Abstract
Cisplatin (cis-diaminedichloroplatinum-II) is an extensively used chemotherapeutic agent, and one of its most adverse effects is ototoxicity. A number of studies have demonstrated that these effects are related to oxidative stress and DNA damage. However, the precise mechanism underlying cisplatin-associated ototoxicity is still unclear. The cofactor nicotinamide adenine dinucleotide (NAD+) has emerged as a key regulator of cellular energy metabolism and homeostasis. Here, we demonstrate for the first time that, in cisplatin-mediated ototoxicity, the levels and activities of SIRT1 are suppressed by the reduction of intracellular NAD+ levels. We provide evidence that the decrease in SIRT1 activity and expression facilitated by increasing poly(ADP-ribose) transferase (PARP)-1 activation and microRNA-34a through p53 activation aggravates cisplatin-mediated ototoxicity. Moreover, we show that the induction of cellular NAD+ levels using β-lapachone (β-Lap), whose intracellular target is NQO1, prevents the toxic effects of cisplatin through the regulation of PARP-1 and SIRT1 activity. These results suggest that direct modulation of cellular NAD+ levels by pharmacological agents could be a promising therapeutic approach for protection from cisplatin-induced ototoxicity.
Cisplatin (cis-diamminedichloroplatinum (II)) is a chemotherapeutic agent extensively used to treat a variety of solid tumors in the head and neck, bladder, lung, ovaries, testicles, and uterus.1 However, progressive irreversible side effects of cisplatin, including nephrotoxicity and ototoxicity, greatly impair the patient's quality of life and frequently result in the need to lower the dosage during treatment or discontinuation of the treatment. Cisplatin ototoxicity primarily occurs in the cochlea and is generally caused by apoptotic damage to the outer hair cells (OHCs), spiral ganglion cells, and the marginal cells of the stria vascularis. In recent years, studies have demonstrated that cisplatin ototoxicity is also closely related to the damage of cochlear tissue by increased production of reactive oxygen species (ROS) and accompanied by the depletion of antioxidant substances and increased lipid peroxidation.2, 3 ROSs, particularly the hydroxyl radical, have a critical role in cisplatin-induced p53 activation through DNA damage.4 Although it is not easy to differentiate the cause from the consequence, a positive feedback loop between inflammatory cytokines and oxidative stress that worsen the cochlear damage is considered as one of the major mechanisms that facilitate cisplatin-induced hearing impairment.5 Interestingly, p53 and NF-κB have been described as key mediators of cisplatin-induced toxicity because of their involvement in oxidative stress, DNA damage, and inflammation through a mutual feedback process of ‘cause and effect.'6, 7 In addition, activities of p53 and NF-κB could be regulated by post-translational modifications, including phosphorylation and acetylation. Recent studies have reported that acetylated p53 and NF-κB are correlated with cisplatin-induced toxicity. Furthermore, acetylation of p53 and NF-κB is critically involved in cisplatin-induced renal injury.8, 9
Cellular nicotinamide adenine dinucleotide (NAD+) and NADH levels have been shown to be important mediators of energy metabolism and cellular homeostasis.10, 11 As NAD+ acts as a cofactor for various enzymes, including sirtuins (SIRTs), poly(ADP-ribose) transferases (PARPs), and cyclic ADP (cADP)-ribose synthases,12, 13 the regulation of NAD+ level may have therapeutic benefits through its effect on NAD+-dependent enzymes. SIRTs, NAD+-dependent protein deacetylases, are present as seven homologs of Sir2 (SIRT1-7) that show differential subcellular localizations in mammals.11 Among these, nuclear SIRT1 is activated under energy stress conditions, such as fasting, exercise, or low glucose availability. 14 SIRT1 has a key role in metabolism, development, stress response, neurogenesis, hormone responses, and apoptosis15, 16 by deacetylation of substrates, such as NF-κB, FOXO, p53, and histones.17, 18, 19
