Abstract
Apolipoproteins of the L family are lipid-binding proteins whose function is largely unknown. Apolipoprotein L1 and apolipoprotein L6 have been recently described as novel pro-death BH3-only proteins that are also capable of regulating autophagy. In an in-silico screening to discover novel putative BH3-only proteins, we identified yet another member of the apolipoprotein L family, apolipoprotein L2 (ApoL2), as a BH3 motif-containing protein. ApoL2 has been suggested to behave as a BH3-only protein and mediate cell death induced by interferon-gamma or viral infection. As previously described, we observed that ApoL2 protein was induced by interferon-gamma. However, knocking down its expression in HeLa cells did not regulate cell death induced by interferon-gamma. Overexpression of ApoL2 did not induce cell death on its own. ApoL2 did not sensitize or protect cells from overexpression of the BH3-only proteins Bmf or Noxa. Furthermore, siRNA against ApoL2 did not alter sensitivity to a variety of death stimuli. We could, however, detect a weak interaction between ApoL2 and Bcl-2 by immunoprecipitation of the former, suggesting a role of ApoL2 in a Bcl-2-regulated process like autophagy. However, in contrast to what has been described about its homologs ApoL1 and ApoL6, ApoL2 did not regulate autophagy. Thus, the role, if any, of ApoL2 in cell death remains to be clarified.
Keywords: apolipoproteins L, Bcl-2 family proteins, BH3-only, interferon-gamma, autophagy
Bcl-2 family proteins regulate mitochondrial permeability to control apoptosis. These proteins induce or inhibit cell death, and they are associated with a growing number of pathologies, including cancer and immune diseases.1, 2 For this reason, the search of new members of this family is of crucial importance. A subfamily of Bcl-2 homologs termed ‘BH3-only proteins' comprises a growing number of proteins that only share a small motif of 15–21 amino acid residues.3, 4 This region, known as the ‘BH3-domain', is essential for the apoptotic function of Bcl-2 family proteins. The homology in this region is relatively loose, and only a few residues are conserved among the members of the family. For this reason, BH3-only proteins have been identified by functional means rather than by sequence homology.
To identify novel BH3-only proteins we used a bioinformatics approach known as profile-based homology search. In brief, we constructed a so-called Hidden Markov Model (HMM) of the BH3-domain from the alignment of a set of proteins known to bear this domain. This HMM describes the probabilities of finding a given amino acid at a given position of the domain. This probabilistic model is then used to search in a sequence database for proteins that are likely to encode the same domain.
One of the proteins identified by this method was the apolipoprotein L2 (ApoL2). Two other members of this family, ApoL1 and ApoL6 have been described to behave as proapoptotic BH3-only proteins.5, 6, 7 Although the functions of these proteins are still unclear, proteins of this family have been shown to bind lipids and they have been suggested to work as pore-forming proteins in intracellular membranes, based on the ability of ApoL1 to form pores in the lysosomal membrane of trypanosomes.8, 9 ApoL2 is highly homologous to ApoL1, and its BH3-like domain is very similar to those of ApoL1 and ApoL6. For these reasons, we explored the function of ApoL2 as a putative new BH3-only protein.
Results
Identification of novel BH3-containing proteins by using a profile-based homology search
Profile-based searches with profiles of BH3 domains as defined in ProSite10 and PFAM,11 as well as regular expression searches with motifs defined in the literature failed to provide satisfactory results in terms of specificity and sensitivity of detecting known human BH3 proteins (Table 1). Therefore, to efficiently identify novel putative BH3-only proteins, we collected the sequences of all human and mouse BH3 motifs annotated in Uniprot as well as those described in the literature, and aligned them to subsequently build an HMM for the BH3-domain (see Materials and Methods) (Figure 1a). This HMM provided better results in finding known BH3 than existing profiles at Pfam (Table 1), and was therefore used to search for putative novel BH3-containing proteins in the human proteome and genome.
Table 1. Summary of the results obtained from direct motif searches in the human proteome when using different strategies.
| Motif search | Hits in human proteome | Known BCL's (TP) | Sensitivity (TP/TP+FN) |
|---|---|---|---|
| 1. Youle et al.4 | 5908 | 13 | 68.4% |
| 2. Liu et al.7 | 152 | 16 | 84.2% |
| 3. Prosite | 28 | 9 | 47.36% |
| 4. Novel HMM | 26 | 19 | 100% |
First column indicates the search strategy: using a direct search with (1) The consensus BH3 motif (LXXXGD) as defined in Youle et al.4 (2) The extended motif (LXXX[GAS][DE]) used by Liu et al.7 in their identification of Apol6; (3) The motif defined by Prosite10 as of December 2008; and (4) an HMM-based search with the profile derived in this work. The following columns indicate, respectively, the number of total hits in the human proteome (Ensembl42 version), the number of known Bcl-2 family members identified of a total of 19 human members described in Youle et al.4 and Uniprot (2008), and, finally the sensitivity of the search as computed by dividing the total number of correctly identified Bcl-2 family members (TP) by the total number of known Bcl-2 family members (TP+FN=19). TP and FN stand for True Positives and False Negatives, respectively
Figure 1.
