Significance
Empathy is of major importance for everyday social interaction. Recent neuroscientific models suggest that pain empathy relies on the activation of brain areas that are also engaged during the first-hand experience of pain. These models rely on rather unspecific and correlational evidence. Here, we show that inducing pain analgesia also reduces pain empathy, and that this is associated with decreased activation of empathy-related brain areas. We then document that blocking placebo analgesia via an opioid antagonist also blocks placebo analgesia effects on pain empathy. This finding suggests that pain empathy is grounded in neural responses and neurotransmitter activity related to first-hand pain.
Keywords: pain, empathy, placebo, fMRI, psychopharmacology
Abstract
Empathy for pain activates brain areas partially overlapping with those underpinning the first-hand experience of pain. It remains unclear, however, whether such shared activations imply that pain empathy engages similar neural functions as first-hand pain experiences. To overcome the limitations of previous neuroimaging research, we pursued a conceptually novel approach: we used the phenomenon of placebo analgesia to experimentally reduce the first-hand experience of pain, and assessed whether this results in a concomitant reduction of empathy for pain. We first carried out a functional MRI experiment (n = 102) that yielded results in the expected direction: participants experiencing placebo analgesia also reported decreased empathy for pain, and this was associated with reduced engagement of anterior insular and midcingulate cortex: that is, areas previously associated with shared activations in pain and empathy for pain. In a second step, we used a psychopharmacological manipulation (n = 50) to determine whether these effects can be blocked via an opioid antagonist. The administration of the opioid antagonist naltrexone blocked placebo analgesia and also resulted in a corresponding “normalization” of empathy for pain. Taken together, these findings suggest that pain empathy may be associated with neural responses and neurotransmitter activity engaged during first-hand pain, and thus might indeed be grounded in our own pain experiences.
There is widespread consensus that empathy recruits brain structures that are also involved in the first-hand experience of the emotion for which one is showing empathy. For example, in the domain of pain, recent image-based and coordinate-based meta-analyses of functional MRI (fMRI) studies have shown that sharing the pain of others consistently activates the bilateral anterior insular (AI) and anterior midcingulate cortex (aMCC) (1). The AI and aMCC are key areas of the network of areas activated by pain, and their activity has been directly related to the affective-motivational component of pain (2). The observation of such shared neural activations has therefore motivated simulationist models, such as the shared representations account of empathy (see refs. 3–5 for review), which propose that we come to understand the feelings of others by engaging the same mental representations as when directly experiencing the emotion with which we are empathizing.
However, neuroimaging alone cannot provide sufficient empirical support for such claims because fMRI activation of the same brain area does not necessarily imply equivalence of mental representations and neural functions (see ref. 6 for review). Areas such as the aMCC and AI, for example, are not only activated by pain, but also by phenomena as distinct as cognitive control and responding to salient events in general (see refs. 7–9 for review). This ambiguity is a result of inherent methodological limitations. First, fMRI has mostly been used as a correlational method that identifies neural responses co-occurring with certain cognitive-psychological functions, thus precluding mechanistic conclusions. Second, the hemodynamic responses fMRI is based upon are only indirect measures of neural activity. Third, each fMRI voxel covers thousands of neurons. In combination, these limitations can generate phenomena, such as that functionally different neuronal firing patterns result in similar fMRI activation maps. Recently, studies using more fine-grained fMRI analysis approaches have attempted to overcome some of these limitations (10–12). Some of these studies have bolstered interpretations that empathy partially relies on shared representations (11). Others (10) have fueled doubts that abstract experiences of pain, such as the “pain” of social rejection or of sharing the pain of others, rely on neural processes equivalent to those underlying direct nociception and the first-hand experience of pain. However, even the most fine-grained and sophisticated fMRI analyses cannot overcome the limitation that fMRI is a correlational method and has imperfect spatial resolution.
To better understand whether empathy and first-hand emotion imply equivalent neural functions therefore requires a conceptually different approach than measuring the neural correlates of people while they are engaging in pain or empathy tasks. One strategy, which we propose here (see also ref. 13), is to test whether experimentally manipulating first-hand nociceptive processing also affects how we empathize with the pain of others. We therefore performed two experiments, combining behavioral and fMRI measures with experimental and psychopharmacological manipulations. In the first experiment, using fMRI, we used placebo analgesia to experimentally reduce the amount of first-hand pain someone perceives, and tested whether this also reduces empathy for pain and the underlying brain processes. In the second experiment, with another group of subjects, we blocked the placebo analgesia effects by administering the opioid receptor antagonist naltrexone. The rationale of this approach was twofold. First, if empathy relies on the recruitment of the representations and neural processes engaged by first-hand pain, then experimentally changing these representations will also affect empathy for pain. Second, directly manipulating opioidergic function enabled us to tap into the link between self- and other-related representations on a neuropharmacological level.
The well-established phenomenon of placebo analgesia describes reduced feelings of pain following administration of an inactive compound promoted as a potent painkiller (14, 15). Placebo analgesia decreases neural activity in the AI and aMCC, among other brain areas (see ref. 16 for meta-analysis). Both areas are strongly associated with the affective-motivational component of pain, and are also central in empathy for pain (see ref. 1 for meta-analysis). Moreover, these areas show a particularly high density of opioid receptors (17) and are activated during both opioid and placebo analgesia (15, 18, 19). This finding implies that these areas play an important role in opioidergically mediated pain regulation (20). Given our primary hypothesis that first-hand pain and empathy for pain share basic mechanistic features, we therefore expected that a placebo analgesia manipulation of first-hand pain would decrease the activation in the AI and aMCC in self-directed pain, and that this would similarly reduce activation during empathy for pain. Moreover, we predicted that both placebo analgesia effects would be suppressed by naltrexone, which we tested on the behavioral level.
Results
Placebo Analgesia Affects First-Hand Pain and Empathy for Pain in Similar Ways (fMRI Experiment).
We first carried out an fMRI experiment with 102 participants, in half of which we experimentally induced analgesia using a well-tested placebo analgesia induction procedure (see Experimental Procedures for details). We measured self-reported affect ratings and fMRI activation in response to painful electrical stimulation delivered to either the participant him- or herself, or to another person present in the scanner room. To increase the specificity of results, all responses were compared with nonpainful control stimulation.
Self-Report Results.
Self-report included “pain” ratings, which either assessed the amount of pain participants experienced during self-directed stimulation, or how much pain the other person supposedly felt during other-directed stimulation. Additional “unpleasantness” ratings assessed the amount of unpleasant affect experienced by participants when witnessing painful stimulation of the other person. Whereas pain ratings targeted cognitive-evaluative aspects associated with pain, unpleasantness ratings tapped into responses related to affective sharing and vicarious distress: that is, whether the other person’s negative affect also increased negative affect in the participant.
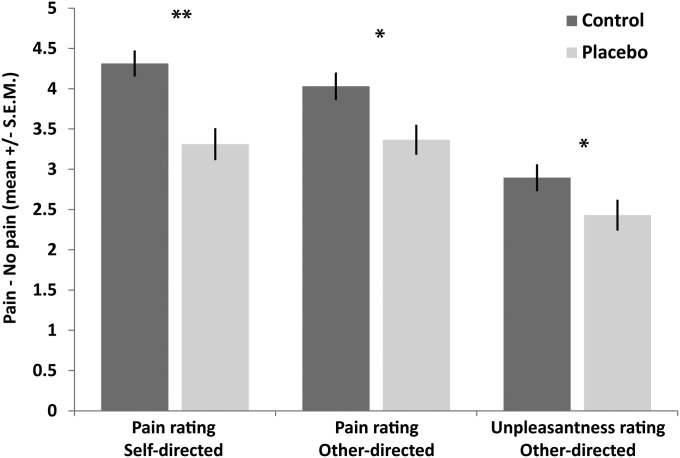
Planned comparisons comparing the difference in pain ratings (painful vs. nonpainful control stimulation) between the two groups revealed placebo analgesia effects for both self-directed [first-hand pain; t test, t(1,100) = 3.940, P < 0.001 one-tailed, Cohen’s d = 0.79] and other-directed stimulation [empathy for pain; t(1,100) = 2.618, P = 0.005 one-tailed, Cohen’s d = 0.52]. The magnitude of these effects did not differ significantly [t(1,100) = 1.491, P = 0.139, Cohen’s d = 0.30], demonstrating that the placebo analgesia induction reduced first-hand pain and its empathic evaluation to a similar extent. Analysis of the unpleasantness ratings revealed lower unpleasantness in the placebo group, suggesting that this group experienced less unpleasant affect than the control group, when witnessing the other person’s pain [t(1,100) = 2.180, P = 0.034 one-tailed, Cohen’s d = 0.44] (see also Fig. 1 and Supporting Information for complimentary ANOVA analyses).
Fig. 1.
Self-report results in the control (n = 53) and placebo group (n = 49), for ratings of self-directed pain (“how painful was this stimulus for you?”), other-directed pain (“how painful was this stimulus for the other person?”), and self-experienced negative affect (unpleasantness) when witnessing other-directed pain (“how unpleasant did it feel when the other person was stimulated?”). Asterisks (*P < 0.05, **P < 0.01) mark significant planned comparisons (independent samples t tests) of the main hypothesis that placebo analgesia reduced both empathy for pain and its first-hand experience.
fMRI Results.
