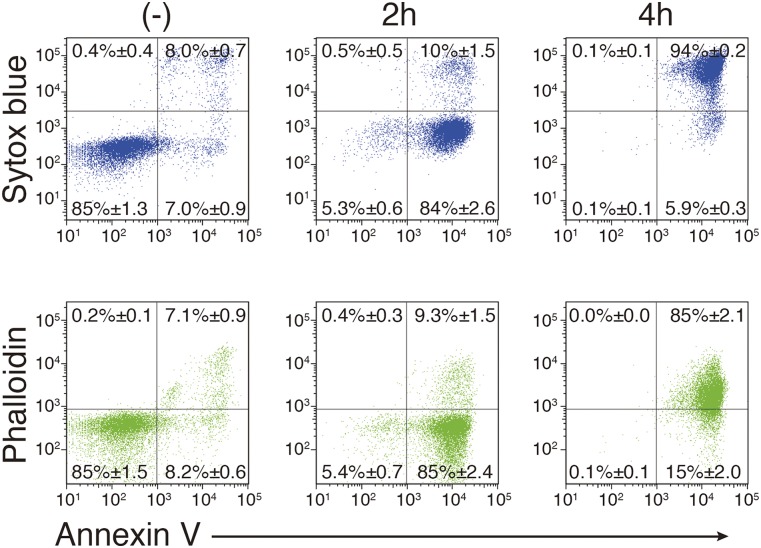
W3 cells, which are mouse T-cell leukemia WR19L cells expressing Fas (23), were treated with FasL (24) and stained with SYTOX Blue or phalloidin, together with Annexin V. Resting W3 cells were negative for Annexin V and SYTOX Blue staining (Fig. S1). At 2 h after the addition of FasL, 85% of the cells were Annexin V-positive but SYTOX Blue-negative, indicating that most of the cells underwent apoptosis. As the incubation continued, SYTOX Blue-positive cells were detected, and, at 4 h after the FasL addition, more than 90% of the cells were positive for Annexin V and SYTOX Blue, indicating that they were undergoing necrosis. Staining with Annexin V and phalloidin resulted in similar cell profiles; W3 cells treated with FasL for 2 h were Annexin V-positive and phalloidin-negative, whereas the 4-h treated cells were positive for Annexin V and phalloidin, indicating that phalloidin staining can be used to exclude plasma membrane-compromised cells.
Fig. S1.
Apoptotic PtdSer exposure. W3 cells were left untreated or treated for 2 or 4 h with 30 U/mL human FasL. The cells were stained by Cy5-labeled Annexin V, SYTOX Blue, and Alexa Fluor 488-conjugated phalloidin and analyzed by flow cytometry.
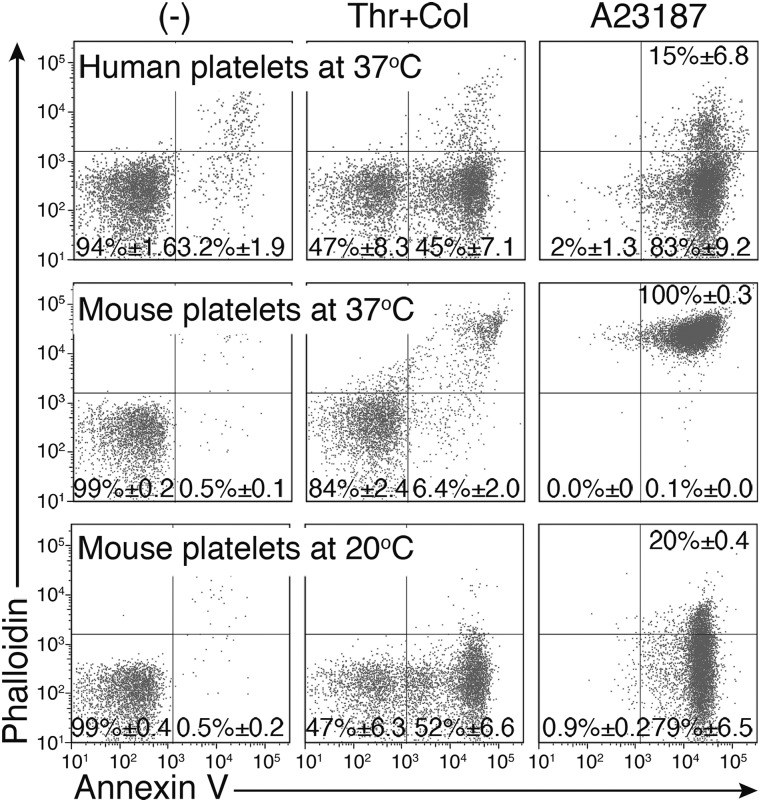
Human and mouse platelets were stimulated for 5 min at 37 °C with 0.5 U/mL thrombin and 10 μg/mL collagen, or with 1.0 μM A23187 in the presence of 1.0 mM CaCl2, and stained with Annexin V and phalloidin. As shown in Fig. 1, the stimulated human platelets exposed PtdSer and were Annexin V-positive, but they remained phalloidin-negative. In contrast, the stimulated mouse platelets were Annexin V- and phalloidin-positive, indicating that they had undergone necrosis. However, when the mouse platelets were treated with thrombin and collagen, or A23187 at 20 °C instead of 37 °C, Annexin V- but not phalloidin-positive cells were detected at 5 min after treatment. When the 20 °C-activated cells were permeabilized with 0.3% saponin, phalloidin-positive cells were detected, consistent with the premise that membrane rupture of the activated platelets was prevented by lowering the incubation temperature. These results suggested that the activated mouse platelets exposing PtdSer were fragile and susceptible to membrane rupture at 37 °C. Therefore, the in vitro effect of TMEM16F on PtdSer exposure in mouse platelets was examined at 20 °C throughout the rest of the study.
Fig. 1.
PtdSer exposure on activated mouse platelets. Human and murine platelets were treated for 5 min at 20 °C or 37 °C with 0.5 U/mL thrombin plus 10 μg/mL collagen or 1 μM A23187 in Tyrode-H buffer containing 1 mM CaCl2. The cells were stained with Cy5-Annexin V and Alexa Fluor 488-phalloidin and analyzed by flow cytometry. The experiments were performed three times, and the average values are shown with SD.
PtdSer Exposure in Activated Mouse Platelets Requires TMEM16F.