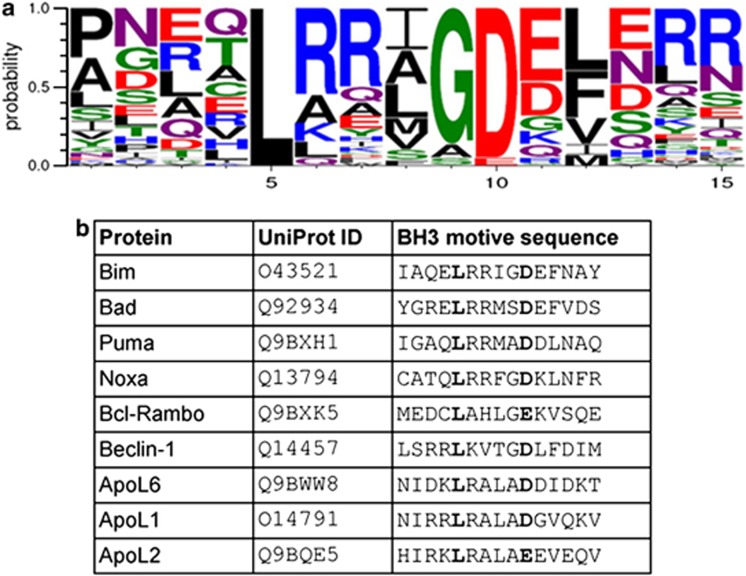
ApoL2 contains a BH3-like motif. (a) Logo representation of the protein profile used in the search for new BH3-domain proteins. The logo indicates the probability of finding a given amino acid at each of the 15 positions of the BH3-domain. Amino acids are represented by the one-letter code, and their height is proportional to the probability of appearing at a given position in the BH3-domain. (b) Alignment of ApoL2 BH3 motif with other BH3 motifs
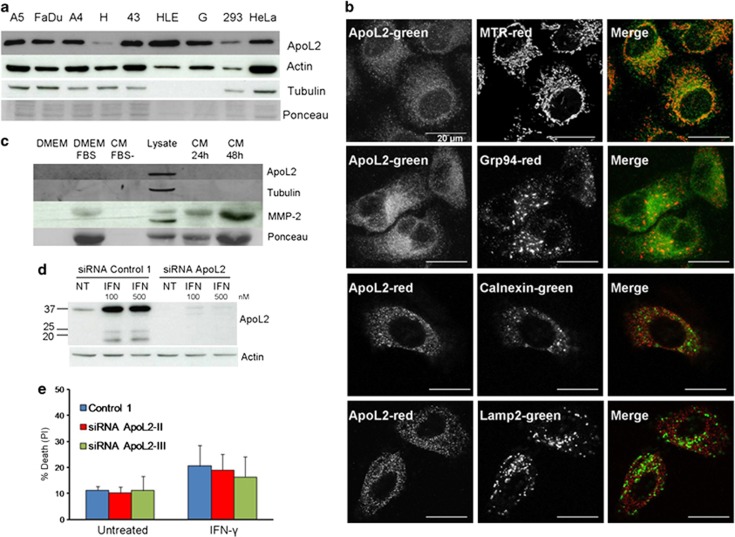
Our screening identified BFK, a known Bcl-2 homolog originally left out from the list of proteins used to build the model,12 and PXT1, a protein that has been recently described as a BH3-only protein that kills HeLa cells in a manner dependent of its BH3 motif13 (Table 2). Another protein identified by this screening was the apolipoprotein L2 protein (Figure 1b, Table 2). Two apolipoproteins of the L family, ApoL1 and ApoL6, have been previously identified as BH3-only proteins.5, 6, 7 ApoL2, due to its homology with ApoL1 and ApoL6 has indeed been proposed to be a BH3-only protein.14 ApoL2 mRNA is ubiquitously expressed, according to the database IST Online (Supplementary Figure 1). We checked that this protein is expressed in a variety of cell lines of different origins (Figure 2a), and highly expressed in HeLa cervical cancer cell line, as predicted due to its high expression in cervical cancer.15 ApoL2 is localized in HeLa cells outside the nucleus in a punctate state (Figure 2b) and it is not secreted (Figure 2c). Although it had been predicted to interact with membranes,16 we observed that it did not colocalize with mitochondrial, endoplasmic reticulum or lysosomal markers (Figure 2b).
Table 2. Summary of the putative BH3-only proteins predicted using HMM.
| Name | UniProt ID | NM ID | Amino acid number | Ensembl ID |
|---|---|---|---|---|
| Apolipoprotein L2 (ApoL2) | Q9BQE5 | NM_030882.2 and NM_145637.1 | 337 | ENSG00000128335 |
| Arf-GAP with coiled-coil, ANK repeat and PH domain-containing protein 3 (ACAP3) | Q96P50 | NM_030649.2 | 834 | ENSG00000131584 |
| GDP-fucose protein O-fucosyltransferase 2 (POFUT2) | Q9Y2G5 | NM_015227.4 | 429 | ENSG00000186866 |
| Phenylalanine-4-hydroxylase(PHA) | P00439 | NM_000277.1 | 452 | ENSG00000171759 |
| Peroxisomal testis-specific protein 1 (PXT1) | J3KR74 | NM_152990.3 | 134 | ENSG00000179165 |
| Uncharacterized protein C19orf55 (C19orf55) | Q2NL68 | NM_001039887 | 480 (putative) | ENSG00000167595 |
The table shows the different identification codes of the gene from the major databases. Uniprot ID (http://www.uniprot.org/), NM ID (http://www.ncbi.nlm.nih.gov/) and Ensembl ID (http://www.ensembl.org/) as well as the number of amino acids of the protein
Figure 2.
Cytosolic ApoL2 is widely expressed in different cell lines and induced by interferon-gamma. (a) ApoL2 expression was tested in different cell lines by western blot: A549 (A5), FaDu, A-431 (A4), HCT116 (H), 435P (43), HLE, HepG2 (G), HEK293 (293) and HeLa cells. (b) Intracellular localization of ApoL2. HeLa cells were stained with MitoTracker red (MTR) as mitochondrial marker. Antibodies against Grp94 and Calnexin were used as endoplasmic reticulum markers. Lysosomal localization was studied using Lamp-2 antibody. Scale bars of 20 μm are shown. (c) ApoL2 is not secreted. Western blot of trichloroacetic acid (TCA)-concentrated medium or cell lysate is shown. DMEM and DMEM complemented with FBS were used as controls. CM FBS−: conditioned medium of HeLa cells grown for 48 h in DMEM without FBS. Lysate: cell lysate of HeLa cells grown in FBS containing medium for 48 h. CM: conditioned medium of HeLa cells grown in FBS containing medium for indicated times. Antibodies against ApoL2, tubulin, actin and the secreted protein metalloproteinase-2 (MMP-2) were used for immunoblotting. (d) HeLa cells were transfected with siRNA ApoL2-II, treated with interferon-gamma (IFN) at 100 or 500 nM for 24 h and collected for western blot. NT means non treated. (e) HeLa cells were transfected with siRNA control 1 or siRNA against ApoL2 and treated with IFN-γ 100 nM for 72 h. Cell death was measured by PI incorporation at the flow cytometer. Figure shows average and S.E.M. of three independent experiments
ApoL2 is transcriptionally induced by interferon-gamma in a number of non-transformed tissues.14 In human bronchial epithelial cells its downregulation sensitized cells to cell death induced by IFN-γ, indicating that ApoL2 is an antiapoptotic protein in this context.14 We observed that in HeLa cells IFN-γ induces ApoL2 (Figure 2d). However, when we downregulated ApoL2 using two different silencing sequences, we could not observe sensitization to cell death (Figure 2e).