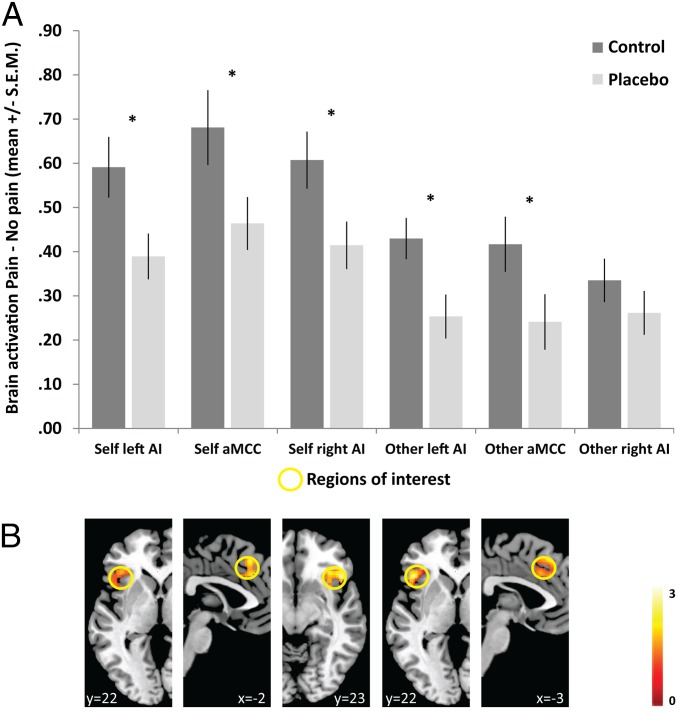
Following three successful initial control analyses (see Supporting Information for details), we tested our main hypothesis that placebo analgesia led to significant changes in the previously identified core empathy for pain network (1). To this end, we applied a region of interest (ROI) approach, which analyzed activation in three independently determined ROIs (in the aMCC and bilateral AI) taken from a meta-analysis of empathy for pain studies (1). Assessing group differences as a network by performing planned comparisons of pooled ROI activation revealed that the placebo group showed lower activation compared with the control group for both self- and other-directed stimulation [self: t(1,100) = 2.373, P = 0.01 one-tailed, Cohen’s d = 0.46; other: t(1,100) = 2.026, P = 0.023 one-tailed, Cohen’s d = 0.40], and that the magnitude of these effects was indistinguishable [t(1,100) = 0.644, P = 0.521, Cohen’s d = 0.12]. Motivated by a significant main effect of ROI (see full ANOVA results in Supporting Information), we also performed analyses testing these effects separately within each ROI.
This process revealed significant group differences in the left AI and aMCC for both self-directed and other-directed stimulation [self-directed: left AI: t(1,100) = 2.324, P = 0.011 one-tailed, Cohen’s d = 0.46; aMCC: t(1,100) = 2.067, P = 0.021 one-tailed, Cohen’s d = 0.41; other-directed: left AI: t(1,100) = 2.595, P = 0.006 one-tailed, Cohen’s d = 0.52; aMCC: t(1,100) = 1.990, P = 0.025 one-tailed, Cohen’s d = 0.39]. Moreover, the magnitudes of these effects were indistinguishable [left AI: t(1,100) = 0.278, P = 0.781, Cohen’s d = 0.06; aMCC: t(1,100) = 1.312, P = 0.192, Cohen’s d = 0.26]. Results for the right AI were somewhat mixed. Planned comparisons revealed group differences only for the self-directed [t(1,100) = 2.281, P = 0.013 one-tailed, Cohen’s d = 0.46] but not for the other-directed condition (P = 0.146 one-tailed, Cohen’s d = 0.21). However, the magnitude of these effects did not differ significantly [t(1,100) = 0.323, P = 0.747, Cohen’s d = 0.26]. See Fig. 2 for illustration of fMRI ROI results.
Fig. 2.
Group differences in brain activation in the empathy network. (A) Bar-plot of mean contrast estimates within ROIs (arbitrary units), plotted separately for self- and other-directed conditions and the two groups, in three ROIs (aMCC, left and right AI). Asterisks (*P < 0.05) mark significant planned comparisons (independent samples t tests) of the main hypothesis that placebo analgesia reduced activation during both self-directed and other-directed stimulation. (B) Activation maps displaying the spatial distribution of brain activity within the ROIs (taken from a two-sample t test contrasting the two groups, for the contrast pain > no pain, separately for self- and other-directed conditions). The yellow circles mark the ROI sphere used to extract the mean activation. Note that these maps are shown for illustration purposes only (and for this reason are thresholded at P = 0.05 uncorrected) and that they are not independent of the ROI results (59).
Naltrexone Blocks Placebo Analgesia Effects on Empathy for Pain (Psychopharmacological Experiment).
The fMRI experiment had shown that experimentally reducing the first-hand experience of pain also reduces empathy for pain. This was accompanied by diminished neural activity in core areas of the pain matrix, and of pain empathy. Although these findings suggest that empathy for pain recruits partially the same neural functions as first-hand pain, fMRI remains naïve with respect to the underlying neurochemical mechanisms mediating the effects. To directly test whether the placebo analgesia effects on self-pain are affected by an opioidergic pain-regulation mechanism, we performed a second placebo analgesia experiment in which one group of participants received the opioid antagonist naltrexone. This experiment provided evidence that placebo analgesia of self-experienced pain was affected by opioidergic activity, as naltrexone blocked the analgesic effects on self-directed pain. Notably, this also blocked the effects placebo analgesia had on other-directed pain, resulting in a “normalization” of pain empathy.
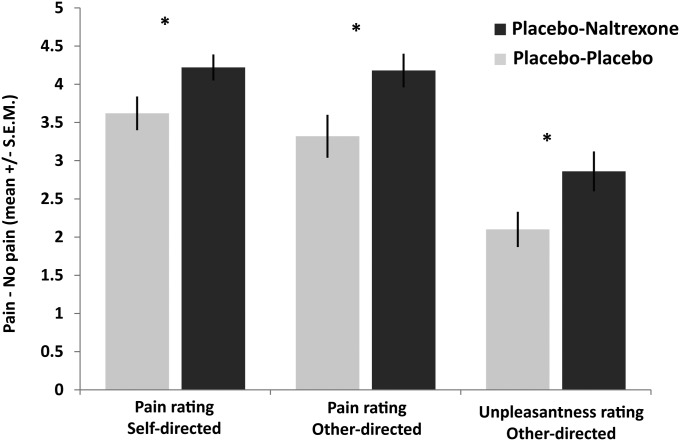
More specifically, self-report pain ratings indicated that naltrexone abolished the placebo analgesic effects for the first-hand experience of pain as well as for empathy for pain. Planned comparisons showed significant group differences in pain ratings for both self-directed [t(1,48) = 2.158, P = 0.018 one-tailed, Cohen’s d = 0.62] and other-directed stimulation [t(1,48) = 2.437, P = 0.010 one-tailed, Cohen’s d = 0.70] with higher ratings in the naltrexone compared with the placebo group, thus indicating a blockage of placebo effects in both conditions. The magnitude of these effects did not differ [t(1,48) = 1.390, P = 0.431, Cohen’s d = 0.23], documenting that the first-hand experience of pain and its empathic evaluation were similarly affected by the placebo-naltrexone manipulation. Furthermore, analysis of the unpleasantness ratings revealed higher values in the naltrexone compared with the placebo group [t(1,48) = 2.180, P = 0.017, Cohen’s d = 0.63], indicating that naltrexone also blocked placebo effects on the amount of unpleasant affect experienced by participants when witnessing the other person’s pain (for illustration of all effects, see Fig. 3).
Fig. 3.
Self-reported affect ratings of the psychopharmacological experiment in the placebo-placebo (n = 25) and the placebo-naltrexone group (n = 25), for the different types of ratings (self-directed pain, other-directed pain, and unpleasantness in response to other-directed pain). Asterisks (*P < 0.05) mark significant planned comparisons (independent samples t tests) of the main hypothesis that naltrexone reduced the effects of placebo analgesia for both empathy for pain and its first-hand experience.
To ascertain placebo analgesia responses of equivalent size in the two experiments, and to test whether the naltrexone manipulation indeed blocked the effects of placebo analgesia, we carried out three additional analyses (see Supporting Information for full details). We first compared self-report data of the placebo group of the fMRI experiment with those of the placebo-placebo group of the psychopharmacological experiment, as these groups had undergone essentially identical manipulations. This revealed no significant difference for either type of rating (all P values > 0.298). Assessing whether effect sizes were similar in both experiments revealed no significant differences between corresponding effect sizes either (all P values > 0.58). Finally, comparing the control group to the naltrexone-placebo group revealed no differences either (all P values > 0.595). Together, these results allow the interpretation of a blockage of the placebo analgesic effect by naltrexone.
Discussion
The aim of the present work was to demonstrate that empathy for pain recruits neural processes that are functionally equivalent to those engaged by the first-hand experience of pain. Functional equivalence, as defined here, refers to the hypothesis that two conditions recruit neural processes subserving the same kind of neurocomputational functions. This concept has so far predominantly been used in visual cognitive neuroscience, addressing the question whether abstract forms of visual perception, such as object imagery, rely on the same functions as direct visual perceptions (e.g., ref. 21). In the present context, we pursued the analogous question whether empathy for pain, as an example of an abstract representation of pain, relies on the functions underpinning direct pain processing. To test this hypothesis, we manipulated the first-hand experience of pain, using placebo analgesia, and observed that this led to a decrease in self-reported empathy and a reduction of fMRI activation in areas considered as the core network of empathy for pain. In a second psychopharmacological experiment using self-report measures, we revealed that empathy “normalizes” when blocking placebo analgesia by means of an opioid antagonist.