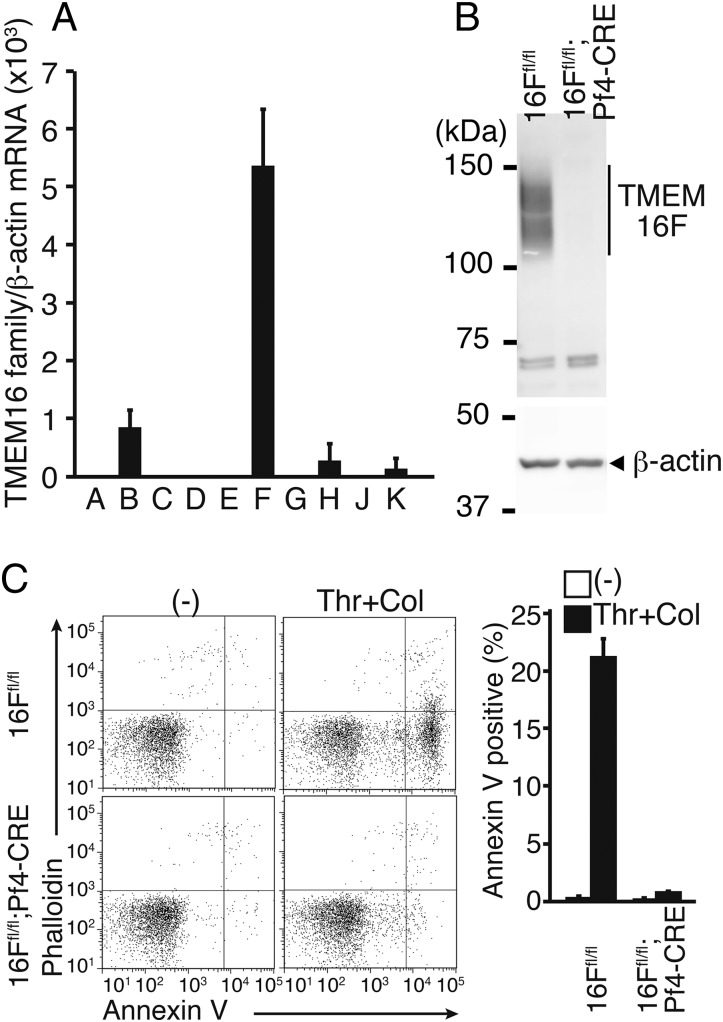
Among 10 TMEM16 members, TMEM16C, 16D, 16F, 16G, and 16J support phospholipid scramblase activity (5). Human platelets expressed a high level of TMEM16F, but not other members (Fig. S2). Accordingly, real-time RT-PCR analysis showed that mouse platelets expressed a high level of TMEM16F and low levels of TMEM16B, 16H, and 16K mRNAs (Fig. 2A), suggesting that TMEM16F could play a role in phospholipid scrambling in mouse platelets. Mice carrying TMEM16F-floxed alleles (TMEM16Ffl/fl) (5) were crossed with Platelet factor 4 (Pf4)-CRE mice, which express CRE recombinase under the control of the megakaryocyte/platelet-specific Pf4 promoter (25). A Western blot analysis with anti-TMEM16F antibody showed the presence of a broad band of ∼120 kDa in platelets from TMEM16Ffl/fl mice (Fig. 2B), which was absent in the platelets from TMEM16Ffl/fl;Pf4-CRE mice. Mouse TMEM16F contains three N-glycosylation sites (6), which could explain the heterogeneity of the TMEM16F protein on Western blots.
Fig. S2.
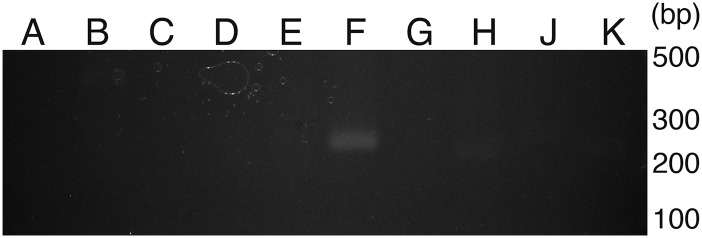
RT-PCR analysis for TMEM16 family in human platelets. RNA from human platelets was reverse-transcribed. Using an aliquot of cDNA corresponding to 5 × 105 platelets, PCR was carried out with a set of primers for the indicated TMEM16 family members. The PCR products were separated by electrophoresis through 2% (wt/vol) agarose gel and visualized by staining with ethidium bromide.
Fig. 2.
Requirement of TMEM16F for PtdSer exposure on activated mouse platelets. (A) TMEM16 family expression in mouse platelets. RNA from mouse platelets was subjected to real-time RT-PCR for the 10 TMEM16 family members. Each mRNA level is expressed relative to β-actin mRNA. The experiments were performed three times, and the average values are shown with SD (bars). (B) TMEM16F Western blotting of mouse platelets. Cell lysates (5 μg protein) from platelets of TMEM16Ffl/fl and TMEM16Ffl/fl;Pf4-CRE mice were analyzed by Western blotting with anti-TMEM16F. The TMEM16F band is indicated. (C) Platelets from TMEM16Ffl/fl and TMEM16Ffl/fl;Pf4-CRE mice were treated at 20 °C for 5 min with 0.5 U/mL thrombin plus 10 μg/mL collagen (Thr + Col) in Tyrode-H buffer with 1 mM CaCl2. The activated platelets were stained with phycoerythrin/cyanin 7 (PE/Cy7)–anti-CD41 mAb, Cy5-Annexin V, and Alexa Fluor 488-phalloidin (C) and analyzed by flow cytometry. FACS profiles for phalloidin and Annexin V staining in the CD41-positive population are shown. The experiments were performed three times, and the percentages of Annexin V-positive and phalloidin-negative populations are plotted (Right) with SD (bars).
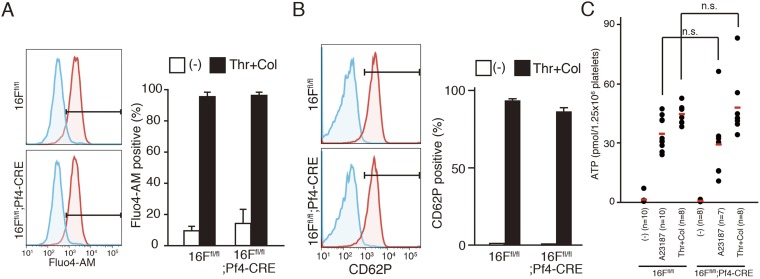
Platelets from TMEM16Ffl/fl and TMEM16Ffl/fl;Pf4-CRE mice were then stimulated for 5 min at 20 °C with thrombin and collagen in the presence of 1.0 mM CaCl2. As shown in Fig. 2C, PtdSer exposure was detected in ∼20% of the stimulated TMEM16F-expressing platelets, whereas the stimulated TMEM16F-deficient platelets showed minimal PtdSer exposure. In contrast, these stimuli increased the intracellular Ca2+ concentrations of the control and TMEM16F-deficient platelets (Fig. S3A), indicating that TMEM16F plays an essential role in PtdSer exposure in activated mouse platelets. Activated platelets also release various granules, such as α-granules containing P-selectin and dense granules containing ATP (26, 27). When platelets from TMEM16Ffl/fl and TMEM16Ffl/fl;Pf4-CRE mice were activated with thrombin and collagen, more than 90% of the platelets with both genotypes expressed P-selectin (Fig. S3B). In addition, both TMEM16F-expressing and -deficient platelets released similar amounts of ATP in response to thrombin/collagen or A23187 (Fig. S3C). These results indicated that TMEM16F was not involved in the release of α- and dense granules.
Fig. S3.