ApoL2 is not a proapoptotic BH3-only protein
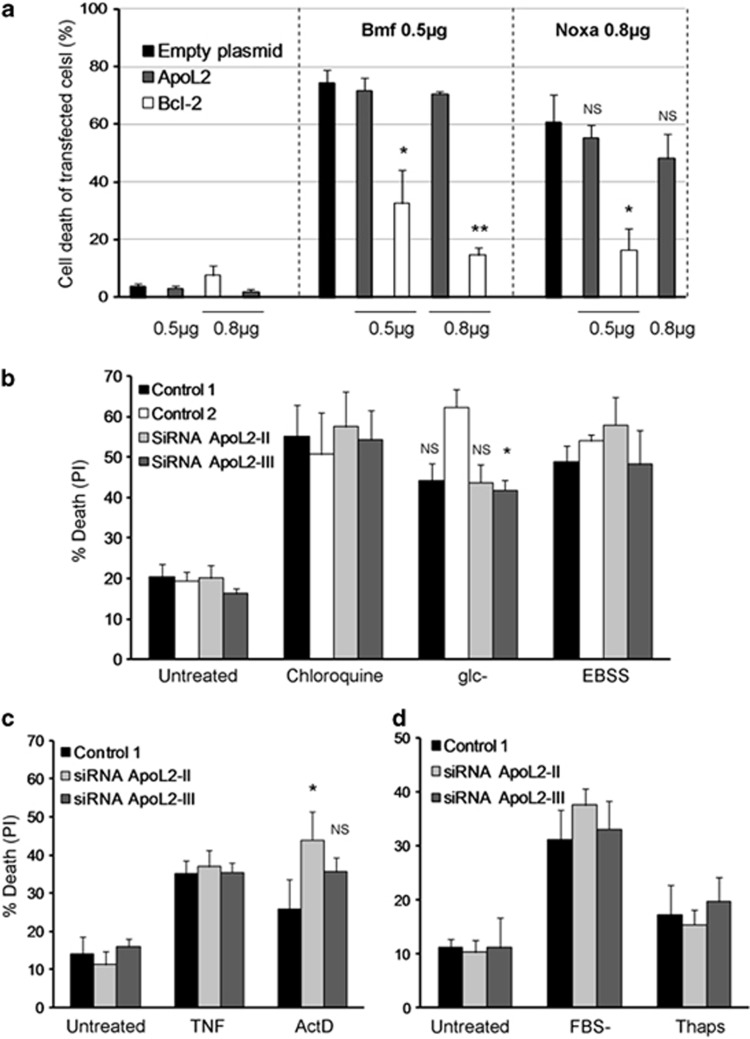
To check whether ApoL2 behaves as a proapoptotic Bcl-2 family member, we overexpressed ApoL2 in HeLa cells. Overexpression was confirmed by immunofluorescence (Supplementary Figure 2) and western blot (Supplementary Figure 3). We used Noxa and Bmf as proapoptotic BH3-only proteins, and verified that these proteins killed HeLa cells (Figure 3a). However, ApoL2 did not. We observed a trend of lower background death in cells overexpressing ApoL2, suggesting that ApoL2 is an antiapoptotic Bcl-2 family protein. To test this we overexpressed ApoL2 in combination with Noxa or Bmf. Our results indicate that ApoL2 confers a minor protection from Noxa (Figure 3a). However, this did not reach statistical significance (n=3). Bcl-2 was employed as a control (expression checked in Supplementary Figure 3) and it protected from Noxa and Bmf.
Figure 3.
ApoL2 does not regulate cell death of HeLa cells. (a) Plasmids encoding two different BH3-only proteins (0.5 μg of Bmf and 0.8 μg of Noxa plasmids) were cotransfected with ApoL2 or Bcl-2 and analyzed by microscopy. GFP (0.3 μg) was used as transfection marker. ApoL2 and Bcl-2 plasmids were used at amounts shown. Empty plasmid was used to normalize the amount of transfected DNA. Dead green cells were scored by shrunk morphology and counted from images using fluorescence microscopy. Figure shows average and S.E.M. of three experiments. For statistical analysis, each ApoL2 or Bcl-2 overexpressing condition has been compared with the empty plasmid condition transfected with the same BH3-only protein. NS, nonsignificant. (b, c, d). HeLa cells were transfected with different control siRNAs or siRNA against ApoL2 and then treated with chloroquine, deprived of glucose (glc−), incubated in starvation buffer (EBSS) or treated with tumor necrosis factor (TNF) or actinomycin D (ActD) for 24 h (b, c), or deprived of serum (FBS−) or treated with thapsigargin (Thaps) for 72 h (d). Cell death was measured by PI incorporation by flow cytometry. Figure shows average and S.E.M. of three (d) or five experiments (b, c). Asterisks or NS (nonsignificant) denote significance versus Control 2 (b) or Control 1 (c)
Next we analyzed whether ApoL2 would regulate cell death induced by a variety of stimuli, either by behaving as an antiapoptotic protein as described14 or as a proapoptotic BH3-only protein like ApoL1 and ApoL6. We knocked down ApoL2 using different siRNA sequences and treated HeLa cells with the endoplasmic reticulum stressor thapsigargin, the DNA damaging agent actinomycin D, the lysosomal inhibitor chloroquine, or starvation of serum, glucose or serum/amino acid/vitamins (culture in EBSS buffer) (Figures 3b–d). We only observed a minor difference in cell death induced by actinomycin D that was significant when cells were depleted of ApoL2 using one siRNA oligo but not the second one. ApoL2 has been shown to be induced by TNF.6 We did not observe induction of ApoL2 upon TNF treatment in HeLa or 293T cells (Supplementary Figure 4). In addition, we treated HeLa cells with TNF in the presence of cycloheximide to induce cell death, and we did not observe any difference when ApoL2 was silenced (Figure 3c).