Self-report and neural data of the fMRI experiment confirm and extend the results of a recently published study by our group (13). In that study, which followed a similar rationale as the present work, we had tested the effects of placebo analgesia on pain-related event-related potentials (ERPs) and self-report. Both the ERP findings, which indicated modulation of the P2-ERP, a component associated with pain and pain regulation, and the self-report were directly in line with the present findings. Moreover, self-report data of the three placebo analgesia groups (one in the ERP study, two in the present two experiments) showed identical patterns in three independent samples. Hence, we now have consistent and multimethod evidence from three experiments, speaking to the robustness of the newly discovered phenomenon of “placebo empathy analgesia.” This phenomenon indicates that reducing one’s own pain also reduces how we represent the pain of others. Importantly, the placebo effect on other-directed pain is a carryover effect of the manipulation of self-directed pain, because the placebo induction explicitly targeted self-experienced pain only, and because participants were explicitly instructed that the pain of the other person was left unchanged. This finding documents that placebo effects can also affect expectancies in a related but not explicitly manipulated domain, extending recent models of placebo as well as empathy as predictive phenomena (22, 23). The fMRI results show that this carryover effect is associated with neural activation changes in the aMCC and AI, two brain areas that are of direct and extensively documented relevance for first-hand and empathic pain alike (1, 2). Importantly, placebo analgesia affected the first-hand experience of pain and empathy for pain to a similar extent, as shown by similar effect sizes for self- and other-directed conditions, for both self-report and brain activation data.
More specifically, the self-report data indicated a reduction of empathy based on two different types of ratings, one tapping more into cognitive-evaluative aspects of empathy (i.e., the pain ratings), and the other tapping into more affective aspects (i.e., the unpleasantness ratings). The pain ratings indicate a change in empathic pain evaluation. This is an important finding because it suggests that placebo analgesia even affected the more cognitive evaluation of others. The unpleasantness ratings document that negative affect experienced by participants in response to painful stimulation of the other person was lowered by placebo analgesia. This finding is of major relevance for theoretical models placing a central role for affect sharing in empathy. It indicates that placebo analgesia does not only affect how much pain we “think” that others are experiencing, but also how much of that pain we feel or vicariously ”re-experience” ourselves. This finding is also in line with emerging consensus on how placebo analgesia modulates the first-hand experience of pain, which is not only by top-down regulation of nociceptive input (24) and early sensory information processing, but also and probably more importantly so by regulating higher-level and in particular the emotional consequences of painful stimulation (see refs. 25 and 26 for reviews; see also ref. 27). The fMRI results provide a neural foundation for this interpretation, as they illustrate that placebo empathy analgesia was observed in a network that has predominantly been associated with the affective-motivational component of pain. Importantly, both the self-report and fMRI results show high specificity as our experimental design allowed for analyses in which unspecific responses to the nonpainful control condition were eliminated from the specific responses to painful stimulation. Hence, the results cannot be explained as an unspecific main effect of the placebo analgesia induction, such as, for example, reducing affective responses to all kinds of stimulation (13).
These findings are in line with social cognition theories proposing that empathy relies on a simulation of others’ feelings, which is directly and specifically grounded in one’s own bodily and neural emotion systems (3, 5, 28, 29). Here, we show that this seems to hold for the affective-motivational component of pain. The fact that placebo analgesia also affected how participants responded to the pain of others provides strong and causal-experimental support for the idea that empathy for pain may rely on neural processes and psychological functions grounded in those engaged by the first-hand experience of pain. Moreover, these findings are well in line with clinical studies also tapping into the causality question of whether shared activations are necessary for empathy, and not just a correlational finding (see ref. 30 for review). For example, the observation that patients with AI lesions show reduced empathy for pain suggests that an intact insular cortex is necessary for empathy (31). Moreover, in the domain of touch, patients unable to have the first-hand experience of a specific type of pleasant touch were unable to accurately empathize with this type of touch (32). Taken together, these and the present findings suggest that the first-hand experience of emotions is crucial and possibly even necessary for an accurate representation of other people’s emotions (but see also ref. 33). However, the notion of functional equivalence and shared representations should not mistakenly imply that empathic and first-hand pain experiences rely on fully shared neural processes and psychological functions. Rather, empathy relies only on a partial equivalence (see also refs. 4, 6, and 34), which in the present case and paradigm encompasses aspects of affective sharing associated with the aMCC and AI.
The specific functions and computations of a certain brain area are difficult to infer from neuroimaging data alone. This challenge is also reflected in the ongoing intense debate (7, 12) of whether brain activation during social rejection, which includes areas of the pain network such as the aMCC, provides evidence that rejection “hurts” in the same way as physical pain does (35). This interpretation and the validity of overlapping fMRI activations for inferences on the similarity of neural processes in general has recently been questioned using more fine-grained analyses of fMRI data for social rejection. Woo et al. (10) used multivoxel pattern analyses that exploit fMRI activation in a multivariate fashion, and hence might overcome some of the limitations of univariate fMRI analyses. Their results demonstrated that previous claims about the similarity of fMRI activation between physical pain and the pain of social rejection do not hold unequivocally when using such an analysis approach. The present fMRI results both complement and extend these findings for pain empathy, as another type of abstract pain. The results show that whereas empathy indeed does not recruit the full set of processes underlying pain processing, those processes attributed to its affective-motivational component were equivalently affected by the placebo manipulation. However, even the experimental manipulation approach we used cannot overcome some of the inherent limitations of fMRI.
The psychopharmacological experiment therefore provides an important new angle to the debate about the nature of shared representations, as it explicitly helps to address some of these limitations. We chose to manipulate opioidergic function for three very specific reasons. First, pain and in particular pain affect is directly related to the release of endogenous opioids, which serve a regulatory analgesic function. Second, the aMCC and AI are particularly rich in opioid receptors, and play a crucial role in analgesia (2, 17). Third, it is a standard procedure in placebo analgesia research to test whether a placebo specifically affects pain, and not just other more unspecific aspects (such as attention), by administering an opioid antagonist (see ref. 36 for review). Our findings indicate that blocking opioidergic neurotransmission abolished the effects of the placebo analgesia induction and led to a “normalization” of both self-directed and other-directed pain. Importantly, this was not only the case for the more cognitive empathic pain evaluations, but also for feelings of unpleasantness when witnessing the other person’s painful stimulation.
The demonstration that placebo empathy analgesia is modulated by an opioid antagonist suggests that one of the hallmark features of pain (i.e., the involvement of the endogenous opioid system in pain processing) is also relevant for empathy for pain. However, although suggestive of such an interpretation, our findings are preliminary. An alternative interpretation of the present dataset is that the effect of naltrexone on placebo empathy analgesia is indirect: because our placebo manipulation only targeted first-hand pain, the effect of naltrexone on empathy might be a result of the effects it had on the subjective experience of self-experienced pain, and how this affected empathy for pain. Building on these insights, additional pharmacological studies teasing apart the indirect effects from placebo analgesia from potential direct effects of the opioid system on placebo empathy analgesia will have to be designed. Moreover, manipulating opioidergic activity during empathy for pain where treatment expectations have not been experimentally distorted seems to be warranted to clarify whether the opioid system may play a general role in empathy for pain.
Our study design and some of its limitations open up additional important questions. With respect to the generalizability of our results, it needs to be noted that placebo manipulations do not only have analgesic effects, but also modulate other types of affective processes (37–39) and engage additional neurotransmitter systems (36). Because the present design was not tailored to tease apart these effects, this calls for further research. The conceptual approach of our study also lays the foundation for additional studies addressing the functional equivalence debate of other types of abstract pain, such as social rejection (7, 10). Moreover, our paradigm was not tailored to disentangle processes related to pain anticipation from those related to pain delivery. In line with previous research providing evidence for shared activations (e.g., see ref. 1 for meta-analysis, and ref. 40), we thus modeled the whole epoch of a trial with one regressor, ranging from the anticipation cue to the end of stimulus delivery. Thus, processes such as pain anticipation, anticipatory anxiety, or avoidance suppression might have been engaged as well. However, these processes might also be vicariously shared in the other-directed pain condition (if assuming that the AI and aMCC underpin the same functions in self-pain and pain empathy). Hence, it might be that empathy-related activations in our own and in previous studies are not only related to the sharing of pain itself, but also to sharing pain-anticipatory processes. Future studies teasing apart pain anticipation from pain delivery are therefore needed to provide more information on which of these processes are specifically affected by placebo empathy analgesia. Finally, our paradigm included only two stimulus levels (“pain vs. no pain”). This might result in activation differences when contrasting pain and no pain related to differences in magnitude estimation (which might be more difficult for the painful stimuli) and preparation of responses (which might take place earlier for the no pain condition). Note, however, that both conditions revealed similar individual variation in the ratings. Nevertheless, future studies might want to incorporate more levels or parametric variation to allow the assessment of effects on a broader range of pain experiences.
Although simulative mechanisms will be of high predictive value in many cases, they can also lead to egocentrically biased evaluations of others, resulting in misunderstandings and social conflict (16, 41, 42). The fact that experimentally reducing self-related pain also reduces empathy for pain is crucial to understand why egocentrism in social judgments may be difficult to regulate. It suggests that empathic simulation taps into neural and possibly also neurochemical mechanisms, which are so strongly grounded in our own experiences that this may lead to the misperception that we are in pain ourselves. This may then result in failures of self-other distinction, which is crucial for accurate empathy (43). The case of placebo empathy analgesia is another illustration of egocentricity bias in empathy, with potentially broad implications and specific implications for health care professionals confronted with the suffering of others on a daily basis. It suggests that a pain killer we take to reduce our own pain may have the unwanted side effect to also blunt our response to the suffering of others. However, this interpretation should be specifically tested as the present study did induce analgesia only indirectly, and not by means of direct administration of analgesics.
Experimental Procedures
Participants.
The study was approved by the Ethics Committee of the Medical University of Vienna, and performed in line with the Declaration of Helsinki (1964). Participants were informed about the experiment (apart from the elements of deception: placebo and confederate) and gave written consent before participating. This was approved by the responsible ethics committee and was necessary in order not to spoil the induction of placebo effects. Participants received a reimbursement of €105 for their participation.
fMRI experiment.