Activation of mouse platelets. (A and B) Platelets from TMEM16Ffl/fl and TMEM16Ffl/fl;Pf4-CRE mice were treated at 20 °C for 5 min with 0.5 U/mL thrombin plus 10 μg/mL collagen (Thr + Col) in Tyrode-H buffer containing 1 mM CaCl2. The activated platelets were stained with PE/Cy7–anti-CD41 mAb and Fluo4-AM (A) or FITC-anti-CD62P mAb (B) and analyzed by flow cytometry. FACS profiles for Fluo4 and CD62 in the CD41-positive population are shown. The experiments were performed three times, and the percentages of the Fluo4-, and CD62-positive populations are plotted (Right) with SD (bars). (C) Platelets from TMEM16Ffl/fl or TMEM16Ffl/fl;Pf4-CRE mice (n = 7–10 mice per group) were stimulated with 1 μM A23187 or 0.5 U/mL thrombin plus 10 μg/mL collagen in the presence of 1 mM CaCl2, and the ATP level in the supernatants was determined. The red bar indicates the average value. Two-tailed Student t tests were used for statistical testing between two groups. n.s., not significant.
Involvement of TMEM16F in the Release of Microparticles.
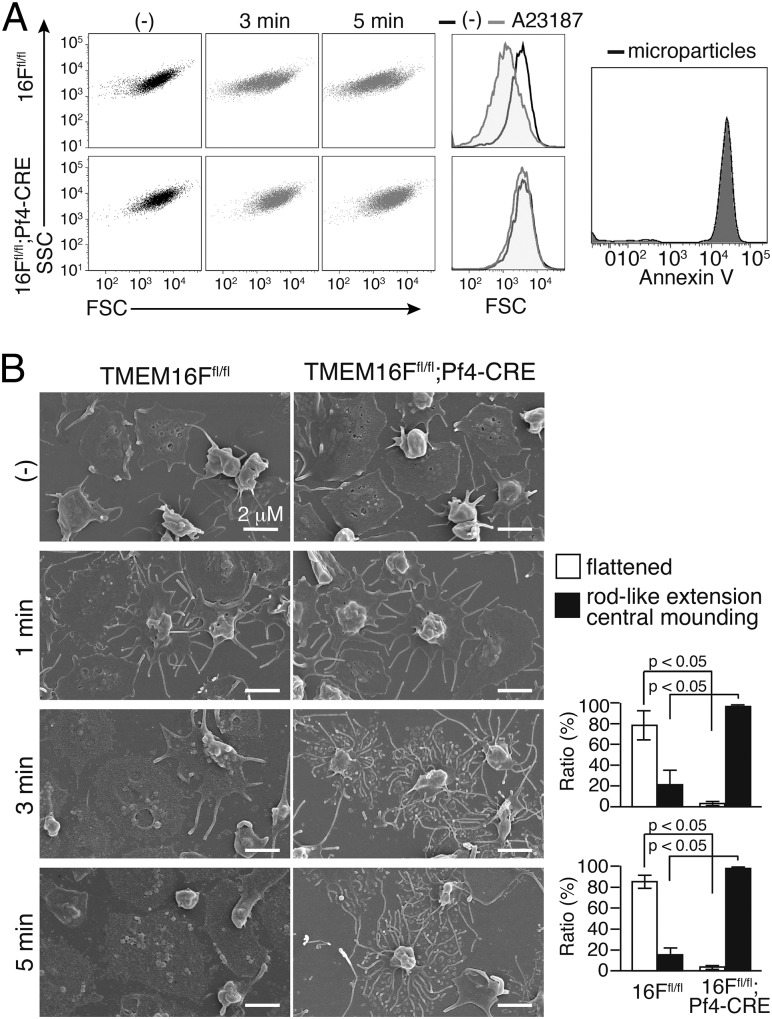
Activated platelets also release microparticles (28), and this process depends on calpain and/or phospholipid scramblase(s) (9, 10). To examine the contribution of TMEM16F to this process, platelets from TMEM16Ffl/fl or TMEM16Ffl/fl;Pf4-CRE mice were treated with thrombin and collagen in the presence of 1.0 mM CaCl2 and analyzed by flow cytometry. As shown in Fig. 3A, the activation of TMEM16F-expressing platelets for 5 min resulted in generation of a population of particles characterized by reduced forward scatter (FSC). In contrast, stimulated TMEM16F-deficient platelets showed little change in FSC, supporting the idea that TMEM16F-mediated phospholipid scrambling plays an important role in microparticle shedding from activated platelets.
Fig. 3.
TMEM16F-dependent microparticle release from activated platelets. (A) TMEM16F-dependent microparticle formation. TMEM16Ffl/fl and TMEM16Ffl/fl;Pf4-CRE platelets (4 × 105) were left untreated or treated with 1 μM A23187 in 0.2 mL of Tyrode-H buffer with 1 mM CaCl2 at 20 °C for 3 or 5 min and stained with CD41-PE/Cy7. The FSC/side scatter (SSC) profiles of CD41-positive populations are shown. (Center) Merged FSC profiles in unstimulated or 5-min stimulated platelets. (Right) Microparticles were collected by centrifugation from the stimulated WT platelets and stained with Cy5-Annexin V. (B) Scanning EM images of activated platelets. TMEM16Ffl/fl and TMEM16Ffl/fl;Pf4-CRE platelets (3 × 105) were left unstimulated or stimulated as in A with 1 μM A23187 for 1, 3, or 5 min in the presence of 1 mM CaCl2. The adherent platelets were observed by scanning EM. (Scale bar: 2 µm.) The 3-min or 5-min activated platelets (approximately 300 platelets in three fields for each) were classified into flattened or rod-like extension and central mounting categories, and their percentages are plotted (Right). The Student t test was used for statistical analysis, and P values are shown.
Platelets undergo dramatic changes in morphology upon binding to immobilized adhesive proteins (29). As shown in Fig. 3B, when purified TMEM16F-expressing or -deficient platelets were allowed to adhere to fibrinogen-coated plates and observed by scanning EM, within 2 h they exhibited a marked central mounding. There was no difference in the morphologies of resting TMEM16F-expressing and -deficient platelets. When the platelets on fibrinogen-coated dishes were stimulated with 1.0 μM A23187 in the presence of 1.0 mM CaCl2, both types of platelets generated many rod-like extensions arranged in a concentric pattern within 1 min (Fig. 3B). However, 3–5 min later, whereas most of the rod-like extensions and central moundings of the TMEM16F-expressing platelets had been replaced by flattened structures, the rod-like extensions continued to develop in the TMEM16F-deficient platelets, and the central moundings remained in most of them. These results indicated that the microparticle shedding and breakdown of the rod-like membrane extensions in platelets were similarly regulated by TMEM16F.
TMEM16F-Dependent Thrombin Generation.
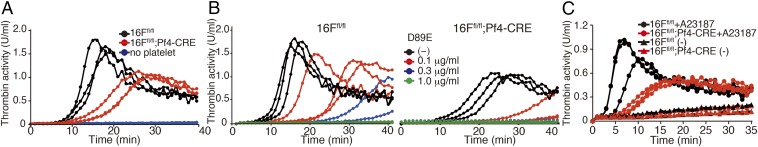
PtdSer exposed on the platelets serves as the membrane scaffold for tenase (Factors VIII and IX) and prothrombinase (Factors V and X), resulting in the generation of thrombin for blood clotting (17). To investigate the possible involvement of TMEM16F in thrombin generation, the tissue factor-induced thrombin generation was assayed at 20 °C with platelet-free plasma (PFP) and washed TMEM16F-expressing or -deficient platelets. As shown in Fig. 4A, the thrombin activity generated with TMEM16F-expressing platelets began to increase at 8 min and peaked ∼15–18 min after the addition of tissue factor. TMEM16F-deficient platelets also supported the thrombin generation to some extent. This is probably because of the necrosis that occurred in the activated platelets after the longer incubation. Notably, the initial rate of thrombin production mediated by the TMEM16F-deficient platelets was 5–10 fold lower than that associated with the TMEM16F-expressing platelets. Furthermore, addition of the D89E mutant of MFG-E8, which masks PtdSer (21), to the thrombin generation assay dose-dependently inhibited thrombin generation by the TMEM16F-expressing and -deficient platelets (Fig. 4B), confirming that PtdSer exposed on platelets played an essential role in activating tenase and prothrombinase. The prothrombin and activated partial thromboplastin times were comparable when PFP from the TMEM16F-expressing or -deficient mice was used (Table S1), confirming that the TMEM16F required for thrombin generation was intrinsic to platelets.