PARPs, the most abundant ADP-ribosyl transferases, also use NAD+ to generate large amounts of poly(ADP-ribose) (PAR), which facilitate the recruitment of DNA repair factors. In particular, PARP-1 is a DNA damage sensor that can be activated in response to DNA damage by various pathophysiological conditions, including oxidative stress and inflammatory injury. However, excessive hyperactivation of PARP-1 causes the depletion of intracellular NAD+ and ATP levels, which eventually leads to cell death.20, 21 PARP-1 activation is also known as one of the important pathogenic mechanisms in cisplatin-induced toxicity.22, 23
A cytosolic antioxidant flavoprotein NADH:quinone oxidoreductase 1 (NQO1) catalyzes the reduction of quinones to hydroquinones by utilizing NADH as an electron donor, which consequently increases intracellular NAD+ levels.24, 25 In addition, accumulation evidence suggests that NQO1 has a role in other biological activities, including anti-inflammatory processes, scavenging of superoxide anion radicals, and stabilization of p53 and other tumor suppressor proteins.26, 27, 28 Several substrates of NQO1 enzyme, including mitomycin C, RH1, AZQ, Coenzyme Q10, and idebenone, have been identified,29, 30 of which β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione; β-Lap) is recently well studied as a substrate of NQO1.31, 32 β-Lap was first isolated from the bark of the lapacho tree and reported to inhibit tumor cell line growth.33 However, recent reports indicate that the conversion of NADH to NAD+ by NQO1 and β-Lap has beneficial effects on several characteristics of metabolic syndrome, for example, prevention of health decline in aged mice, amelioration of obesity or hypertension, prevention of arterial restenosis, and protection against salt-induced renal injury.34, 35, 36, 37, 38 Furthermore, we recently have demonstrated that conversion of NADH to NAD+ by NQO1 and β-Lap suppresses cisplatin-induced acute kidney injury by downregulating potential damage mediators such as oxidative stress and inflammatory responses.9
Although a link between NAD+-dependent molecular events and cellular metabolism is evident, it remains unclear whether modulation of NAD+ levels has an impact on cisplatin-induced hearing impairment. Therefore, herein we investigated the role of NAD+ metabolism on cisplatin-induced cochlear dysfunction, and the effect of increased levels of intracellular NAD+ facilitated by β-Lap on cisplatin-induced hearing impairment with a particular interest in NAD+-dependent enzymatic pathways including SIRTs and PARPs.
Results
β-Lap ameliorates cisplatin-induced hearing loss in mice
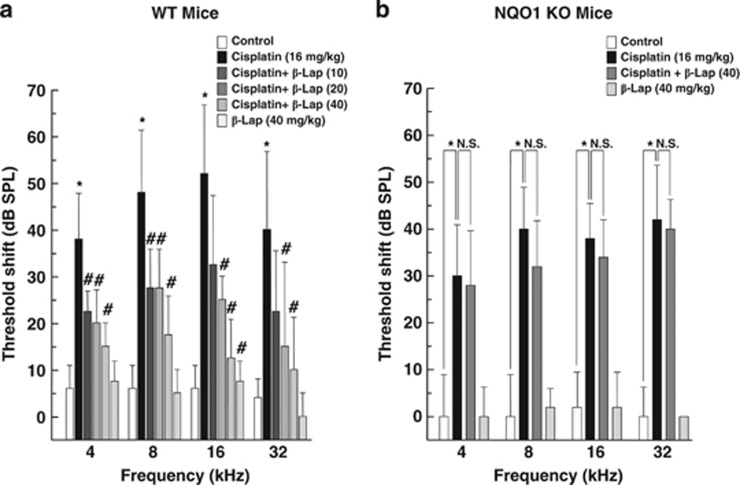
To investigate the effect of β-Lap on cisplatin-induced hearing loss, C57BL/6 wild-type (WT) and NQO1−/− mice were treated with β-Lap, cisplatin, or β-Lap plus cisplatin as indicated in Figure 1. We measured the hearing function of the animals in experimental groups using auditory brainstem response (ABR) 1 day before and 4 days after cisplatin exposure. Marked shift in ABR threshold was observed in cisplatin-treated C57BL/6 WT mice at 4, 8, 16, and 32 kHz. However, this cisplatin-induced ABR threshold shift was strongly attenuated in the β-Lap-treated WT mice at all frequencies examined (4, 8, 16, and 32 kHz, Figure 1a). ABR threshold was not altered in the phosphate-buffered saline (PBS) control or β-Lap only treated group. Next, we investigated whether β-Lap-induced protective effects are mediated through the conversion of NADH to NAD+ by NQO1. We performed a series of experiments using NQO1−/− mice with the same experimental scheme. The ABR thresholds were markedly shifted in cisplatin-treated NQO1−/− mice at 4, 8, 16, and 32 kHz, whereas this shift in ABR threshold was not attenuated by β-Lap in the NQO1−/− mice (Figure 1b). These results strongly indicate that NQO1 has a critical role in the protective effect of β-Lap in cisplatin-induced hearing loss in mice.