ApoL2 interacts weakly with Bcl-2 but it does not regulate autophagy
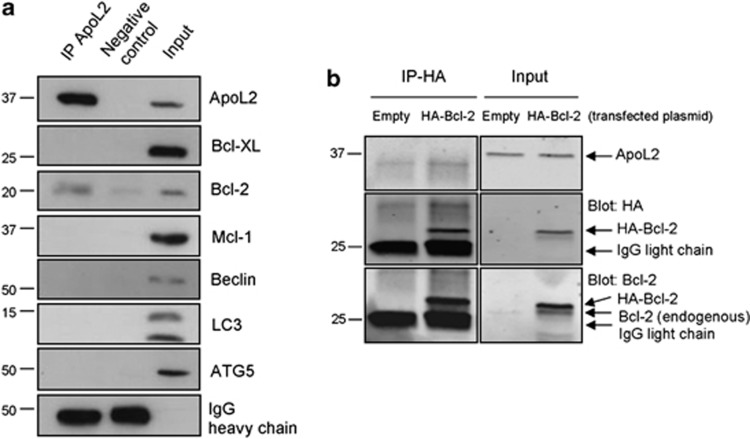
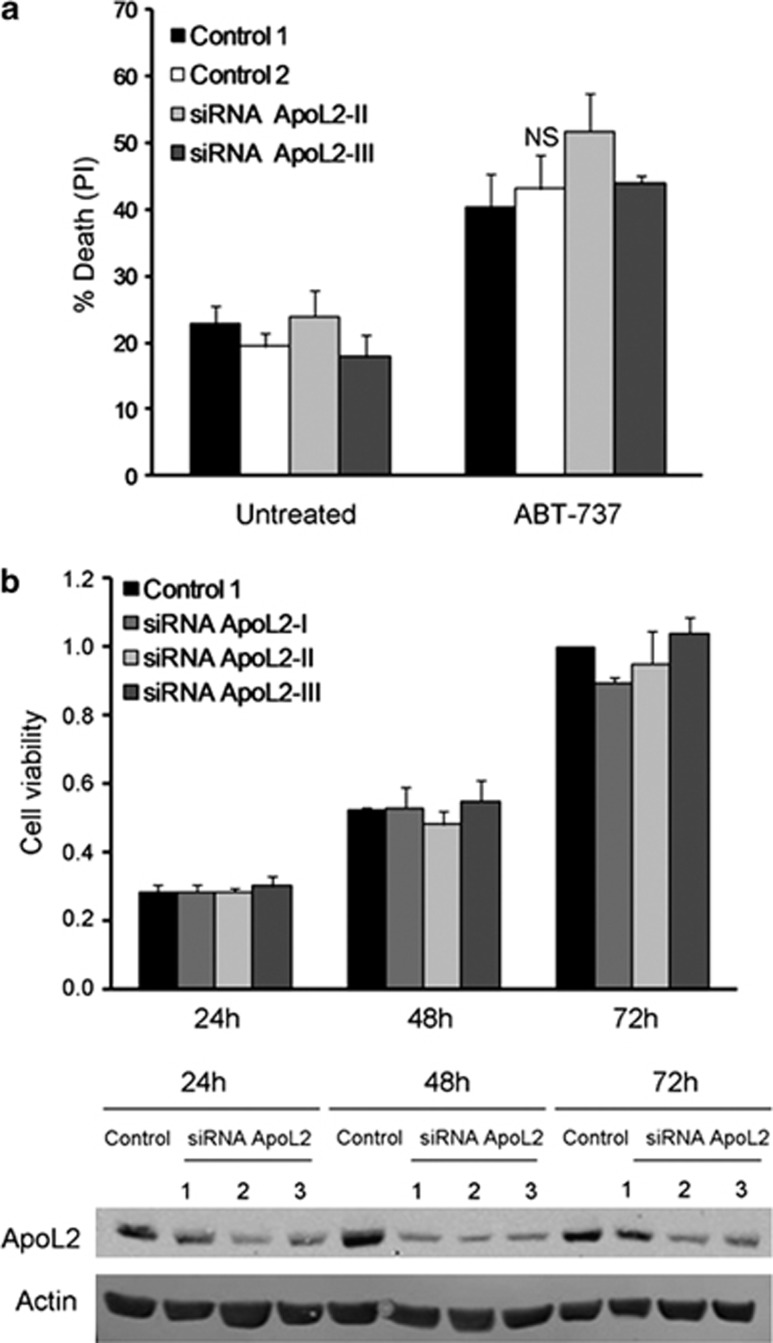
We could not detect a role of ApoL2 in cell death. However, not all BH3-only proteins described to date regulate cell death. Some proteins like Beclin-1 regulate autophagy through its interaction with Bcl-2 family proteins. ApoL6, which induces cell death and inhibits autophagy, has been shown to bind Bcl-xL.17 We thus tested whether endogenous ApoL2 interacted with other BH3-containing proteins. We immunoprecipitated ApoL2 and blotted for multidomain Bcl-2 family proteins. Bcl-2 was reproducibly immunoprecipitated with ApoL2 (Figure 4a). We were unable to immunoprecipitate endogenous Bcl-2 under the same conditions (not shown). For these reasons, to confirm these interactions in a different manner we overexpressed HA-tagged Bcl-2.18 Under these conditions, we were unable to immunoprecipitate ApoL2 with anti-HA antibody (Figure 4b) or to detect HA upon immunoprecipitation of ApoL2, neither in HeLa nor in 293T cells (not shown). We next checked whether the weak interaction between ApoL2 and Bcl-2 (detected only using endogenous proteins) would alter the sensitivity of HeLa cells to the Bcl-2 and Bcl-xL inhibitor ABT-737. Downregulation of ApoL2 did not alter the amount of cell death induced by ABT-737 (Figure 5a).
Figure 4.
Immunoprecipitation of ApoL2 in HeLa cells. (a) Endogenous ApoL2 was immunoprecipitated (IP) and the presence of the indicated proteins was assayed by western blot. Blots from a single experiment representative of three independent experiments are shown. (b) HeLa cells were transfected with HA-Bcl-2 or empty vector. Anti-HA was used for immunoprecipitation and the presence of ApoL2, Bcl-2 and HA was assayed by western blot. Panel shown is representative of three independent experiments. Left and right panels were cropped from the same films
Figure 5.