Fig. 4.
Contribution of TMEM16F to PtdSer-mediated thrombin formation by platelets and microparticles. (A and B) Thrombin generation assay with TMEM16F-expressing or -deficient platelets. Washed platelets (1.2 × 107, three independent preparations each) from TMEM16Ffl/fl or TMEM16Ffl/fl;Pf4-CRE mice were mixed with 6 μL of PFP in 120 μL Tyrode-H buffer containing 8 mM CaCl2 in the absence (A) or presence of 0.1, 0.3, or 1.0 μg/mL D89E (B) and incubated at 20 °C with 8.3 pM human tissue factor. Thrombin activity was assayed with Z-GGR-AMC. (C) Thrombin generation by microparticles. Platelets (1.2 × 107) from TMEM16Ffl/fl or TMEM16Ffl/fl;Pf4-CRE platelets (three independent preparations each) were stimulated at 20 °C for 5 min with 1 μM A23187 or left unstimulated in Tyrode-H buffer containing 1 mM CaCl2. The microparticles were collected and used for thrombin generation.
Table S1.
Hematologic analysis of megakaryocyte/platelet-specific TMEM16F−/− mice
| Measurement | TMEM16Ffl/fl (n = 8) | TMEM16Ffl/fl;Pf4-CRE (n = 9) |
| WBC, × 103/µL | 6.9 ± 3.3 | 6.1 ± 1.8 |
| RBC, × 106/µL | 8.9 ± 0.7 | 9.0 ± 0.4 |
| HGB, g/dL | 13.3 ± 0.8 | 13.2 ± 0.5 |
| HCT, % | 48.4 ± 3.4 | 47.8 ± 2.0 |
| MCV, fL | 53.8 ± 0.9 | 53.5 ± 0.9 |
| MCH, pg | 14.8 ± 0.3 | 14.7 ± 0.4 |
| MCHC, g/dL | 27.5 ± 0.4 | 27.5 ± 0.3 |
| PLT, × 105/µL | 9.5 ± 2.2 | 10.5 ± 1.3 |
| PT, s | 8.9 ± 0.5 | 8.9 ± 0.4 |
| aPTT, s | 34.0 ± 5.9 | 35.6 ± 8.2 |
aPTT, activated partial thrombin time; HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; PLT, platelet; PT, prothrombin time; RBC, red blood cell; WBC, white blood cell.
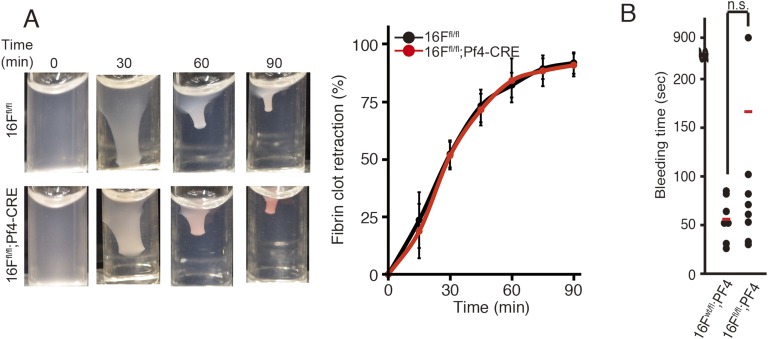
Microparticles released from activated platelets can also activate the tenase and prothrombinase complexes to promote coagulation (30). Microparticles collected from A23187-treated TMEM16F-expressing platelets constitutively exposed PtdSer on their surface (Fig. 3A) and generated thrombin in the presence of PFP with no or minimal delay (Fig. 4C). Consistent with the finding that few microparticles were released from the TMEM16F-deficient platelets (Fig. 3A), the ability of microparticles collected from A23187-treated TMEM16F-deficient platelets to support thrombin generation was low. Another role of platelets in thrombus formation involves their aggregation and subsequent contribution to fibrin clot retraction. When TMEM16F-expressing and -deficient platelets were stimulated with thrombin, the clot retraction occurred in less than 90 min, and there was no clear difference in their rates or levels (Fig. S4A). Accordingly, the tail bleeding times were similar in the TMEM16F-expressing and -deficient mice (Fig. S4B).
Fig. S4.
TMEM16F-indepdendent fibrin clot retraction and tail breeding. (A) Fibrin clot retraction. Platelets (4.5 ×107 /mL) from TMEM16Ffl/fl and TMEM16Ffl/fl;Pf4-CRE mice were washed and resuspended in 0.3 mL Tyrode-H buffer containing 15% (vol/vol) PFP and transferred to an aggregometer cuvette. Fibrin clotting was initiated by adding 1 U/mL thrombin and 8 mM CaCl2. (Left) Retracting clot at the indicated times. (Right) Clot surface area was assessed by digital processing at the indicated times, and clot retraction kinetics were determined as percentages of the maximum retraction (volume of platelet-free area; n ≥ 4). The average values are shown with SD (bars). (B) Tail bleeding times of TMEM16Ffl/+;Pf4-CRE and TMEM16Ffl/fl;Pf4-CRE mice (n ≥ 7) are shown with average values (bars). Two-tailed Student t tests were used for statistical testing between two groups. n.s., not significant.
TMEM16F-Dependent Thrombus Formation in Vivo.
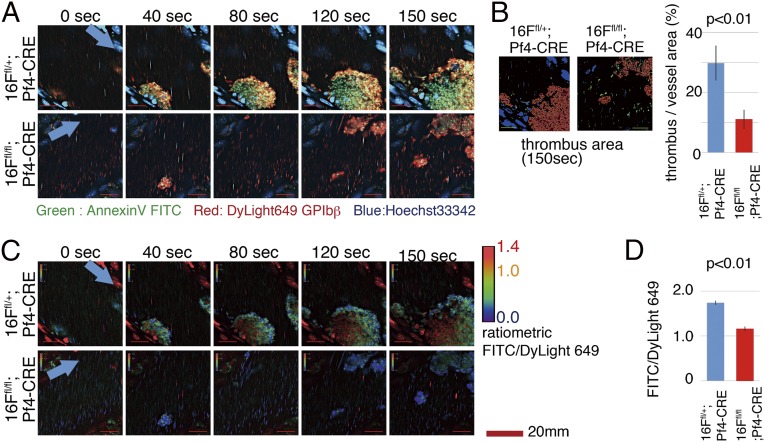
The numbers of platelets, erythrocytes, and white blood cells, as well as the hematological parameters, were comparable between TMEM16F-expressing and -deficient mice (Table S1). The contribution of TMEM16F to in vivo thrombus formation and PtdSer exposure in vivo was examined by using intravital visualization technique (31). Platelet aggregations in testicular veins were induced by reactive oxygen species (ROS) produced by photochemical reactions with laser irradiations, and the developed thrombus area within vessel lumens was quantified. In TMEM16F-deficient mice, near-normal platelet aggregation responses were observed in the early phase (40 s after laser irradiation; Fig. 5 A and B and Movies S1A and S1B and S2A and S2B), and thrombus development in TMEM16F-deficient mice was similar to that in control mice. However, developed thrombus became fragile in a later phase, and was frequently collapsed by blood flow. Reflecting this instability, the thrombus area of TMEM16F-deficient mice was smaller when examined at 150 s after laser irradiation. In addition, we directly evaluated PtdSer exposure by injecting FITC-labeled Annexin V. In the WT mice, FITC signal within the thrombus area was gradually increased, indicating PtdSer exposure after platelet aggregations in vivo. However, this FITC signal increase was attenuated in TMEM16F-deficient mice (Fig. 5 C and D and Movies S1A and S1B and S2A and S2B). These results indicated that TMEM16F was indispensable for PtdSer exposure on activated platelets in vivo.