Figure 1.
Effect of β-Lap on cisplatin-induced hearing loss in mice. β-Lap (10, 20, 40 mg/kg body weight) was administered orally once a day for 4 consecutive days. Cisplatin (16 mg/kg body weight) was injected once at 12 h after the first β-Lap administration. The changes in ABR threshold at 4, 8, 16, and 32 kHz were measured at one day before and 4 days after cisplatin treatment in WT (a) and NQO1−/− mice (b). *,#P<0.05, by one-way ANOVA when compared with the control (*) or cisplatin (#) group (n=5)
β-Lap treatment protects against cisplatin toxicity in the cochlear tissue and HEI-OC1 auditory cells
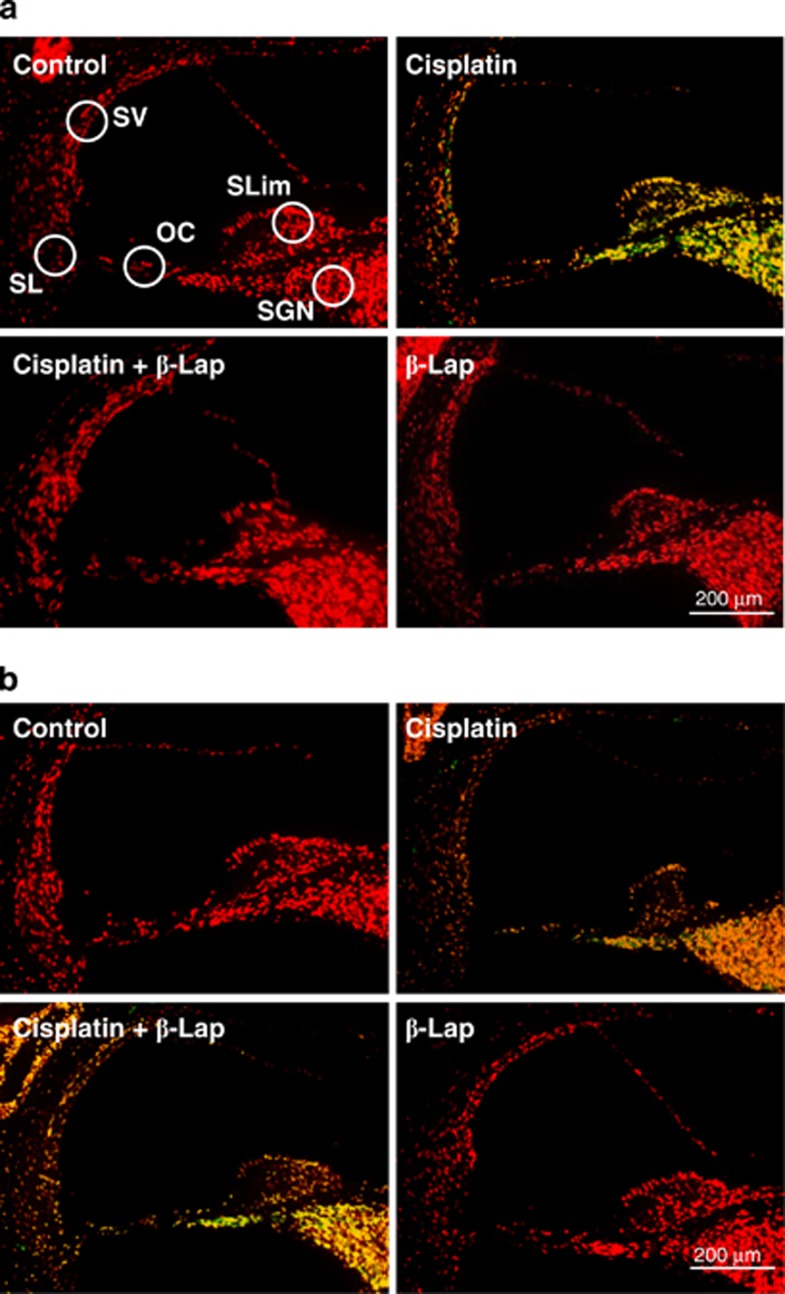
As the hearing functions in C57BL/6 WT mice were severely affected by cisplatin, but not in the β-Lap group (Figure 1), we next examined whether the apoptotic cell death in cochlear tissues correspond to the observed loss of hearing function. TUNEL analysis indicated that the total number of TUNEL-positive nuclei clearly increased in the stria vascularis, spiral ligament, spiral ganglion neurons, and organ of Corti of the cisplatin alone group, whereas the TUNEL-positive nuclei in the whole cochlea of ciapltin+β-Lap mice were either not detected or negligible. Interestingly, this protective effect of β-Lap from the cisplatin-induced apoptotic cell death in the cochlea from WT mice (Figure 2a) was not observed in cochlear tissue from NQO1−/− mice (Figure 2b). These results strongly indicate that NQO1 has a critical role in the protective effect of β-Lap from cisplatin-induced cochlear damage and hearing deterioration. We next analyzed the effect of β-Lap on the viability of cisplatin-treated HEI-OC1 auditory cells. β-Lap treatment significantly prevented cisplatin toxicity in a dose-dependent manner (Supplementary Figure 1A), which was attenuated after transfection of cells with NQO1-specific siRNA (Supplementary Figure 1B). These results strongly indicated that β-Lap acts through NQO1 to block cisplatin-induced toxicity.