For the fMRI experiment, 120 healthy right-handed volunteers (Vienna university students; 81 female, 39 male, mean age ± SEM = 24.63 ± 0.36 y) were randomly assigned to a control (n = 60; 38 females, 22 males) or a placebo group (n = 60, 43 females, 17 males). Eighteen participants in total had to be excluded from the analysis, mainly because of nonresponding to the placebo manipulation (10 exclusions; see Experimental Procedures subsection, below), but also because of technical problems (such as partial malfunctioning of the pain stimulator; eight exclusions, seven of them in the control group). All analyses reported in the report were carried out for the remaining 102 participants (control group: n = 53, 34 females, 19 males, mean age ± SEM = 26.19 ± 0.58 y; placebo group: n = 49, 36 females, 13 males, mean age ± SEM = 24.57 ± 0.41 y).
Psychopharmacological experiment.
Using a double-blind, placebo-controlled between-subjects design, 57 healthy right-handed volunteers (Vienna university students; 28 female, 29 male, mean age ± SEM = 25.05 ± 0.41 y) were randomly assigned to a placebo-placebo (n = 29, 13 females, 16 males) or a placebo-naltrexone group (n = 28, 15 females, 13 males). Seven participants in total had to be excluded from the analysis, because of nonresponding to the placebo manipulation (five exclusions, four of them in the placebo-placebo group; see Experimental Procedures subsection, below), or because of technical problems (two exclusions in the placebo-naltrexone group). All analyses reported in the paper were carried out for the remaining 50 participants (placebo-placebo group: n = 25, 12 females, 13 males, mean age ± SEM = 25.28 ± 0.75 y) or placebo-naltrexone group (n = 25, 12 females, 13 males, mean age ± SEM = 25.00 ± 0.52 y).
Experimental Task and Trial Structure.
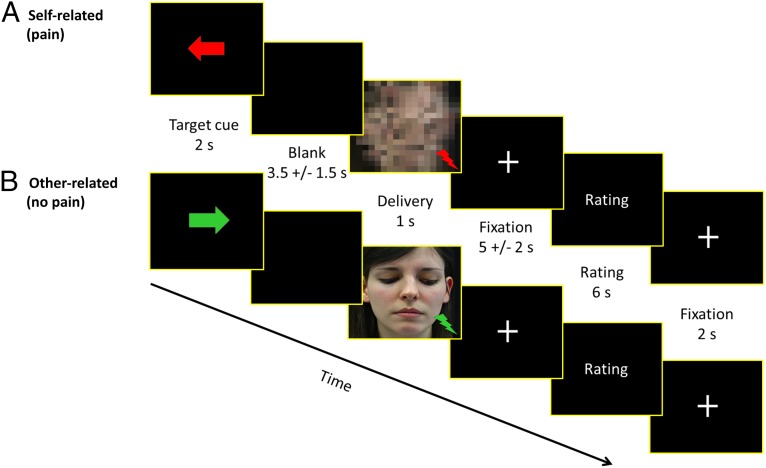
The following task description applies to both experiments. We used a well-established empathy for pain paradigm (1, 40) in which participants were either exposed to short-lasting (500 ms) and individually calibrated painful or nonpainful electrical stimulation themselves, or witnessed another person (a confederate of the experimenters) being exposed to such stimulation. Electrical stimulation was delivered using a Digitimer DS5 Isolated Bipolar Constant Current Stimulator (Digitimer Ltd, Clinical & Biomedical Research Instruments) via electrodes attached to the back of the left hand. In the fMRI experiment, average stimulation intensity was 0.16 mA (SD 0.15) for nonpainful sensations and 0.74 mA (SD 0.59) for painful sensations. In the psychopharmacological experiment, average stimulation intensity was 0.11 mA (SD 0.10) for nonpainful sensations and 0.89 mA (SD 0.78) for painful sensations. There were no group differences in either study. Stimulation intensities were comparable to previous studies in our laboratory using the same equipment (13, 44). During the experiment, the trial structure and timing was as follows (Fig. 4). First, the target (i.e., the participant or the other person) of the upcoming stimulus was indicated on the computer screen by an arrow pointing either to the participant or to the other person (duration = 2,000 ms). The color of this arrow indicated the intensity of the upcoming stimulus (red: painful vs. green: nonpainful). After a blank screen with jitter (3,500 ms ± 1,500 ms), the electrical stimulus (500 ms) was delivered while another visual stimulus (1,000 ms) was simultaneously shown on the screen. In case of stimulation of the other person, this stimulus consisted of a picture of the other person’s face, shown with either a painful or a neutral expression. In case of stimulation of the participant, scrambled versions of these pictures were shown to control for visual stimulation. All pictures in addition were accompanied by either a red (painful) or green (nonpainful) flash in the lower right corner of the picture, depending on the stimulus category. Importantly, this set-up enabled participants to know with which intensity and when the other person was stimulated, without requiring direct observation of her actual reactions (as in reality the confederate never received actual stimulation). The delivery cue was then followed by a fixation cross, presented with a jittered duration (5,000 ms ± 2,500 ms) and an optional rating (self-directed: one rating question; other-directed: two rating questions; 6,000-ms answering time provided per each rating question). Jitter was added to optimize the estimation of hemodynamic responses (45).
Fig. 4.
Structure and timeline of two exemplary trials. (A) Self-directed pain trial, (B) Other-directed no pain trial. An anticipation cue indicated the target (left = self, right = other) and intensity (red = painful, green = nonpainful) of the upcoming electrical stimulus. After a variable interval of 3.5 ± 1.5 s, electrical stimulation (duration = 500 ms) was delivered concurrently with visual presentation (duration = 1,000 ms) of either a scrambled face (self-directed trials) or the other person’s face (other-directed trials) accompanied by a red (in case of painful stimulation) or green (nonpainful stimulation) flash. Afterward, affect ratings (6 s per rating) were collected, but only in about one-third of all trials.
If a rating was requested, the questions to be answered differed according to who was the target of stimulation. After stimulation of themselves, participants rated their own pain (self-directed pain ratings), using the question “How painful was this stimulus for you?” on a seven-point rating scale ranging from “not at all” to “extremely painful.” After stimulation of the other person, participants rated the other person’s pain (other-directed pain ratings; “How painful was this stimulus for the other person?” answered using the same seven-point rating scale as for the self-directed pain ratings), as well as their own affect during stimulation of the other (unpleasantness ratings; “How unpleasant did it feel when the other person was stimulated?”; seven-point scale, from “not at all” to “extremely unpleasant”). We used two different affect rating scales to tap into different aspects of empathy (see e.g., refs. 6 and 46). Self- and other-directed pain ratings targeted cognitive-evaluative aspects. Ratings of unpleasantness were used to indicate the degree of negative affect triggered by witnessing the other participant’s stimulation, and targeted aspects of empathy related to affective sharing and vicarious distress.
Ratings were collected only in about one-third of the trials in a pseudorandomized fashion. Before the next trial started, another fixation cross was presented (2,000 ms). In total, 15 trials of each condition (i.e., self-directed pain/no pain; other-directed pain/no pain) were presented.
Experimental Procedures.
fMRI experiment.
Before the day of the experiment, participants filled in online versions of empathy and emotion contagion questionnaires [Interpersonal Reactivity Index (47); Emotional Contagion Scale (48)]. After arrival to the laboratory in pairs of two, participants were introduced to each other. One of them was a confederate, who was always female. Both participants then underwent a psychophysical pain calibration procedure (similar to the one used in ref. 49). This process enabled us to determine reliable values for painful and nonpainful stimulation by asking participants to rate each stimulus on a seven-point scale ranging from 1 = “perceptible, but clearly nonpainful sensation” to 7 = “unbearable pain.” The painful stimuli used in the experiment later on were consistently rated on that scale with a value of 6 (corresponding to “extremely painful, but bearable”), and nonpainful stimuli were rated with 1 (“perceptible, but clearly nonpainful sensation”). A major asset of this design is that it included closely matched control conditions with nonpainful stimulation. Such a control condition has rarely been used in previous placebo analgesia experiments (but see ref. 19). By subtracting rating or neural responses during nonpainful stimulation, our effects can therefore be much more specifically attributed to pain processing. Domain-general aspects (such as generalized perceptual or behavioral responses, including stimulus-directed attention) related to stimulus presentation are explicitly eliminated by this analysis approach.
After calibration, participants of the placebo group were introduced to a medical doctor. She administered the placebo pill and informed participants about the “medication” by explaining that it was an approved, highly effective as well as expensive pain killer [because placebos perceived as more expensive have been shown to be more effective (50)]. She also conveyed that the purpose of the study was not to test the effectiveness of the medication (“This is an approved pain medication, which can be purchased in Austrian pharmacies without prescription”). Participants were then asked to rate the question “Do you expect this medication to be effective in reducing your pain?” on a scale ranging from 1 (“not at all”) to 7 (“very effective”). Because the aim of this study was to assess the effects of placebo analgesia on empathy for pain, it was crucial to ensure that each participant in the placebo group was clearly responding to the placebo analgesia manipulation (i.e., that it had substantial analgesic effects on self-directed pain). Based on a set of criteria outlined in detail below (see Supporting Information, Nonresponder Identification), 10 subjects were classified as nonresponders.
After 15 min waiting time (allegedly for the medication to take effect), the placebo analgesic effect was amplified by a classic conditioning procedure commonly used and proven to be effective in placebo analgesia studies (51). More specifically, participants were exposed to a series of four stimuli delivered with an intensity of stimuli rated between “3” and “4” during the calibration procedure. However, participants were led to believe that they received stimuli they had previously rated as “6.” After obtaining the participant’s rating of these stimuli, participants were asked again to answer the question, “How effective is this medication?” The confederate did not receive any medication, which was explicitly made clear to participants. Participants and confederate were jointly brought into the scanner room, where the confederate was seated on a table with a computer screen and keyboard placed next to the scanner. After the participant had been positioned in the scanner, the confederate left the room without the participant being able to notice it. Before entering the room, the confederate was told to communicate with hand gestures with the experimenters while the experiment was running because it would be impossible to hear her during scanning. These instructions were given in front of the participant to intensify the illusion that the confederate was actually a second participant. For the same reason, during the experiment the experimenter was always pretending to communicate with both participant and confederate. Then the experimental task was performed, which took about 20 min. Notably, painful stimuli were delivered with the individually calibrated intensity of “6” (extremely painful, but bearable) to participants of both groups. After completion of the experiment, postexperimental questionnaires were filled in and participants were debriefed.