Fig. 5.
Analysis of PtdSer exposure on platelets aggregated at a thrombus in a laser/ROS injury model. TMEM16Ffl/+;Pf4-CRE and TMEM16Ffl/fl;Pf4-CRE mice were injected with DyLight 649-anti-GP1bβmAb, FITC-Annexin V, Hoechst 33342, and hematoporphyrin. After anesthesia, the testicular vein was exposed and visualized by using a high-speed resonance scanning confocal microscope with highly sensitive GaAs detectors. Platelet aggregations were induced by photochemically induced ROS from hematoporphyrin within vessel lumens by laser irradiations. (A) Representative snap-shot raw images of developed thrombus. Note that the early responses of platelet aggressions were comparable in TMEM16F-deficient mice and control mice (40 s). However, the developed thrombus in TMEM16F-deficient mice was fragile, frequently collapsed by blood flow (80 s), and smaller in later phase (150 s) compared with the WT mice. Arrows indicate blood flow direction. (Scale bars: 20 μm.) (B) Quantification of thrombus area at 150 s using automatic software analysis (n = 20 vessels from n = 5 animals). (C and D) Ratiometric views and quantification analysis. Ratios are displayed in pseudocolor. Note that the FITC/DyLight 649 ratio increased within thrombus area in the WT mice, which was attenuated in TMEM16F-deficient mice. The FITC/DyLight 649 ratios were determined in 100 regions from 20 vessels from five animals, and the average values were plotted (D). We used ratiometric analysis to minimize the photobleaching effect by laser irradiation. The original videos are supplied as Movies S1A and S1B and S2A and S2B.
Discussion
Here, we report on the generation of mice with a platelet-specific deletion in TMEM16F and the effect of this deletion on the various functions of activated platelets. We demonstrated that TMEM16F was required for activation-induced PtdSer exposure, thrombin production, and microparticle release, but not for activation-induced α- and dense-granule release or thrombin-induced clot retraction. It did not affect tail vein bleeding time. These phenotypes are very similar to the phenotypes associated with human patients with Scott syndrome (7) and dogs with a similar hereditary syndrome (15), but significantly differ from the phenotypes previously associated with TMEM16F-null mice (13).
By using a convenient method to distinguish between ruptured platelets and intact activated platelets, we found that 37 °C-activated mouse platelets were fragile and their plasma membranes ruptured easily, in comparison with human platelets, which remained intact under those conditions. Notably, Yang et al. (13) activated mouse platelet at 37 °C for 30 min and did not see clear TMEM16F dependency on PtdSer exposure. PtdSer exposure in activated mouse platelets is also mediated by cyclophilin D-dependent Ca2+-induced openings of the mitochondrial permeability transition pore (MPTP) (17, 32). These changes in MPTP lead to cell death, and mouse platelets may undergo MPTP more easily than human platelets. As PtdSer exposed on the compromised platelet membranes could contribute to thrombin generation, this may also account for the different thrombin phenotypes of TMEM16-deficient platelets reported here and by Yang et al. (13).
Platelet activation leads to dramatic changes in platelet morphology and the release of α- and dense granules. These granules are present in the cytoplasm, and are released as multivesicular bodies with exosome-like properties (33). Exosomes are transported from the cytoplasm to the plasma membrane by the actions of both RabGTPase and SNARE, and are released after fusion with the plasma membrane (34). On the contrary, microparticles express platelet membrane receptors, expose PtdSer on their surfaces, and play an important role in hemostasis and thrombosis (35). Microparticles are generated from platelet plasma membranes by shedding (36) in response to intracellular Ca2+ (37). We found that, when microparticle release was assessed by using activation conditions in which PtdSer exposure was strictly dependent on TMEM16F (20 °C and low Ca2+ concentrations), it was severely reduced in the absence of TMEM16F, although the release of α- and dense granules remained intact. Sims et al. (28) previously reported that the platelets from a patient with Scott syndrome exhibit defective microvesicle release, whereas another report suggested the involvement of calpain in the release of microparticles (38). We found that, when mouse platelets were treated with A23187 in the presence of 1 mM Ca2+, microparticles were released from platelets in a TMEM16F-dependent manner. However, when the platelets were treated with A23187 in the presence of higher concentrations of Ca2+ (3 mM), the microparticles were released in a TMEM16F-independent, but calpain-dependent, manner. These results suggest that, under physiological conditions, TMEM16F plays an important role in microparticle shedding.
TMEM16F dependency has been similarly observed during bone tissue mineralization (39), during which PtdSer-rich matrix vesicles containing concentrated phosphate and Ca2+ ions bud off from the plasma membranes (40). Our EM images revealed that the rod-like extensions that formed on the surface of the activated platelets were released in a TMEM16F-dependent manner, consistent with the possibility that microparticle release from the activated platelets is associated with morphological changes. Given that the PtdSer-exposing plasma membranes of mouse platelets appear to be fragile, microparticle release may be a secondary event that follows PtdSer exposure.
As described earlier, the deletion of TMEM16F in mouse platelets had little effect on clot retraction or tail bleeding times. The apparently normal bleeding time observed in mice containing a platelet-specific TMEM16F deletion differs from the results of Yang et al. (13). This difference may result from the role of TMEM16F-mediated PtdSer scrambling in endothelial or vascular cells. However, this is unlikely because a patient with Scott syndrome who carries a defective TMEM16F gene in all tissues has a normal bleeding time (14). Munnix et al. (41) reported that there are two populations in activated platelets. One population of platelets that controls the generation of thrombin and fibrin exposes PtdSer, but does not express the activated αIIbβ3 integrin. On the contrary, the other population of platelets that controls clot retraction expresses the activated αIIbβ3 integrin, but does not expose PtdSer (41). Our results, showing that TMEM16F-null platelets were associated with reduced thrombin generation but normal clot retraction times, are consistent with this finding.