ApoL2 does not regulate cell proliferation or sensitivity to ABT-737. (a) HeLa cells were transfected with control siRNA or siRNA targeting ApoL2 and then they were treated with the BH3 mimetic ABT-737 at 30 μM for 24 h. Cell death was measured by PI incorporation and flow cytometry. Panel shows average and S.E.M. of five independent experiments. (b) HeLa cells were transfected with control 1 siRNA or siRNAs against ApoL2 and growth analysis was performed at indicated time points by crystal violet coloration. The lower panel shows western blot analysis of ApoL2 silencing over time
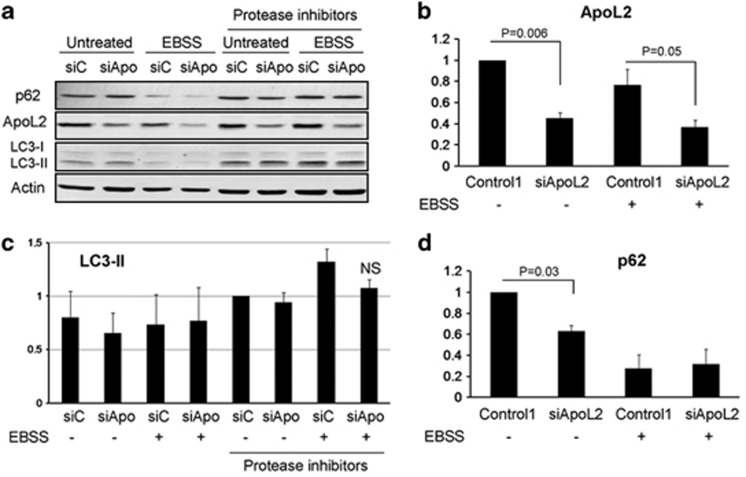
One possibility is that ApoL2, by interacting with Bcl-2 or signaling lipids, would regulate cell proliferation. We tested this and could not observe any effects on cellular proliferation by downregulation of ApoL2 (Figure 5b). We next investigated whether ApoL2 could act like Beclin-1 or ApoL6 regulating autophagy.17 We downregulated ApoL2 (Figures 6a and b) and measured basal autophagy (lipidation of LC3 and degradation of p62 in the presence or absence of protease inhibitors) and starvation-induced autophagy (same measurements after incubation in starvation buffer EBSS). Our results indicate that ApoL2 does not alter basal or starvation-induced autophagic flux as measured by levels of LC3-II (Figures 6a and c). We did observe a significant reduction of p62 levels after ApoL2 was downregulated, suggesting that this protein regulates basal autophagy (Figures 6a and d), but this was not accompanied by a difference in levels of LC3-II at these conditions (Figure 6c).
Figure 6.
ApoL2 does not regulate autophagy. HeLa cells were transfected with siRNA Control 1 (labeled as siC) or siRNA against ApoL2-II (labeled as siApo) for 48 h and then the medium was changed or they were incubated with EBSS for 6 h to induce autophagy. The protease inhibitors pepstatin and E64D (10 μM each) were used to block autophagic flux. A representative western blot is shown in a. ApoL2 levels were quantified and are shown in b: data were weighted to ponceau or actin and then normalized against Control 1-transfected untreated cells. (c) Quantification of relative LC3-II levels: data were weighted to ponceau or actin and then normalized against Control 1-transfected HeLa cells with protease inhibitors as control of basal autophagy. (d) Quantification of relative p62 levels: data were weighted to ponceau or actin and then normalized against Control 1-transfected untreated cells. Graphs show average and S.D. of three independent experiments
Altogether, our data indicate that ApoL2 is not a classical BH3-only protein, and its exact function in cell death by interferon treatment remains to be determined.
Discussion
BH3-only proteins do not share a high degree of homology between them, and it is possible that the BH3-domain arose either randomly during evolution or by a process of convergent evolution.19 Moreover, the BH3-domain is not extremely well conserved even among Bcl-2 family proteins that share more domains than the BH3.3 Many BH3-only proteins have not been identified by sequence, but on the basis of their interaction with Bcl-2 family proteins. Other members of this family have been found to be proapoptotic proteins and the putative BH3-motif was identified later. We have performed here a search based on a newly-generated protein composition profile that was shown to identify all known BH3-only proteins plus few additional candidates in the human genome.
Our screening identified the protein PXT1, which has a BH3-like domain. This protein has been described to induce cytochrome c release and apoptosis in HeLa cells in a manner dependent on its BH3 motif.13 In addition, apolipoprotein L2 (ApoL2) caught our attention due to the recent description of ApoL2 homologs as BH3-only proteins. ApoL1, the founding member of the family, was identified as a component of a class of high density lipoproteins (HDL) in human blood.20 In subsequent years, a number of homologous proteins have been described: the apolipoprotein L family comprises six members in humans and 8–14 members in rodents.16, 21 ApoL1 is the only member of the family expected to be secreted, and when internalized by trypanosomes it generates pores in their lysosomal membrane.8 ApoL1 and ApoL6 also kill mammalian cells when overexpressed, and it has been proposed that all members of the family could share this ability with these two proteins.9 Induction of cell death by ApoL1 and ApoL6 was prevented when their BH3 motif was deleted.5, 7 Both proteins bind lipids;5, 7 interestingly, ApoL1 binds cardiolipin which is a lipid required for permeabilization of liposomes by Bcl-2 family members.22 ApoL6 binds Bcl-xL and it regulates autophagy.17 So indeed, many similarities exist between some members of the apolipoprotein L family and ‘classic' Bcl-2 family proteins.
ApoL2 has been shown to be antiapoptotic in primary cells treated with interferon-gamma.14 Recently, ApoL2 has been identified as a protein that translocates to mitochondria in cells infected with H3N2 swine influenza virus.23 These two facts, together with the description of other proteins of the family as BH3-only proteins has led to propose that ApoL2 has a role in apoptosis, which we have not been able to confirm. It is possible that the aspartic residue in position 10 of the motif (Figure 1) is essential for their proapoptotic function. The function of other Bcl-2 family members with a glutamic acid in that position, Bcl-Rambo (Bcl2L13) and Bcl-G (Bcl2L14), is still not fully defined, but their main function may be unrelated to cell death.24, 25
We have detected a weak interaction between Bcl-2 and ApoL2. However, this did not alter apoptosis induced by many stimuli or starvation-induced autophagy. We did observe a basal regulation of p62, an autophagic protein, which could suggest that this protein regulates autophagy under certain conditions due to its interaction with Bcl-2. On the other hand, Bcl-2 regulates multiple metabolic pathways, Ca2+ stores in the endoplasmic reticulum, mitochondrial morphology and DNA repair.26, 27 We have not explored here the possibility that ApoL2 regulates these functions of Bcl-2. Nonetheless, it is also possible that the ApoL2 has a cell-type or stimulus-dependent role on cell death that according to our data is not general or ubiquitous.