Psychopharmacological experiment.
This experiment was largely identical to the fMRI experiment, in terms of design and procedures, but differed in two aspects: First, it involved administration of a pharmacological compound to half of the participants, in a double-blinded fashion. Second, the placebo analgesia induction procedure differed from the one in the fMRI experiment in one respect, which was that after the initial administration of a placebo pill, participants received another pill following the conditioning procedure (supposedly to strengthen the effects). This pill was the one that either included naltrexone or placebo. The rationale of this procedure was directly motivated by previous placebo analgesia research (24), and served to block opioidergic placebo analgesia effects once induced by the administration of the inert pill and the conditioning procedure. Moreover, administration of naltrexone already before the conditioning procedure might have counteracted the placebo analgesic effects. For details on nonresponders (n = 5) in this experiment see Supporting Information, Nonresponder Identification.
fMRI Acquisition and Statistical Analysis.
MRI data were acquired using a 3 Tesla Siemens Tim Trio MRI system (Siemens Medical) using a 32-channel head coil for signal reception. Blood oxygen level-dependent sensitive functional imaging was performed using a multiband accelerated echoplanar imaging sequence with the following parameters: echo time (TE)/repetition time (TR) = 33/1,800 ms, flip angle 60°, interleaved acquisition, 54 axial slices coplanar the connecting line between anterior and posterior commissure, field of view 192 mm × 192 mm × 108 mm, matrix size 128 × 128, voxel size 1.5 × 1.5 × 2 mm; 509 volumes were acquired within one run with a total duration of 916 s. Structural images were acquired after functional scanning using a magnetization-prepared rapid gradient-echo sequence (TE/TR = 4.21/2,300 ms, 160 sagittal slices, voxel size = 1.0 × 1.0 × 1.1 mm, field of view = 256 mm).
Data preprocessing was carried out in SPM12 (Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk/spm) using standard algorithms and parameters unless specified differently. This included slice timing correction (reference = first slice), motion correction, spatial normalization to MNI (Montreal Neurological Institute) stereotactic space using an in-house scanner-specific echoplanar imaging template, and spatial smoothing (6-mm Gaussian kernel). We applied a 2-mm threshold for excessive head movement. Data analysis was performed based on a general linear model approach. The first-level design matrix of each subject contained five regressors: self-directed pain, self-directed no pain, other-directed pain, other-directed no pain, rating. For each condition (self-directed pain, self-directed no pain, other-directed pain, other-directed no pain) we modeled the whole time period from the onset of the anticipation cue until 1 s after the onset of the delivery of the painful stimulus and convolved them with SPM12’s standard canonical hemodynamic response function. Additional nuisance regressors included realignment parameters. Group statistics were calculated using second-level random effects analyses in SPM12. Our analysis steps consisted of three “manipulation checks,” followed by a specific test of our main hypothesis.
As a first check, we needed to make sure that our design robustly activated the network of areas involved in pain responses (52). This was tested only in the control group, using the contrast self-directed pain > self-directed no pain. This analysis is presented and interpreted at a statistical threshold of P < 0.05 [family-wise error (FWE)-corrected on voxel level, as implemented in SPM12].
Second, we needed to determine that the placebo analgesia induction procedure activated the wide-spread network identified by previous placebo analgesia fMRI studies (as summarized, for example, in ref. 16). To maximize comparability of our results to those of these previous studies, the following analysis procedure was directly motivated by the approach taken in most previous studies on placebo analgesia with regard to correction for multiple comparisons based on FWE rate (P < 0.05) using small volume correction (as implemented in SPM12; initial height threshold P < 0.005, uncorrected) (e.g., refs. 24, 53, 54). First, we restricted the group comparison analysis to specific a priori defined ROIs. Second, we analyzed only painful conditions: self-directed pain: control group > placebo group and placebo group > control group. ROIs were drawn using spheres centered on peak coordinates used in previous pain and placebo analgesia studies (15, 24, 53, 55–58) in the following regions (bilaterally): dorso-lateral prefrontal cortex (DLPFC) (coordinates: x = 36, y = 13, z = 39), secondary somatosensory cortex (x = 39, y = −15, z = 18), insula [anterior (x = 33, y = 18, z = 6) and posterior (x = 44, y = −15, z = 4) part], dorsal anterior cingulate cortex (x = 3, y = 6, z = 36), rostral anterior cingulate cortex (rACC) [pregenual (x = 10, y = 32, z = −8) and subgenual (x = 6, y = 30, z = −9) parts], ventral striatum (x = 9, y = 6, z = −3), thalamus (x = 12, y = −18, z = 3), and the periaqueductal gray (x = 0, y = −32, z = −10). In accordance with the aforementioned studies, we also varied the size of the ROIs, using spheres of 10-mm radius for cortical regions (except for the DLPFC: 15 mm) and 6-mm radius for the smaller subcortical regions.
In a third check, we had to make sure that our design evoked empathic responses in line with previous reports, based on comparing the present results to those of previous fMRI studies identifying the aMCC and bilateral AI as part of a “core network” underlying empathy for pain (1). In line with the meta-analysis reported in ref. 1 and the original approach proposed by Singer et al. (40), we performed a conjunction analysis of activation during self-directed and other-directed stimulation, for the control group only [i.e., (self-directed pain > self-directed no pain) ∩ (other-directed pain > other-directed no pain)]. This analysis is presented and interpreted at a statistical threshold of P < 0.05 (FWE-corrected on voxel level, as implemented in SPM12).
Because all these initial analyses were highly conclusive (Supporting Information), we proceeded to test our main hypothesis, which was that placebo analgesia also reduces empathy for pain, and that this is associated with reduced activation in the “core network” associated with empathy for pain (consisting of the aMCC and bilateral AI). To this end, we performed ROI analyses that investigated whether placebo analgesia affected activation in this network in a similar fashion during the first-hand experience of pain and during empathy for pain. More specifically, we extracted parameter estimates from each subject and condition, in predefined 10-mm spherical ROIs covering the left AI, right AI, and aMCC, centered on clusters reported in Lamm et al. (1). For that purpose, we used the self-directed pain > baseline, self-directed no pain > baseline, other-directed pain > baseline, and other-directed no pain > baseline first-level contrasts. We then calculated a mixed-model ANOVA, which aimed at assessing whether the experimental factors (for details, see below) produced significant variation in the data (with the focus being on a significant interaction of group*intensity and a nonsignificant three-way interaction of group*intensity*target, which would indicate that the activation difference for painful vs. nonpainful stimulation differed between placebo and control group). In case of significant effects, planned comparisons were used to specifically test our main hypothesis that the placebo analgesia manipulation resulted not only in a reduction of the first-hand experience of pain, but also of empathy for pain. These planned comparisons consisted of t tests for independent samples, which first tested whether self-directed painful stimulation (vs. nonpainful stimulation) differed between the placebo and the control group (i.e., self-directed pain − self-directed no pain: placebo vs. control), and then whether this was also the case for other-directed painful stimulation (i.e., other-directed pain − other-directed no pain: placebo vs. control). Because both of these tests assessed directed a priori hypotheses (i.e., that activation in the placebo group would be smaller than in the control group), one-tailed significance levels were used to determine their significance. Finally, a third independent samples t test (two-tailed, no directed hypothesis) examined whether the reduction of empathy for pain was similar to the reduction of its first-hand experience [i.e., self-directed pain (placebo < control) vs. other-directed pain (placebo < control)]. Additional post hoc pairwise comparisons were used in case individual conditions showed visible differences that were not covered by the planned comparisons. We first computed a four-way mixed-model ANOVA incorporating all factors of our design, with the between-subjects factor group (placebo vs. control) and the within-subjects factors ROI (left AI, right AI, aMCC), target (self-directed vs. other-directed stimulation), and intensity (painful vs. nonpainful stimulation), followed by planned comparisons incorporating pooled activation across all three ROIs. The planned comparison with pooled activation intended to test whether the full network of areas associated with empathy showed group differences. In addition, motivated by a significant main effect of ROI in this ANOVA, we computed separate mixed-model ANOVAs and planned comparisons for the left AI, right AI, and aMCC. This process also allowed us to more specifically explore the consistency or differences of effects in the three ROIs.
Behavioral Measures Analysis.
Statistical analyses of behavioral measures (as well as for fMRI ROI data) were performed using SPSS 18.0 (Statistical Packages for the Social Sciences, v18.0; SPSS) and the level of significance was set to P < 0.05. For the rating data, our analysis approach paralleled the one we chose for the fMRI ROI analysis. We used two separate ANOVAs and planned comparison analyses for the different types of ratings: in the first, self-directed, and other-directed pain ratings were analyzed using a mixed-model ANOVA with the between-subjects factor group (control vs. placebo), and the within-subjects factors intensity (painful vs. nonpainful stimulation) and target (self- vs. other-directed stimulation). Planned comparisons using independent samples t tests were conducted on the differences pain − no pain, separately for self- and other-directed conditions. The second ANOVA (factors group and intensity) analyzed the unpleasantness ratings delivered in response to stimulation of the other person, followed by another independent samples t test comparing groups for the unpleasantness difference during other-directed pain − other-directed no pain.