Our in vivo analysis showing reduced exposure of PtdSer on TMEM16F-null platelets also showed that damaged white blood cells present in the TMEM16Ffl/fl;Pf4-CRE mice exposed PtdSer. Thus, the PtdSer exposed on these cells may contribute to the generation of thrombin and fibrin in vivo, which could explain the mild coagulation defect in these mice and in patients with Scott syndrome. Taken together, the similarity in the phenotypes of the TMEM16Ffl/fl;Pf4-CRE mice and those of patients with Scott syndrome suggests that these mice may provide an ideal animal model for this disease.
Materials and Methods
Cell lines, reagents, genotyping of mouse mutants, EM, real-time PCR, flow cytometry, Western blotting, and ATP assay are described in SI Materials and Methods.
Mice.
C57BL/6J mice were from Japan SLC. TMEM16Ffl/fl mice were as described previously (5). Mice carrying TMEM16F-null platelets were generated by crossing TMEM16Fflox/+ mice with Pf4-CRE mice (25). The mice were housed in a specific pathogen-free facility at Kyoto University, and all animal experiments were performed in accordance with protocols approved by the animal care and use committees of Kyoto University and Osaka University.
Preparation of Washed Platelets and PFP.
Platelets were prepared as described previously (42). In brief, for human platelets, blood was drawn into a one-sixth volume of acid-citrate-dextrose (ACD) buffer (41.6 mM citric acid, 85.3 mM trisodium-citrate buffer, pH 5.0, and 136 mM d-glucose). For mouse platelets, blood was collected by cardiac puncture from 8–12-wk-old mice that had been euthanized by CO2 asphyxiation, and one-sixth volume of ACD buffer was added. Platelet-rich plasma (PRP) was obtained by centrifugation at 150 × g for 15 min. To obtain washed platelets, an equal volume of modified Tyrode buffer (Tyrode-H, 10 mM Hepes⋅NaOH buffer, pH 7.4, 12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, and 1 mM MgCl2), supplemented with 0.3 µM prostaglandin E1 (PGE1) and 15% (vol/vol) ACD, was added to PRP and centrifuged at 1,000 × g for 5 min. The precipitated platelets were washed with Tyrode-H buffer containing 0.15 µM PGE1 and 1 mM EDTA, suspended in Tyrode-H buffer, and used as washed platelets. To obtain PFP, the ACD-treated mouse blood was successively centrifuged at 22 °C twice at 1,000 × g for 5 min and once at 20,000 × g for 30 min. The supernatant was recovered as PFP.
Assay for Thrombin.
The thrombin generation was assayed according to Wielders et al. (8). In brief, 1.2 × 107 washed platelets in 54 μL of Tyrode-H buffer were mixed with 6 μL of mouse PFP in a 96-well plate and incubated at 20 °C for 15 min. Tyrode-H buffer (60 μL) supplemented with 0.2 mM Z-Gly-Gly-Arg-7-Amino-4-methylcoumarin (Z-GGR-AMC), 16.6 pmoles human tissue factor, and 16 mM CaCl2 was added to each well and incubated at 20 °C, and the resulting fluorescence was detected at an excitation wavelength of 390 nm and emission wavelength of 460 nm by using a microplate reader (Infinite M200; TECAN). For microparticles, 1.2 × 107 washed platelets in 200 μL of Tyrode-H buffer containing 0.05% BSA and 1 mM CaCl2 were treated at 20 °C for 5 min with 1 μM A23187. After stopping the reaction with 600 μL of Tyrode-H buffer containing 0.05% BSA and 2.7 mM EDTA, the platelets were removed by centrifuging twice at 1,000 × g at 4 °C for 5 min. Microparticles were collected from the supernatants by centrifugation at 20,000 × g for 30 min, and the thrombin activity was assayed as described earlier.
Intravital Microscopy and Thrombus Formation.
Imaging analysis of thrombus formation and PtdSer exposure was performed as described previously (31) with some modifications. In brief, a mixture of Hoechst 33342 (10 mg/kg; Invitrogen), DyLight 649-anti-GP1bβmAb (0.2 mg/kg), and FITC-Annexin V (20 μL per mouse; BioLegend) was administered into anesthetized mice. Hematoporphyrin (1.8 mg/kg) was administrated to produce ROS upon laser irradiation. The mice were secured to the heated piezo-drive heating stage (Nikon and Tokai Hit) of an inverted microscope (Eclipse Ti; Nikon). Veins on the testicular epidermis were monitored during laser excitation at 405, 488, and 640 nm (1.5 mW power at objective lens). XYT sequential images were obtained with a resonance confocal microscope (A1; Nikon) using a 100× oil-immersion objective lens (NA 1.15; Nikon). Data were analyzed and visualized using an automatic algorithm in NIS-Elements (Nikon) and custom-designed software (IMAGICA Imageworks). Snap-shot XY and ratiometric images are provided in the figures and videos. Also calculated by the software were the developed thrombus area and time-dependent changes of ratiometric signals. Quantifications were performed automatically by software without parameter adjustment by observers.
SI Materials and Methods
Cell Lines, Antibodies, and Reagents.
W3 cells (23), mouse T-cell lymphoma (WR19L) expressing mouse Fas, were cultured in RPMI1640 containing 10% (vol/vol) FCS. Leucine-zippered human FasLignad (FasL) (24) was produced in COS cells. The Flag-tagged D89E mutant of MFG-E8 was produced in HEK293T and purified with anti-Flag agarose (M2; Sigma-Aldrich) (21). Rabbit anti-mouse TMEM16F was as described previously (5). PE/Cy7–rat anti-mouse CD41 mAb (clone MWReg30) and mouse anti-human CD41 mAb (clone HIP8) were from BioLegend. FITC–anti-mouse CD62P (P-selectin) mAb (clone RB40.34) was from BD Bioscience. DyLight 488–anti-GP1bβ was from Emfret. Mouse anti–β-actin mAb (sc-47778) was from Santa Cruz Biotechnology. Alexa Fluor 488- or Alexa Fluor 647-phalloidin, Fluo4-acetoxymethyl (AM), Texas Red dextran (70,000 MW), and Hoechst 33342 were from Thermo Fisher Scientific. Collagen (Cellmatrix Type-I-C) was from Nitta Gelatin. A23187, CoA, bovine thrombin, PGE1, hematoporphyrin, Photinus pyralis firefly luciferase, and luciferin were from Sigma-Aldrich. FITC-lactadherin and human tissue factor were from Haematologic Technologies. Cy5-Annexin V was from BioVision.
Primers for Genotyping.
Primers used for mouse genotyping are as follows: (i) TMEM16F WT-specific sense primer, 5′-CTCCAGAGTTTGTAAGTAACACAT; (ii) TMEM16F mutant specific sense primer, 5′-CAGTCATCGATGAATTCATAACTT; (iii) TMEM16F common anti-sense primer, 5′-AAGACTGATTTCCAAGG TTATCGAA; (iv) Pf4-CRE sense primer, 5′- CCCATACAGCACACCTTTTG; and (v) Pf4-CRE anti-sense primer, 5′-TGCACAGTCAGCAGGTT (25).
Scanning EM.