Materials and Methods
Building the HMM
Sequences of vertebrate proteins annotated with the BH3-domain in Uniprot and the literature as of November 2008 (Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bcl-Rambo, Bcl-G, Bax, Bak, Bok, Bim, Bid, Bad, Bmf, Noxa, Hrk, Puma, Bik, Blk, Mule, Spike, Nix, BNIP3, Map-1, Cul7, Beclin-1, p53, ApoL6, ApoL1 and AVEN) were aligned with MAFFT.28 A 15-residue long region from the alignment containing the annotated BH3 domains was selected using trimAl 1.3,29 and a HMM model was built for the region using HMMER v1.8.5.30 HMMER was used to search in the entire human proteome, as retrieved from Ensembl database version 50.31 To detect the domain in putative unpredicted proteins we ran Exonerate32 using that profile over the genome sequence. The results were compared with similar searches using the profiles available at PFAM (which rendered only already-annotated proteins) and Prosite databases, as well as with regular expression searches with motifs described in the literature (Table 1).
Cell culture and treatments
HeLa cells from American Type Culture Collection and 293T were cultured in pyruvate-free high glucose Dulbecco's Modified Eagle's Medium (DMEM; Gibco Life Technologies, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 200 mg/ml of penicillin, 100 μg/ml of streptomycin and glutamine 2 mM (hereafter referred to as PSQ). Cells were maintained at 37 °C and in a 5% CO2 atmosphere. Cell maintenance is based on three splits per week using trypsin EDTA-Solution 0.05% (Invitrogen). HeLa cells were plated at a concentration of 150 000/ml in 6- or 12-well plates and treated 24 h later, when they reached the concentration of 500 000/ml. 293T cells were plated at a concentration of 1 × 106/ml in 10 cm plates for transfections.
Before glucose-deprivation or FBS-deprivation treatments, cells were washed twice with FBS-free, pyruvate-free DMEM medium without glucose (Gibco Life Technologies) or high-glucose, FBS-free DMEM, respectively. Glucose-deprivation treatment is performed in PSQ-containing, glucose-free DMEM medium without glucose supplemented with 10% dialyzed FBS. FBS-deprivation treatment was performed in PSQ-containing high-glucose DMEM. For induction of cell death by starvation in Earle's Balanced Salt Solution (EBSS, Gibco Life Technologies), cells were washed twice with EBSS before treating with EBSS supplemented with Hepes 25 mM.
ABT-737 (Selleck Chemicals, Houston, TX, USA) is used at 30 μM, chloroquine, thapsigargin and actinomycin D (Sigma-Aldrich, St. Louis, MO, USA) at 100μM, 100 μg/ml and 50 nM, respectively, interferon-gamma (Novus Bionova, Madrid, Spain) at 100 ng/ml. TNF-α (Peprotech, Le-Perray-en-Yvelines, France, 10 ng/ml) is added in combination with 10 μM cycloheximide (Sigma-Aldrich) to induce cell death.
For autophagy induction, cells were incubated in home-made EBSS (potassium chloride 400 mg/ml, sodium bicarbonate 2.2 g/ml, sodium chloride 6.8 g/ml, NaH2PO4-H2O 140 mg/ml, D-Glucose 1 g/ml) supplemented with 25 mM Hepes. EBSS-Hepes treatment is performed after washing the cells twice with EBSS.
Autophagy flux was blocked by adding the protease inhibitors pepstatin A and E64d (Sigma, St. Louis, MO, USA, 10 μM each) simultaneously with the treatments.
Cell viability
For analysis of viability, cells were harvested by combining floating cells in the medium and adherent cells detached by trypsinization, and subjected to FACS analysis to detect incorporation of propidium iodide 1 μg/ml (10 min incubation in PBS) using Gallios Flow Cytometer Beckman Coulter. Data were analyzed using FlowJo software, version 7.6.4.
Cell viability and number was additionally measured by crystal violet coloration. After the indicated treatments, cells were covered with staining solution (0.2% crystal violet, 2% EtOH solution) and incubated for 20 min at room temperature. Cells were rinsed twice with PBS and once with water and let dry for 16 h. Crystal violet-stained cells were then resuspended in 10% SDS and absorbance was read at 595 nm in a BioTek (Winooski, VT, USA) PowerWave XS microplate spectrophotometer.
Western blotting
Cell pellets were resuspended in RIPA buffer (Thermo Scientific, Waltham, MA, USA) or lysis buffer (0.06 M Tris, 2% SDS) containing protease inhibition cocktail (Roche, Basel, Switzerland) and phosphatase inhibitors (PhosSTOP, Roche) and they were then sonicated.
For trichloroacetic acid (TCA) precipitation, the acid (Merck, Darmstadt, Germany) was added to the sample at a final concentration of 13%, mixed thoroughly and incubated overnight at 4 °C under rotation. The mixture was centrifugated (16 000 × g, 15 min, 4 °C), and the supernatant was discarded. The pellet was resuspended in RIPA buffer.
Protein quantification was performed using Pierce BCA protein assay kit, following the manufacturer's instructions. 40 μg of protein were diluted in 10 μl of laemmli buffer 4 × (63 mM Tris-HCl, 10% glycerol, 2% SDS, 0.01% bromophenol blue and 5% 2-mercaptoethanol), and PBS was added until 40 μl of total volume. Lysates were boiled for 10 min at 96 °C and loaded in a 12% acrylamide gel. Mini-protean (Bio-Rad, Hercules, CA, USA) electrophoresis tank was used to perform the electrophoresis assay. Proteins were transferred to polyvinylidene fluoride (PVDF, Millipore, Darmstadt, Germany) or nitrocellulose membranes (Bio-Rad) through semi-dry transfer (1 h at 0.2 A/membrane). Transfer validation and loading charge control was checked by ponceau dye (Sigma). PVDF membranes were blocked with 5% nonfat dry milk in Tween Tris-buffered saline (TTBS) and processed for immunoluminiscence. Nitrocellulose membranes were blocked with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE, USA) and processed for immunofluorescence using Odyssey Fc Imaging system. Primary and secondary antibodies were incubated for 1 h at room temperature or overnight at 4°C, in 5% milk TTBS. Three 10-min TTBS washes in the shaker were performed before developing by enhanced chemiluminescence (ECL; Pierce, Waltham, MA, USA) or scanning the membrane using Odyssey Imaging System. Quantification of band intensity was performed with Fiji/Image J software 1.47b.