Differences between groups in trait empathy questionnaire data and postexperimental ratings (self-perceived similarity, liking, affiliation with the other person, attributed strength, neediness, and agreeableness) were tested with independent samples t tests (two-tailed). Correlations were assessed using the Pearson correlation coefficient.
Psychopharmacological Experiment.
Data were analyzed in exactly the same way as the behavioral and the questionnaire data of the fMRI experiment. In addition, to test more specifically whether naltrexone indeed led to a blockage of placebo analgesia, we performed a number of additional analyses for the three types of ratings. First, to ensure comparability between the experiments, we tested whether placebo analgesia resulted in similar ratings in the two experiments. To this end ratings of the placebo group in the fMRI and ratings of the placebo-placebo group in the psychopharmacological experiment were compared using independent samples t tests. Second, we statistically compared the effect sizes of the two experiments by comparing the rating differences of the respective groups (control group − placebo group vs. placebo-naltrexone group − placebo-placebo group), by means of a generalized linear regression model. This analysis tested whether the observed effects in the fMRI experiment (control > placebo) were of equal size as the reversed effects (naltrexone > placebo) in the psychopharmacological experiment. As a final complimentary check, we compared ratings of the control group in the fMRI experiment and of the placebo-naltrexone group in the psychopharmacological experiment using independent samples t tests. Taken together, the latter two analyses if significant would allow the interpretation that naltrexone not only decreased placebo analgesic effects, but blocked them to an extent that the naltrexone-placebo group became indistinguishable from the control group.
Additional Results of fMRI Experiment
Rating ANOVAs.
The ANOVA of pain ratings revealed main effects of intensity [F(1,100) = 1144.587, P < 0.001, partial-η2 = 0.920; indicating generally higher ratings for painful than for nonpainful stimuli] (note that all supplemental analyses have been conducted two-tailed because of nonspecific expectations regarding the results), target [F(1,100) = 38.741, P < 0.001, partial-η2 = 0.279; determined by higher ratings for self-directed compared with other-directed stimuli], and group [F(1,100) =7.506, P = 0.009, partial-η2 = 0.067; indicating higher ratings in the control group]. Crucially, the interaction intensity*group was significant [F(1,100) = 13.812, P < 0.001, partial-η2 = 0.121, see planned comparisons in the main text], and so was the interaction target*group [F(1,100) = 4.853, P = 0.030, partial-η2 = 0.046]. As simple effects analyses were not informative about the directionality of this interaction (all pairwise-comparisons > 0.001), we computed an independent sample t test on the difference scores (self – other) pooled over painful and nonpainful stimulation, revealing this difference being larger in the placebo compared with the control group [t(100) = 2.203, P = 0.030]. The target*intensity interaction and the target*intensity*group three-way interaction were nonsignificant (P = 0.364 and P = 0.139, respectively).
The ANOVA of unpleasantness ratings, by which participants indicated the degree of negative affect triggered by witnessing the other participant’s stimulation, revealed a main effect of intensity [F(1,100) = 446.080, P < 0.001, partial-η2 = 0.817; higher empathy ratings for pain compared with no pain stimuli], but no main effect of group (P = 0.742). There was a trend for an intensity*group interaction [F(1,100) = 3.393, P = 0.068, partial-η2 = 0.033; see planned comparisons in the main text].
fMRI Data Manipulation Checks.
Testing whether our design reliably activated the network of areas involved in pain responses (contrast: self-directed pain > no pain) in the control group, revealed extensive activation of the brain, including all parts of the “classic” pain network (52). Areas activated were, among others (see Table S1 for details), the aMCC and ACC, bilateral anterior and posterior insular cortex and the cerebellum, but also the DLPFC, orbitofrontal cortex (OFC), inferior frontal gyrus, periaqueductal gray (PAG), putamen, thalamus, primary motor cortex, and supplementary motor area (SMA).
Table S1.
fMRI results (neural activation related to first-hand pain)
| Brain region | x | y | z | t value | P value |
| Cluster 1 (k = 79,131) | |||||
| L ACC | −4 | 20 | 30 | 12.30 | P < 0.001 |
| R insula | 36 | 6 | 8 | 12.10 | P < 0.001 |
| L MCC | −8 | 14 | 34 | 12.00 | P < 0.001 |
| R ACC | 4 | 26 | 28 | 11.82 | P < 0.001 |
| L insula | −40 | 4 | 2 | 11.77 | P < 0.001 |
| L cerebellum | −22 | −68 | −20 | 11.75 | P < 0.001 |
| Inferior frontal gyrus | −52 | 4 | 2 | 11.56 | P < 0.001 |
| R cerebellum | 34 | −54 | −28 | 11.51 | P < 0.001 |
| Cerebellar vermis | −2 | −62 | −8 | 11.43 | P < 0.001 |
| R MCC | 8 | −24 | 46 | 11.32 | P < 0.001 |
| PAG | 6 | −14 | −12 | 11.19 | P < 0.001 |
| R SMA | 6 | −6 | 58 | 11.14 | P < 0.001 |
| R putamen | 10 | 8 | 0 | 10.89 | P < 0.001 |
| R thalamus | 4 | −12 | 12 | 10.59 | P < 0.001 |
| L primary motor cortex | −40 | −8 | 58 | 10.56 | P < 0.001 |
| Cluster 2 (k = 1,114) | |||||
| R DLPFC | 32 | 46 | 28 | 7.97 | P < 0.001 |
| Cluster 3 (k = 70) | |||||
| R middle temporal gyrus | 54 | −52 | 6 | 5.78 | P < 0.001 |
| Cluster 4 (k = 44) | |||||
| L OFC | −26 | 42 | −10 | 6.20 | P < 0.001 |
| Cluster 5 (k = 33) | |||||
| L hippocampus | −20 | −6 | −38 | 6.38 | P < 0.001 |
Significant clusters resulting from the contrast self-directed pain > self-directed no pain, in the control group are given, including MNI coordinates, cluster size (k), t value, and P value (whole-brain, FWE-corrected, P < 0.05, voxel-level, k > 15). Only the highest peak is included in the case of several peaks in a cluster.
Testing whether the placebo analgesia induction procedure modulated activation in similar areas as in previous research revealed significantly stronger activation in the bilateral AI and posterior insular cortex, bilateral dorsal ACC, secondary somatosensory cortex (SII), and left thalamus in the control group. For the placebo group, we found stronger activations in bilateral subgenual ACC. See Table S2 for detailed results.
Table S2.
fMRI results (placebo analgesia network)
| Brain region | x | y | z | k | t-value | P value |
| Control > placebo | ||||||
| R anterior insula | 28 | 26 | 10 | 325 | 6.62 | P < 0.001 |
| L anterior insula | −26 | 24 | 10 | 272 | 5.63 | P < 0.001 |
| L dACC | −8 | 14 | 32 | 177 | 5.17 | P < 0.001 |
| R posterior insula | 38 | −14 | 0 | 164 | 4.51 | P = 0.001 |
| R SII | 30 | −18 | 16 | 100 | 4.57 | P = 0.001 |
| R dACC | 8 | 2 | 40 | 88 | 4.40 | P = 0.001 |
| L Thalamus | −16 | −14 | 6 | 20 | 3.62 | P = 0.006 |
| Placebo > control | ||||||
| L sgACC | −4 | 30 | −2 | 28 | 3.46 | P = 0.030 |
| R sgACC | 4 | 28 | −4 | 27 | 3.34 | P = 0.038 |
Significant brain activation clusters of the group comparisons during painful stimulation of the self (self-directed pain: control group > placebo group and self-directed pain: placebo group > control group) are given, including MNI coordinates, cluster size (k), t value, and P value (small volume-corrected, FWE-corrected, P < 0.05). Only the highest peak is included in the case of several peaks in a cluster.
To assess whether our study reliably activated those areas previously identified in empathy for pain studies using a conjunction analysis [(self-directed pain > self-directed no pain) ∩ (other-directed pain > other-directed no pain)] in the control group identified shared activations in the following areas: aMCC, bilateral AI, bilateral cerebellum, bilateral DLPFC, and bilateral SMA. See Table S3 for results. Notably, activation in the aMCC and bilateral AI extensively overlapped with meta-analytic activation clusters reported previously (1).
Table S3.
fMRI results (neural activation during empathy)
| Brain region | x | y | z | k | t value | P value |
| R SMA | 8 | 16 | 66 | 1,753 | 8.46 | P < 0.001 |
| L insula | −30 | 20 | −10 | 1,737 | 8.02 | P < 0.001 |
| R insula | 32 | 20 | −14 | 767 | 7.72 | P < 0.001 |
| R cerebellum | 42 | −60 | −30 | 2,412 | 7.48 | P < 0.001 |
| R caudate nucleus | 16 | 14 | 6 | 347 | 7.07 | P < 0.001 |
| L DLPFC | −24 | 54 | 30 | 227 | 6.93 | P < 0.001 |
| L MCC | −2 | −18 | 36 | 232 | 6.84 | P < 0.001 |
| PAG | −4 | −14 | −16 | 100 | 6.82 | P < 0.001 |
| L cerebellum | −42 | −66 | −26 | 844 | 6.81 | P < 0.001 |
| R middle temporal gyrus | 48 | −26 | −6 | 244 | 6.68 | P < 0.001 |
| L temporo-parietal junction | −58 | −44 | 28 | 90 | 6.47 | P < 0.001 |
| R primary motor cortex | 50 | 4 | 44 | 83 | 5.99 | P < 0.001 |
| R DLPFC | 18 | 56 | 34 | 69 | 5.98 | P < 0.001 |
| R temporo-parietal junction | 62 | −44 | 22 | 63 | 5.95 | P < 0.001 |
| L cerebellum | −6 | −80 | −22 | 28 | 5.90 | P < 0.001 |
| L cerebellum | −4 | −56 | −36 | 15 | 5.75 | P = 0.002 |
| L SMA | −44 | −2 | 44 | 22 | 5.67 | P = 0.001 |
| Thalamus | −4 | −18 | 10 | 67 | 5.57 | P < 0.001 |
Significant brain activation clusters during both painful stimulation of the self and painful stimulation of the other are given, including MNI coordinates, cluster size (k), t value, and P value (whole-brain conjunction analysis, self-directed pain > self-directed no pain) ∩ (other-directed pain > other-directed no pain, FWE-corrected, P < 0.05, k > 15). Only the highest peak is included in the case of several confluent peaks.
fMRI Data: Testing of Main Hypothesis.