Platelets were activated on fibrinogen-coated coverslips as described previously (19). In brief, the washed mouse platelets (1 × 108/mL) in Tyrode-H buffer containing 0.05% BSA were allowed to adhere for 2 h at 20 °C to glass coverslips (14 mm; Matsunami) that had been incubated at 37 °C for 2 h with 0.1 mg/mL bovine fibrinogen (Fraction 1; Tokyo Chemical Industry). The nonadherent platelets were removed, and the adherent platelets were incubated at 20 °C for 15 min in Tyrode-H buffer containing 0.05% BSA and 1 mM CaCl2 and stimulated with 1 μM A23187. One or three minutes later, the platelets were fixed overnight at 4 °C with 4% (vol/vol) glutaraldehyde in 60 mM Hepes⋅HCl buffer (pH 7.4) containing 200 mM NaCl. The coverslips were successively dehydrated by 10-min incubations in serially diluted ethanol [50% (vol/vol), 60% (vol/vol), 70% (vol/vol), 80% (vol/vol), 90% (vol/vol), and 99% (vol/vol) ethanol], followed by two 15-min incubations in 100% (vol/vol) ethanol. The coverslips were incubated in tert-butyl alcohol for 30 min twice and coated with a thin layer of platinum palladium. The specimens were examined with a scanning electron microscope (S-4700; Hitachi).
Real-Time RT-PCR.
RNA was prepared from the washed platelets using the RNeasy mini kit (Qiagen), and reverse-transcribed by using a High Capacity RNA-to-cDNA kit (Thermo Fisher Scientific). Aliquots of the products were amplified in a reaction mixture containing LightCycler 480 SYBR Green Master (Roche Diagnostics). The mouse primers used for real-time PCR were as follows: TMEM16A, 5′-ACCCCGACGCCGAATGCAAG and 5′-GCTGGTCCTGCCTGACGCTG; 16B, 5′-GAGGCGCACACCTGGGTCAC and 5′-ATGGGGCGTGGATCCGGACA; 16C, 5′-GCCAGCAATTGCCAACCCCG and 5′-GCAGTCCGACTCCTCCAGCTCT; 16D, 5′-ACAGGCATGCTCTTCCCCGC and 5′-GCGATCACTGCTCGGCGTCT; 16E, 5′-AGCAGCTCCAGCTTCGGCCT and 5′-TTCACGCTCTGCAGGGTGGC; 16F, 5′-CCCACCTTTGGATCACTGGA and 5′-TCGTATGCTTGTCTTTTCCT; 16G, 5′-ACATGTGCCCGCTGTGCTCC and 5′-GGGCCGAGGCCTCTCCTCAA; 16H, 5′-TGGAGGAGCCACGTCCCCAG and 5′-GCGGGGCAGACCCTTCACAC; 16J, 5′-GCTGTGGTGGTGACTGGGGC and 5′-CCAGGCGCGTGGATTTCCCA; 16K, 5′-TGGGGGCAGAAGCAGTCGGT and 5′-GGCCTGTGGGTAGCCAGGGAT; and β-actin, 5′-TGTGATGGTGGGAATGGGTCAG and 5′-TTTGATGTCACGCACGATTTCC. The mRNAs were quantified at the point where the LightCycler System detected the upstroke of the exponential phase of PCR product accumulation, with the respective linearized plasmid DNAs as references.
The RT-PCR for TMEM16 members in human platelets was carried out with the following primers: TMEM16A, 5′-ACCATGTGCCCGCTTTGCGA and 5′-CCCGTGAGGTCCCAGCGGTA; 16B, 5′-AGGTCTGCAGGGCGGTTCCA and 5′-CAGCGAGTGGCCAGGGAAGC; 16C, 5′-CTGGCCCCTTCAGCCGTGC and 5′-CAGCGTGCCCAGCGCTCATA; 16D, 5′-CACTGCCCCTTTCAGCCAGCA and 5′-CCCAGGAGGCCCAGCACTCA; 16E, 5′-GACGCAGAGTTAAAGGCGGAAAGA and 5′-AGGGCGGGGAATATCACTCTCCT; 16F, 5′-TCCGTCCCTCCCTACGGGGA and 5′-AGCCAGCTTGGCTGCAATCACA; 16G, 5′-GCCACTGTGCCAGGAGCAGG and 5′-ATCCGGGGCTTGGCAGGACT; 16H, 5′-TCCACGTGGCCATCCCCGAT and 5′-GCCTCCTCTCGGCCACCAGA; 16J, 5′-GCGTGGTCCTGCACTGGGAC and 5′-GGAGGCCAGGACGCGGTAGA; and 16K, 5′-AGTTTGAGGAGCCCCGGCCA and 5′-TGGTCCACTCAGACCCGCTGT.
Induction of Apoptosis.
Apoptosis was induced as described previously (3). In brief, W3 cells (1 × 105) were treated with 30 U/mL FasL at 37 °C, stained with 1,000-fold diluted Cy5-Annexin V, 250 nM SYTOX Blue, and 1.0 U/mL Alexa Fluor 488-conjugated phalloidin, and then analyzed by flow cytometry by using the FACSAria II system.
Flow Cytometry.
Washed platelets were suspended at a concentration of 2 × 106 cells per milliliter in Tyrode-H buffer containing 0.05% BSA, 1 μg/mL PE/Cy7-conjugated anti-CD41 mAb, 1,000-fold diluted Cy5-labeled Annexin V, and 1.0 U/mL Alexa Fluor 488-conjugated phalloidin. The platelets were stimulated for 5 min with 0.5 U/mL thrombin and 10 μg/mL collagen or 1 μM A23187 in the presence of 1 mM CaCl2, and analyzed with the use of a FACSAria II system. To examine the intracellular Ca2+ level or the exposure of CD62P, platelets were incubated at 20 °C for 5 min, stimulated with 0.5 U/mL thrombin and 10 μg/mL collagen, and analyzed by flow cytometry as described earlier.
Western Blotting.
The platelets were lysed in RIPA buffer (50 mM Hepes⋅NaOH buffer, pH 8.0, containing 1% Nonidet P-40, 1% SDS, 0.5% sodium deoxycholate, 150 mM NaCl, and a mixture of protease inhibitors [Complete Mini; Roche Diagnostics]). After removing debris by centrifugation at 20,000 × g for 30 min, the lysates were mixed with a one-fourth volume of 5× SDS sample buffer [200 mM Tris⋅HCl, pH 6.8, 10% (wt/vol) SDS, 25% (vol/vol) glycerol, 5% (vol/vol) β-mercaptoethanol, and 0.05% bromophenol blue], incubated at room temperature for 30 min, and subjected to 7.5% (wt/vol) SDS/PAGE (Bio Craft). The separated proteins were transferred to a PVDF membrane (Merck). The membrane was incubated with rabbit anti-mouse TMEM16F Ab in Immunoreaction Enhancer Solution (Can Get Signal; Toyobo Life Science), followed by incubation with HRP-labeled goat anti-rabbit Igs (Dako). The peroxidase activity was detected with an Immobilon Western Chemiluminescent HRP Substrate (Merck).
Assay for ATP Released from the Activated Platelets.
Mouse platelets (1.25 × 107 cells in 50 μL Tyrode-H buffer containing 1 mM CaCl2) were stimulated at 20 °C for 5 min with 1 μM A23187 or 0.5 U/mL thrombin plus 10 μg/mL collagen. After platelets and microparticles were removed by successive centrifugation twice at 1,000 × g for 5 min and once at 20,000 × g for 30 min at 4 °C, the ATP concentration in the supernatant was determined with the luciferase assay system essentially as described previously (43). In brief, the supernatant was treated with 10 mM Tris⋅HCl (pH 8.0)/1 mM EDTA-saturated phenol and extracted with ethyl ether. A 5-μL aliquot was incubated with 0.5 μg/mL firefly luciferase and 50 μg/mL luciferin in 0.2 mL of 16 mM Tricine, 316 mM EDTA, 341 mM Na3PO4, 10.3 mM MgSO4, 0.95 mM Triton X-100, 4.4 mM DTT, and 2.7 mM CoA. The resulting bioluminescence was measured with a microplate reader.