Primary antibodies used for western blotting were: anti-actin (ICN clone 4), polyclonal anti-ApoL2 (Sigma, HPA001078), anti-tubulin (Sigma, Clone TUB 2.1). polyclonal antibody against p62 (Progen, Heidelberg, Germany), polyclonal anti-LC3 (Abcam, Cambridge, UK), anti-HA (Sigma, clone HA-7), anti-Bcl-xL (Cell Signaling, Beverly, MA, USA, 54H6), anti-Bcl-2 (Santa Cruz, Dallas, TX, USA, 100), polyclonal anti-Mcl-1 (Santa Cruz, sc-819), polyclonal anti-Atg5 (CosmoBio, Tokyo, Japan), anti-beclin-1 (BD Biosciences, Franklin Lakes, NJ, USA, 20/Beclin). HRP secondary antibodies were: antimouse and anti-rabbit (Zymax, Bideford, UK) or anti-guinea pig (Abcam). IRDye secondary anti-bodies against mouse or rabbit (IRDye 800CW donkey anti-rabbit IgG1 1/15.000 or IRDye 680LT anti-mouse IgG 1/20 000) were from LI-COR Biosciences.
Plasmids and transient transfection
ApoL2 cDNA (NM_030882.2) was purchased from Origene and subcloned into ampicillin resistant pcDNA3.1 plasmid using EcoR1 and BamH1 restriction enzymes. Invitrogen PureLink kit was used to extract the plasmid from competent bacteria (Promega, Fitchburg, WI, USA).
For death experiments, HeLa cells were transfected in six-well plates, using 4 μl of Genejuice (Novagen, Darmstadt, Germany) and 2 μg of total DNA. To normalize until 2 μg, we completed with the empty plasmid pcDNA 3.1. pcDNA 3.1-Bcl-2 plasmid was generously provided by Dr. Jean-Ehrland Ricci (Nice, France). HA-Noxa and pcDNA 3.1-Bmf were provided by Professor Seamus Martin (Dublin, Ireland). pcDNA 3.1-HA-Bcl-2 and pcDNA 3.1-HA-Bcl-xL plasmids from Dr. Douglas Green's laboratory (Memphis, TN, USA) were used in the immunoprecipitation assay. Cell death was analyzed by counting GFP positive dead cells against total GFP positive cells using an Olympus IX70 inverted microscope. For immunoprecipitation, HeLa cells were transfected in 10 cm dishes, using 3 μg of polyethylenimine linear (PEI; Polyscience Europe, Heidelberg, Germany) per μg of DNA. Cotransfection was performed using 10 μg of ApoL2 and 10 μg of HA-Bcl-xL or HA-Bcl-2 plasmid.
siRNA transfection
Cells were transfected at a density of 300 000/ml using 1.5 μl of DharmaFECT 1 (Dharmacon, Lafayette, CO, USA) per milliliter of total volume and following manufacturer's instructions. siRNA concentration was 100 nM. After 24 h, medium was replaced with growth medium. Control sequences were: 5′-GUAAGACACGACUUAUCGC[dT][dT] (‘control 1') and an ON-TARGET plus siRNA pool of 4 oligos against mouse RIPK (Dharmacon; ‘control 2'). Three different siRNA sequences were used against ApoL2: ApoL2-I (GCGGCACCAAUGUAGCAAA[dT][dT]), ApoL2-II (CAGUGUGGUAGAACUAGUA[dT][dT]) and ApoL2-III (CAAUGUUCUUACCUUAGUU[dT][dT]).
Immunofluorescence
Cells were cultured on glass coverslips pretreated with poly-L-Lysine (Sigma). After 24 h they were incubated for 15 min in culture medium at 37 °C and 5% CO2 with MitoTracker red 200 nM (Invitrogen) before fixing, or they were directly fixed with a fresh 4% solution of paraformaldehyde for 20 min. Cells were then incubated with blocking buffer: 0.05% Triton, 3% BSA in PBS for 1 h and kept overnight at 4 °C with primary antibodies diluted 1 : 200 in blocking buffer: Ab rabbit anti-ApoL2 (Sigma), mouse anti-Lamp-2 (BD pharmigen, Franklin Lakes, NJ, USA, CD107b, 555803), mouse anti-Calnexin (Santa Cruz, E-10, sc-46669), goat anti-GRP94 (Santa Cruz, C-19, sc-1794). Cells were incubated with secondary antibodies Alexa Fluor 568 red and 488 green (Life Technologies, Carlsbad, CA, USA) diluted 1 : 400 in blocking buffer for 1 h. Then they were mounted in Vectashield solution (Vector laboratories, Burlingame, CA, USA) on microscope slides and visualized on a Leica TCS SP5 Spectral Confocal microscope with a HCX PL APO lambda blue × 63 1.4 oil objective lens. Acquisition software was LEICA (Wetzlar, Germany) Application Suite Advanced Fluorescence (LAS AF) version 2.6.0.7266 and pictures were analyzed with Fiji/Image J software.
Immunoprecipitation
A total of 30 μl of Protein G Magnetic Beads (Millipore) were washed 3 × in immunoprecipitation buffer and then incubated in 1 ml of immunoprecipitation buffer with 1 μg of antibody for 4 h at 4 °C under rotation. 10 × 106 cells were lysed in 500 μl of immunoprecipitation buffer (20 mM Tris-HCl (pH7.5), 137 mM NaCl, 1% Triton X-100, 2 mM EDTA (pH 8)) containing complete protease inhibitor cocktail and incubated for 30 min in ice. A total of 1400 μg of cell extract were incubated overnight in 1 ml of immunoprecipitation buffer with the antibody-coupled beads. The next day, beads were washed five times with immunoprecipitation buffer and eluted with 60 μl of immunoprecipitation buffer containing 2% SDS. Then 20 μl of laemmli buffer 4 × were added, and samples were boiled for 10 min at 95 °C. Eluted proteins were split in two gels of SDS-polyacrylamide gel electrophoresis. Ten percent of the total protein subjected to immunoprecipitation was loaded as input and 30 μl of the remaining supernatant after immunoprecipitation was also loaded to confirm immunodepletion. A total of 1 μg of anti-HA and anti-ApoL2 described above were used for immunoprecipitation.