The mixed-model ANOVA with the between-subjects factor group (control vs. placebo) and the within-subject factors ROI (left AI, right AI, aMCC), intensity (painful vs. nonpainful stimulation) and target (self- vs. other-directed stimulation) revealed a main effect of ROI [F(1,200) = 4.909, P = 0.008, partial-η2 = 0.047; stronger responses in the aMCC than in right AI, trend for stronger responses in the left AI than in the right AI], a main effect of intensity [F(1,100) = 187.478, P < 0.001, partial-η2 = 0.652; stronger response to painful compared with nonpainful stimulation], and a main effect of target [F(1,100) = 93.295, P < 0.001, partial-η2 = 0.483; stronger responses to self-directed compared with other-directed stimuli]. Crucially, there also was a significant intensity*group interaction [F(1,100) = 7.813, P = 0.006, partial-η2 = 0.072; see planned comparisons in the main text] and a nonsignificant target*intensity*group interaction (P = 0.521). Moreover, there was a significant target*intensity interaction [F(1,100) = 17.513, P < 0.001, partial-η2 = 0.149]. Because simple effects analyses were not informative about the directionality of this interaction (all pairwise-comparisons > 0.001), we computed paired t tests on the activation difference scores (painful – nonpainful), which revealed a higher difference for self-directed in comparison with other-directed stimuli [t(101) = 4.226, P < 0.001]. There were also significant interactions of target*ROI [F(1,200) = 13.430, P < 0.001, partial-η2 = 0.118, see follow-up analyses below] and target*intensity*ROI [F(1,200) = 3.621, P = 0.029, partial-η2 = 0.035, see follow-up analyses below]. Furthermore, we found nonsignificant effects of group and target*group (P = 0.103 and P = 0.626, respectively). The ROI*target*intensity*group interaction (P = 0.379) and all other interactions remained nonsignificant (all P values > 0.076). Because of the main effect and interactions with the ROI factor, we performed separate ANOVAs for each ROI.
Left AI ANOVA.
The ANOVA with the between-subjects factor group (control vs. placebo) and the within-subject factors intensity (painful vs. nonpainful stimulation) and target (self- vs. other-directed stimulation) for the left AI revealed a main effect of intensity [F(1,100) = 173.577, P < 0.001, partial-η2 = 0.634; stronger left AI response to painful compared with nonpainful stimulation)], and a main effect of target [F(1,100) = 42.859, P < 0.001, partial-η2 = 0.30; stronger responses to self-directed compared with other-directed stimuli]. Crucially, there also was a significant intensity*group interaction [F(1,100) =8.989, P = 0.003, partial-η2 = 0.082; see planned comparisons in the main text], and a nonsignificant target*intensity*group interaction (P = 0.781). Moreover, there was a significant target*intensity interaction [F(1,100) =10.499, P = 0.002, partial-η2 = 0.095]. As simple effects analyses were not informative about the directionality of this interaction (all pairwise-comparisons > 0.001), we computed paired t tests on the activation difference scores (painful – nonpainful), which revealed a higher difference for self-directed in comparison with other-directed stimuli [t(101) = 3.269, P = 0.001]. There were no significant effects of group and target*group (P = 0.351 and P = 0.999, respectively).
aMCC ANOVA.
The ANOVA revealed a main effect of intensity [F(1,100) = 152.226, P < 0.001, partial-η2 = 0.604; stronger aMCC response to painful compared with nonpainful stimulation] and a main effect of target [F(1,100) = 65.055, P < 0.001, partial-η2 = 0.394; stronger aMCC response to self-directed compared with other-directed stimuli]. Again, the ANOVA also revealed a significant intensity*group interaction [F(1,100) = 7.229, P = 0.008, partial-η2 = 0.067; see planned comparisons in the main text], and a nonsignificant target*intensity*group three-way interaction (P = 0.747). Moreover, there was a significant target*intensity interaction [F(1,100) = 14.518, P < 0.001, partial-η2 = 0.127]. As simple effects analyses were not informative about the directionality of this interaction (all pairwise-comparisons > 0.001), we computed paired t tests on the activation difference scores (painful – nonpainful), which revealed a higher difference for self-directed in comparison with other-directed stimuli [t(101) = 3.843, P < 0.001]. The target*group interaction remained nonsignificant (P = 0.432). There was a main effect of group [F(1,100) = 4.017, P = 0.048, partial-η2 = 0.039] with higher activation in the control compared with the placebo group.
Right AI ANOVA.
The ANOVA revealed a main effect of intensity [F(1,100) =166.064, P < 0.001, partial-η2 = 0.624; stronger right AI response to painful compared with nonpainful stimulation] and a main effect of target [F(1,100) =163.760, P < 0.001, partial-η2 = 0.621; stronger responses to self-directed compared with other-directed stimuli]. We also observed a significant intensity*group interaction [F(1,100) = 4.506, P = 0.036, partial-η2 = 0.043; see planned comparisons in the main text] as well as a nonsignificant target*intensity*group three-way interaction (P = 0.193). Moreover, there was a significant target*intensity interaction [F(1,100) = 21.931, P < 0.001, partial-η2 = 0.180]. As simple effects analyses were not informative about the directionality of this interaction (all pairwise-comparisons > 0.001), we computed paired t tests on the activation difference scores (painful – nonpainful), which revealed a higher difference for self-directed in comparison with other-directed stimuli [t(101) = 4.721, P < 0.001]. The target*group interaction and main effect of group remained nonsignificant (P = 0.589 and P = 0.139, respectively).
Questionnaires.
Neither trait empathy measures (all P values > 0.105) nor postexperimental ratings (all P values > 0.175) showed any significant group differences.
Effectiveness Ratings.
Expectation of effectiveness of the medication had to be rated between 1 (“not at all”) and 6 (“very effective”). Mean ratings were 4.39 (SD: 1.151) after administration of the placebo pill (Question T1: “Do you expect this medication to be effective in reducing your pain?”) and 4.76 (SD: 1.407) after the placebo analgesia conditioning procedure (Question T2: “How effective is this medication?”). The conditioning procedure significantly increased the effectiveness ratings [t(48) = 2.312, P = 0.025].
Additional Results of Psychopharmacological Experiment
Ratings.
The mixed-model ANOVA of pain ratings revealed a main effect of intensity [F(1,48) = 784.587, P < 0.001, partial-η2 = 0.942; indicating higher ratings for painful than for nonpainful stimuli], and a main effect of target [F(1,48) = 6.878, P < 0.001, partial-η2 = 0.294; with higher ratings in the self-directed compared with the other-directed conditions]. We also observed a significant intensity*group interaction [F(1,48) = 7.211, P = 0.010, partial-η2 = 0.131; see planned comparisons in the main text] as well as a significant target*group interaction [F(1,48) = 6.371, P = 0.015, partial-η2 = 0.117; with simple effects analyses revealing significantly higher self-directed compared with other-directed ratings in placebo-placebo (P < 0.001), but not in the placebo-naltrexone group (P = 0.174), whereas all other comparisons were nonsignificant (all P values > 0.070)]. The target*intensity interaction (P = 0.315) and the target*intensity*group 3-way interaction (P = 0.431) were far from reaching significance. There was no main effect of group (P = 0.579).
Analysis of the unpleasantness ratings revealed a similar pattern of results. The ANOVA showed a main effect of intensity [F(1,48) = 202.485, P < 0.001, partial-η2 = 0.808; higher empathy ratings for painful compared with nonpainful stimuli] and a significant intensity*group interaction [F(1,48) = 4.754, P = 0.034, partial-η2 = 0.090; see planned comparisons in the main text]. No main effect of group was observed (P = 0.499).
Questionnaires.
Neither the independent-samples t test on trait empathy measures differences (all P values > 0.183) nor the t tests on postexperimental ratings of self-perceived similarity, affiliation with the other person and attributed strength (all P values > 0.270) showed any significant group differences. The t test on liking showed a trend for a group difference (P = 0.085; higher values in placebo-placebo group). The t tests on neediness [t(1,48) = 2.656, P = 0.011, Cohen’s d = 0.77; higher values in placebo-naltrexone group] and agreeableness [t(1,48) = 2.518, P = 0.015, Cohen’s d = 0.72, higher values in placebo-placebo group] showed significant group differences.
Effectiveness Ratings.
Expectation of effectiveness of the medication had to be rated between 1 (“not at all”) and 6 (“very effective”). Mean ratings were 4.04 (SD: 1.129; placebo-placebo group: 4.12 ± 1.166; placebo-naltrexone group: 3.96 ± 1.108; no significant difference P = 0.621) after delivery of the pill (“Do you expect this medication to be effective in reducing your pain?”) and 4.70 (SD: 1.31; placebo-placebo group: 4.60 ± 1.384; placebo-naltrexone group: 4.80 ± 1.258; no significant difference P = 0.595) after the placebo analgesia conditioning (“How effective is this medication?”). The conditioning procedure significantly increased the effectiveness ratings [t(49) = 3.148, P = 0.003].
Comparison Between Experiments.