Supplementary Material
Acknowledgments
We thank K. Okamoto-Furuta and H. Kohda for the EM analysis, Radek C. Scoda for Pf4-CRE transgenic mice, H. Sakaguchi for software analysis, and M. Fujii for secretarial assistance. This work was supported in part by Grants-in-Aid from the Japan Society for the Promotion of Science (to S. Nagata).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516594112/-/DCSupplemental.
References
- 1.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 3.Segawa K, et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344(6188):1164–1168. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341(6144):403–406. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki J, et al. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem. 2013;288(19):13305–13316. doi: 10.1074/jbc.M113.457937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468(7325):834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 7.Weiss HJ, Vicic WJ, Lages BA, Rogers J. Isolated deficiency of platelet procoagulant activity. Am J Med. 1979;67(2):206–213. doi: 10.1016/0002-9343(79)90392-9. [DOI] [PubMed] [Google Scholar]
- 8.Wielders SJ, et al. Absence of platelet-dependent fibrin formation in a patient with Scott syndrome. Thromb Haemost. 2009;102(1):76–82. doi: 10.1160/TH08-11-0719. [DOI] [PubMed] [Google Scholar]
- 9.Toti F, Satta N, Fressinaud E, Meyer D, Freyssinet JM. Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood. 1996;87(4):1409–1415. [PubMed] [Google Scholar]
- 10.Bevers EM, et al. Defective Ca(2+)-induced microvesiculation and deficient expression of procoagulant activity in erythrocytes from a patient with a bleeding disorder: A study of the red blood cells of Scott syndrome. Blood. 1992;79(2):380–388. [PubMed] [Google Scholar]
- 11.Kojima H, et al. Production and characterization of transformed B-lymphocytes expressing the membrane defect of Scott syndrome. J Clin Invest. 1994;94(6):2237–2244. doi: 10.1172/JCI117586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castoldi E, Collins PW, Williamson PL, Bevers EM. Compound heterozygosity for 2 novel TMEM16F mutations in a patient with Scott syndrome. Blood. 2011;117(16):4399–4400. doi: 10.1182/blood-2011-01-332502. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, et al. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell. 2012;151(1):111–122. doi: 10.1016/j.cell.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss HJ, Lages B. Family studies in Scott syndrome. Blood. 1997;90(1):475–476. [PubMed] [Google Scholar]
- 15.Brooks MB, Catalfamo JL, Brown HA, Ivanova P, Lovaglio J. A hereditary bleeding disorder of dogs caused by a lack of platelet procoagulant activity. Blood. 2002;99(7):2434–2441. doi: 10.1182/blood.v99.7.2434. [DOI] [PubMed] [Google Scholar]
- 16.Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 17.de Witt SM, Verdoold R, Cosemans JMEM, Heemskerk JWM. Insights into platelet-based control of coagulation. Thromb Res. 2014;133(suppl 2):S139–S148. doi: 10.1016/S0049-3848(14)50024-2. [DOI] [PubMed] [Google Scholar]
- 18.Bevers EM, et al. The complex of phosphatidylinositol 4,5-bisphosphate and calcium ions is not responsible for Ca2+-induced loss of phospholipid asymmetry in the human erythrocyte: A study in Scott syndrome, a disorder of calcium-induced phospholipid scrambling. Blood. 1995;86(5):1983–1991. [PubMed] [Google Scholar]
- 19.Jobe SM, et al. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood. 2008;111(3):1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 21.Hanayama R, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 22.Wulf E, Deboben A, Bautz FA, Faulstich H, Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA. 1979;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi T, et al. Increased cytotoxicity of soluble Fas ligand by fusing isoleucine zipper motif. Biochem Biophys Res Commun. 2004;322(1):197–202. doi: 10.1016/j.bbrc.2004.07.098. [DOI] [PubMed] [Google Scholar]
- 25.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109(4):1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 26.Munnix ICA, Cosemans JMEM, Auger JM, Heemskerk JWM. Platelet response heterogeneity in thrombus formation. Thromb Haemost. 2009;102(6):1149–1156. doi: 10.1160/TH09-05-0289. [DOI] [PubMed] [Google Scholar]
- 27.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120(26):5209–5216. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: An isolated defect in platelet procoagulant activity. J Biol Chem. 1989;264(29):17049–17057. [PubMed] [Google Scholar]
- 29.McCarty OJT, et al. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280(47):39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinauridze EI, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(3):425–434. [PubMed] [Google Scholar]
- 31.Nishimura S, et al. In vivo imaging visualizes discoid platelet aggregations without endothelium disruption and implicates contribution of inflammatory cytokine and integrin signaling. Blood. 2012;119(8):e45–e56. doi: 10.1182/blood-2011-09-381400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattheij NJA, et al. Dual mechanism of integrin αIIbβ3 closure in procoagulant platelets. J Biol Chem. 2013;288(19):13325–13336. doi: 10.1074/jbc.M112.428359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 34.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Burnouf T, Goubran HA, Chou M-L, Devos D, Radosevic M. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014;28(4):155–166. doi: 10.1016/j.blre.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 37.Dachary-Prigent J, Pasquet JM, Freyssinet JM, Nurden AT. Calcium involvement in aminophospholipid exposure and microparticle formation during platelet activation: A study using Ca2+-ATPase inhibitors. Biochemistry. 1995;34(36):11625–11634. doi: 10.1021/bi00036a039. [DOI] [PubMed] [Google Scholar]
- 38.Pasquet JM, Dachary-Prigent J, Nurden AT. Calcium influx is a determining factor of calpain activation and microparticle formation in platelets. Eur J Biochem. 1996;239(3):647–654. doi: 10.1111/j.1432-1033.1996.0647u.x. [DOI] [PubMed] [Google Scholar]
- 39.Ehlen HW, et al. Inactivation of anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J Bone Miner Res. 2013;28(2):246–259. doi: 10.1002/jbmr.1751. [DOI] [PubMed] [Google Scholar]
- 40.Damek-Poprawa M, et al. Chondrocytes utilize a cholesterol-dependent lipid translocator to externalize phosphatidylserine. Biochemistry. 2006;45(10):3325–3336. doi: 10.1021/bi0515927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munnix ICA, et al. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: Regulation by transient integrin activation. Arterioscler Thromb Vasc Biol. 2007;27(11):2484–2490. doi: 10.1161/ATVBAHA.107.151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takizawa H, et al. Lnk regulates integrin alphaIIbbeta3 outside-in signaling in mouse platelets, leading to stabilization of thrombus development in vivo. J Clin Invest. 2010;120(1):179–190. doi: 10.1172/JCI39503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chida J, Kido H. Extraction and quantification of adenosine triphosphate in mammalian tissues and cells. Methods Mol Biol. 2014;1098:21–32. doi: 10.1007/978-1-62703-718-1_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.