Statistics
Error bars in the figures represent the standard error of the mean (S.E.M.). Data were statistically analyzed to find significant differences using two-tailed, paired Student's t-test. Significant differences are marked in the figures with * (P≤0.05) or ** (P≤0.0005).
Acknowledgments
We wish to thank Dorothée Walter, Silvia Ramírez-Peinado, Dídac Domínguez and Clara León-Annicchiarico for help with experiments and Jean-Ehrland Ricci, Seamus Martin, Giulio Donati, Albert Tauler, Oscar M Tirado, Fabien Llambi, Pat Fitzgerald and Doug Green for plasmids, reagents and/or advice. This work was supported by the Association for International Cancer Research (AICR), grant number 08-0621 and Fondo de Investigaciones Sanitarias of Spain, grant numbers PI10/00104 and PI13/00139.
Glossary
- ApoL
apolipoprotein L
- FBS
fetal bovine serum
- IFN- γ
interferon-gamma
- TNF
tumor necrosis factor
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Melino
Supplementary Material
References
- 1Droin NM, Green DR. Role of Bcl-2 family members in immunity and disease. Biochim Biophys Acta 2004; 1644: 179–188. [DOI] [PubMed] [Google Scholar]
- 2Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis 2009; 14: 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene 2008; 27: S2–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59. [DOI] [PubMed] [Google Scholar]
- 5Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem 2008; 283: 21540–21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Zhaorigetu S, Wan G, Kaini R, Jiang Z, Hu CA. ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy 2008; 4: 1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Liu Z, Lu H, Jiang Z, Pastuszyn A, Hu CA. Apolipoprotein l6, a novel proapoptotic Bcl-2 homology 3-only protein, induces mitochondria-mediated apoptosis in cancer cells. Mol Cancer Res 2005; 3: 21–31. [PubMed] [Google Scholar]
- 8Perez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, Nolan DP, Lins L, Homble F et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 2005; 309: 469–472. [DOI] [PubMed] [Google Scholar]
- 9Vanhollebeke B, Pays E. The function of apolipoproteins L. Cell Mol Life Sci 2006; 63: 1937–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Sigrist CJA, de Castro E, Cerutti L, Cuche BA, Hulo N, Bridge A et al. New and continuing developments at PROSITE. Nucleic Acids Res 2013; 41: D344–D347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR et al. Pfam: the protein families database. Nucleic Acids Res 2014; 42: D222–D230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Coultas L, Pellegrini M, Visvader JE, Lindeman GJ, Chen L, Adams JM et al. Bfk: a novel weakly proapoptotic member of the Bcl-2 protein family with a BH3 and a BH2 region. Cell Death Differ 2003; 10: 185–192. [DOI] [PubMed] [Google Scholar]
- 13Kaczmarek K, Studencka M, Meinhardt A, Wieczerzak K, Thoms S, Engel W et al. Overexpression of peroxisomal testis specific 1 protein induces germ cell apoptosis and leads to infertility in male mice. Mol Biol Cell 2011; 22: 1766–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Liao W, Goh FY, Betts RJ, Kemeny DM, Tam J, Bay BH et al. A novel anti-apoptotic role for apolipoprotein L2 in IFN-gamma-induced cytotoxicity in human bronchial epithelial cells. J Cell Physiol 2011; 226: 397–406. [DOI] [PubMed] [Google Scholar]
- 15Ahn WS, Bae SM, Lee JM, Namkoong SE, Han S-J, Cho YL et al. Searching for pathogenic gene functions to cervical cancer. Gynecol Oncol 2004; 93: 41–48. [DOI] [PubMed] [Google Scholar]
- 16Page NM, Butlin DJ, Lomthaisong K, Lowry PJ. The human apolipoprotein L gene cluster: identification, classification, and sites of distribution. Genomics 2001; 74: 71–78. [DOI] [PubMed] [Google Scholar]
- 17Zhaorigetu S, Yang Z, Toma I, McCaffrey TA, Hu CA. Apolipoprotein L6, induced in atherosclerotic lesions, promotes apoptosis and blocks Beclin 1-dependent autophagy in atherosclerotic cells. J Biol Chem 2011; 286: 27389–27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell 2011; 44: 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Aouacheria A, Brunet F, Gouy M. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-only, and BNip families of apoptotic regulators. Mol Biol Evol 2005; 22: 2395–2416. [DOI] [PubMed] [Google Scholar]
- 20Duchateau PN, Pullinger CR, Orellana RE, Kunitake ST, Naya-Vigne J, O'Connor PM et al. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem 1997; 272: 25576–25582. [DOI] [PubMed] [Google Scholar]
- 21Monajemi H, Fontijn RD, Pannekoek H, Horrevoets AJ. The apolipoprotein L gene cluster has emerged recently in evolution and is expressed in human vascular tissue. Genomics 2002; 79: 539–546. [DOI] [PubMed] [Google Scholar]
- 22Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002; 111: 331–342. [DOI] [PubMed] [Google Scholar]
- 23Wu X, Wang H, Bai L, Yu Y, Sun Z, Yan Y et al. Mitochondrial proteomic analysis of human host cells infected with H3N2 swine influenza virus. J Proteomics 2013; 91: 136–150. [DOI] [PubMed] [Google Scholar]
- 24Tischner D, Villunger A. Bcl-G acquitted of murder!. Cell Death Dis 2012; 3: e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Giam M, Okamoto T, Mintern JD, Strasser A, Bouillet P. Bcl-2 family member Bcl-G is not a proapoptotic protein. Cell Death Dis 2012; 3: e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Hetz C, Glimcher L. The daily job of night killers: alternative roles of the BCL-2 family in organelle physiology. Trends Cell Biol 2008; 18: 38–44. [DOI] [PubMed] [Google Scholar]
- 27Laulier C, Lopez BS. The secret life of Bcl-2: apoptosis-independent inhibition of DNA repair by Bcl-2 family members. Mutat Res 2012; 751: 247–257. [DOI] [PubMed] [Google Scholar]
- 28Russell DJ, Katoh K, Standley D. MAFFT: iterative refinement and additional methods In: Multiple Sequence Alignment Methods. Humana Press: Suita, Japan, 2014; pp 131–146. [DOI] [PubMed] [Google Scholar]
- 29Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009; 25: 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Eddy SR. Accelerated profile HMM searches. PLoS Comput Biol 2011; 7: e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S et al. Ensembl 2014. Nucleic Acids Res 2014; 42: D749–D755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Slater GS, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics 2005; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.