Figure 2.
Effect of β-Lap on cisplatin-induced toxicity in mice. β-Lap (40 mg/kg body weight) was administered orally once a day for 4 consecutive days. Cisplatin (16 mg/kg body weight) was injected once at 12 h after the first β-Lap administration. Cochlear tissue was isolated at 4 days. Apoptotic cells were identified by TUNEL staining and examination under a fluorescent microscope in WT (a) and NQO1−/− mice (b). The TUNEL-positive nuclei were visualized as green. Counterstaining for nuclei was conducted with propidium iodide and visualized as red. Control, PBS-treated group; Cisplatin, 16 mg/kg cisplatin only group; Cisplatin+β-Lap, cisplatin and 40 mg/kg β-Lap combined group; β-Lap, 40 mg/kg β-Lap only group. OC, organ of Corti; SGN, spiral ganglion neuron; SL, spiral ligament; SLim, spiral limbus; SV, stria vascularis
PARP-1 activation is critically involved in cisplatin-induced damages of cochlear tissues and HEI-OC1 cells
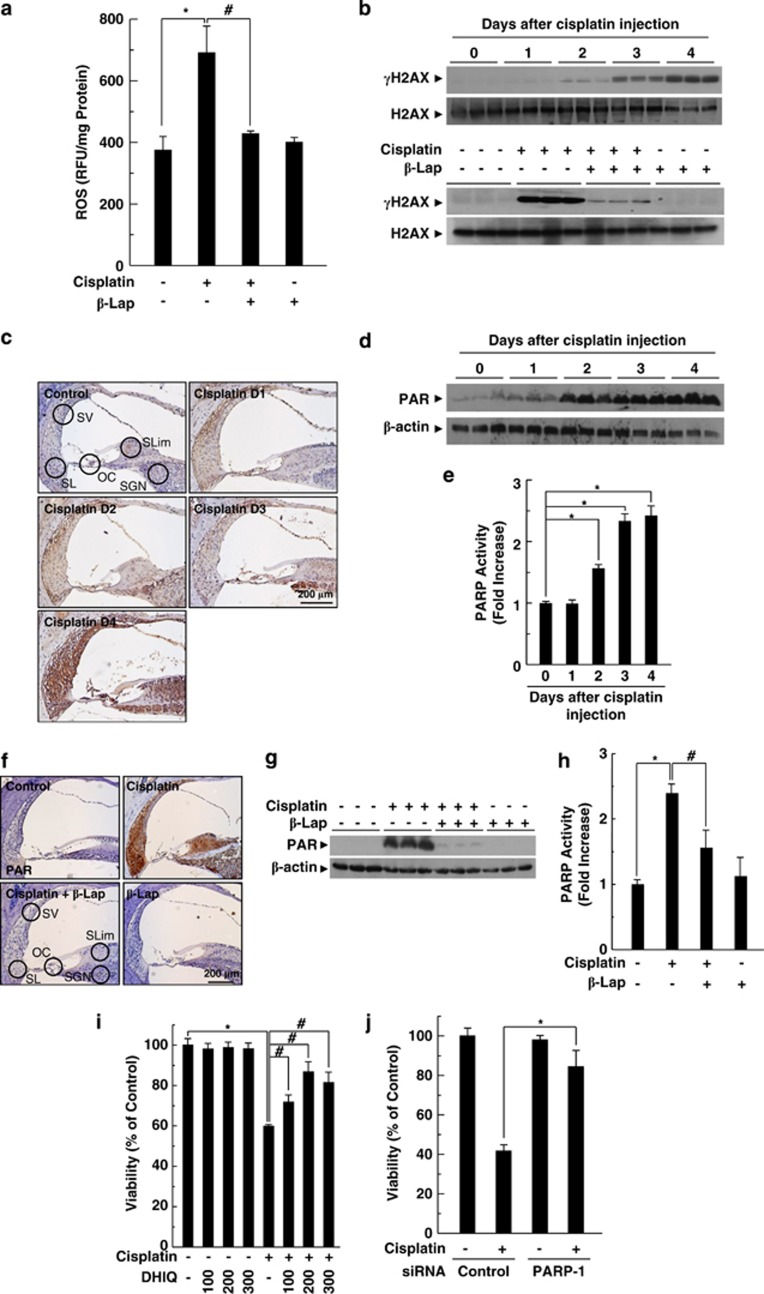
In our previous study, we demonstrated that increased ROS production is one of the major contributors to the pathogenesis of cisplatin ototoxicity in both in vitro and in vivo models by assessing DNA damage.5 Here, we analyzed the effect of β-Lap on intracellular ROS levels in the cochlear tissue of cisplatin-injected mice by using 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA), which is a peroxide-sensitive fluorescent probe, and DNA damage by measuring the phosphorylation of H2AX using western blotting. As expected, cisplatin significantly increased the intracellular