Comparison of ratings in the two experiments showed no significant differences between the placebo group in the fMRI and the placebo-placebo group in the psychopharmacological experiment [self-directed pain: t(72) = −0.954, P = 0.343; other-directed pain: t(72) = 0.192, P = 0.848; unpleasantness: t(72) = 1.049, P = 0.298]. To compare the size of the group effects between the experiments a generalized linear regression model using the method of weighted least squares was used, because of inhomogeneity of variances between the ratings in experiment 1 and 2 (which has different sample sizes). As a hypothesis specific contrast, it was tested whether the difference (E1 − E2) of the mean difference in experiment 1 (E1 = control group − placebo group) and 2 (E2 = placebo-naltrexone group − placebo-placebo group) differed from zero, using an F-test. For each rating, this contrast was not significant: self-directed pain: estimated difference = 0.397, 95% confidence interval (CI) (−1.546, 2.340), F(1,148) = 1.036, P = 0.311; other-directed pain: estimated difference = −0.216, 95% CI (−2.442, 2.010), F(1,148) = 0.250, P = 0.618; unpleasantness: estimated difference = −0.296, 95% CI (−2.504, 1.913), F(1,148) = 0.468, P = 0.495. The comparison between ratings of the control group in the fMRI and the placebo-naltrexone group in the psychopharmacological experiment showed no significant differences either [self-directed pain: t(62.9) = 0.391, P = 0.697; other-directed pain: t(76) = −0.534, P = 0.595; unpleasantness: t(76) = 0.115, P = 0.909].
Nonresponder Identification
We used a combination of three measures to identify and exclude nonresponders. First, and most importantly, doubts expressed about the analgesic effects of the medication or about pain medication in general (such as “usually I don’t respond well to pain killers”) were recorded. Second, differences of the belief scores about the effectiveness of the placebo before and after the placebo induction procedure were analyzed. Exceptionally low total belief scores (sum of both measures <6, on the scale ranging from 1 = “not effective at all” to 7 = “very effective”) and strong decreases between first and second measure (>3) indicated a lack of responding. Third, we took the number of placebo conditioning trials into account: If participants responded with “6” (i.e., “extremely painful, but bearable”) to the conditioning stimulus (delivered at an intensity of “4”), we deemed the conditioning trial as nonsuccessful, told participants to wait another 5 min for the medication to take effect, and then tried again. This was carried out until participants did not respond with “6” to the conditioning stimulus anymore. If three or more such repetitions were necessary, this indicated that the placebo pill had not worked very well (considering that the majority of participants showed analgesic responses after the first trial).
fMRI Experiment.
Six of the 10 excluded participants of the placebo group met criterion 1 (doubts); one subject met all three criteria (doubts; belief score sum 5; three conditioning trials). The remaining three subjects met criteria 1 and 3 (doubts, and number of conditioning trials = 3). Notably, the proportion of nonresponders (16.7%) did not exceed the proportion in other studies (see for example refs. 15, 19, and 54).
Psychopharmacological Experiment.
All of the five excluded subjects in this experiment met criterion 1 (doubts). One of the subjects additionally met criterion 2 (sum of belief scores: 4). The proportion of nonresponders was 9% in this case, which was not significantly lower than in the fMRI experiment (z-test, z = 1.240, P = 0.215).
Acknowledgments
We thank Daniel Graf, Bernadette Hippmann, Julia Hebestreit, Alexander Kudrna, and Andreas Martin (all University of Vienna) for assistance with functional MRI measurements; Fritz Zimprich (Medical University of Vienna) for medical support; Stanislav Katina (Masaryk University Brno) for help with statistical analysis of behavioral data; Andreas Gartus (University of Vienna) for technical support; and two anonymous reviewers for helpful comments on our work. This study was supported by the Vienna Science and Technology Fund (WWTF; Project CS11-016).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.I.E. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511269112/-/DCSupplemental.
References
- 1.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Bastiaansen JA, Thioux M, Keysers C. Evidence for mirror systems in emotions. Philos Trans R Soc Lond B Biol Sci. 2009;364(1528):2391–2404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decety J. To what extent is the experience of empathy mediated by shared neural circuits? Emot Rev. 2010;2(3):204–207. [Google Scholar]
- 5.Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- 6.Lamm C, Majdandžić J. The role of shared neural activations, mirror neurons, and morality in empathy—A critical comment. Neurosci Res. 2015;90:15–24. doi: 10.1016/j.neures.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberger NI. Social pain and the brain: Controversies, questions, and where to go from here. Annu Rev Psychol. 2015;66:601–629. doi: 10.1146/annurev-psych-010213-115146. [DOI] [PubMed] [Google Scholar]
- 8.Shackman AJ, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol. 2011;93(1):111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Woo CW, et al. Separate neural representations for physical pain and social rejection. Nat Commun. 2014;5:5380. doi: 10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corradi-Dell’Acqua C, Hofstetter C, Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. J Neurosci. 2011;31(49):17996–18006. doi: 10.1523/JNEUROSCI.2686-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iannetti GD, Salomons TV, Moayedi M, Mouraux A, Davis KD. Beyond metaphor: Contrasting mechanisms of social and physical pain. Trends Cogn Sci. 2013;17(8):371–378. doi: 10.1016/j.tics.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Rütgen M, Seidel E-M, Riečanský I, Lamm C. Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first-hand emotion experience. J Neurosci. 2015;35(23):8938–8947. doi: 10.1523/JNEUROSCI.3936-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25(45):10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zubieta JK, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atlas LY, Wager TD. How expectations shape pain. Neurosci Lett. 2012;520(2):140–148. doi: 10.1016/j.neulet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Baumgärtner U, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30(3):692–699. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Wager TD, Scott DJ, Zubieta J-K. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—Imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 20.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5(7):565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 21.Bartolomeo P. The neural correlates of visual mental imagery: An ongoing debate. Cortex. 2008;44(2):107–108. doi: 10.1016/j.cortex.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Büchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: A predictive coding perspective. Neuron. 2014;81(6):1223–1239. doi: 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Eippert F, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2013;34(3):738–752. doi: 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16(11):1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 27.Zubieta JK, Yau WY, Scott DJ, Stohler CS. Belief or need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behav Immun. 2006;20(1):15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Goldman A. Simulating Minds: The Philosophy, Psychology and Neuroscience of Mindreading. Oxford Univ Press; New York: 2006. [Google Scholar]
- 29.Mitchell JP. Inferences about mental states. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hillis AE. Inability to empathize: Brain lesions that disrupt sharing and understanding another’s emotions. Brain. 2014;137(Pt 4):981–997. doi: 10.1093/brain/awt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu X, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135(Pt 9):2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison I, et al. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain. 2011;134(Pt 4):1116–1126. doi: 10.1093/brain/awr011. [DOI] [PubMed] [Google Scholar]
- 33.Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61(2):203–212. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Jackson PL, Rainville P, Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125(1-2):5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 36.Benedetti F, Amanzio M. Mechanisms of the placebo response. Pulm Pharmacol Ther. 2013;26(5):520–523. doi: 10.1016/j.pupt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Qin S, Guo J, Luo J. A follow-up fMRI study of a transferable placebo anxiolytic effect. Psychophysiology. 2011;48(8):1119–1128. doi: 10.1111/j.1469-8986.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 38.Ellingsen DM, et al. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc Natl Acad Sci USA. 2013;110(44):17993–17998. doi: 10.1073/pnas.1305050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovic P, et al. Placebo in emotional processing—Induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 41.Silani G, Lamm C, Ruff CC, Singer T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J Neurosci. 2013;33(39):15466–15476. doi: 10.1523/JNEUROSCI.1488-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinbeis N, Bernhardt BC, Singer T. Age-related differences in function and structure of rSMG and reduced functional connectivity with DLPFC explains heightened emotional egocentricity bias in childhood. Soc Cogn Affect Neurosci. 2015;10(2):302–310. doi: 10.1093/scan/nsu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaki J, Bolger N, Ochsner K. It takes two: The interpersonal nature of empathic accuracy. Psychol Sci. 2008;19(4):399–404. doi: 10.1111/j.1467-9280.2008.02099.x. [DOI] [PubMed] [Google Scholar]
- 44.Seidel EM, et al. Uncertainty during pain anticipation: The adaptive value of preparatory processes. Hum Brain Mapp. 2015;36(2):744–755. doi: 10.1002/hbm.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donaldson DI, Buckner RL. Effective paradigm design. In: Jezzard P, Mathews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford Univ Press; Oxford: 2001. pp. 177–195. [Google Scholar]
- 46.Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17(1):18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- 47.Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44(1):113–126. [Google Scholar]
- 48.Doherty RW. The emotional contagion scale: A measure of individual differences. J Nonverbal Behav. 1997;21(2):131–154. [Google Scholar]
- 49.Chan SCC, Chan CCH, Kwan ASK, Ting KH, Chui TY. Orienting attention modulates pain perception: An ERP study. PLoS One. 2012;7(6):e40215. doi: 10.1371/journal.pone.0040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waber RL, Shiv B, Carmon Z, Ariely D. Commercial features of placebo and therapeutic efficacy. JAMA. 2008;299(9):1016–1017. doi: 10.1001/jama.299.9.1016. [DOI] [PubMed] [Google Scholar]
- 51.Price DD, et al. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83(2):147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 52.Derbyshire SWG. Exploring the pain “neuromatrix”. Curr Rev Pain. 2000;4(6):467–477. doi: 10.1007/s11916-000-0071-x. [DOI] [PubMed] [Google Scholar]
- 53.Geuter S, Eippert F, Hindi Attar C, Büchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 2013;67:227–236. doi: 10.1016/j.neuroimage.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wager TD, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 55.Bingel U, Schoell E, Herken W, Büchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131(1-2):21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Eippert F, Bingel U, Schoell E, Yacubian J, Büchel C. Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. J Neurosci. 2008;28(21):5465–5472. doi: 10.1523/JNEUROSCI.5336-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Büchel C, et al. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22(3):970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: Contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31(2):439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]