ROS production compared with that of the control, whereas the cisplatin-induced ROS production was completely attenuated by β-Lap (Figure 3a). Consistently, the phosphorylation of H2AX, indicating DNA damage, was increased by cisplatin in a time-dependent manner (Figure 3b upper panel), and this cisplatin-induced phosphorylation of H2AX was attenuated by β-Lap (Figure 3b lower panel).
Figure 3.
PARP-1 activation in cisplatin ototoxicity. β-Lap (40 mg/kg body weight) was administered orally once a day for 4 consecutive days. Cisplatin (16 mg/kg body weight) was injected once at 12 h after the first β-Lap administration. (a) Cochlear tissue was isolated at 4 days after cisplatin injection, and then tissue extracts were incubated with 20 μM of 2′,7′-dichlorodihydrofluorescein diacetate to determine total ROS at 37 °C for 60 min. Fluorescence intensity was recorded using a fluorometer and normalized to protein content. (b) Cochlear tissue was isolated at indicated times (upper) and 4 days (lower) after cisplatin treatment. (c–h) DNA damage was determined by western blotting using anti-γH2AX antibody. PARP-1 activation was analyzed by IHC (c and f) and western blotting (d and g) using anti-PAR antibody, and PARP activity was assayed using the PARP assay kit (e and h) in the cisplatin-treated cochlear tissue. Control, PBS-treated group; Cisplatin, 16 mg/kg cisplatin only group; Cisplatin+β-Lap, cisplatin and 40 mg/kg β-Lap combined group; β-Lap, 40 mg/kg β-Lap only group. *,#P<0.05 by one-way ANOVA compared with the control (*) and cisplatin only (#) group, (n=5). (i and j) HEI-OC1 cells were pretreated with the indicated doses of DHIQ for 30 min (i) or transfected with 100 nM control or PARP-1-specific siRNAs for 24 h (j), and then further maintained in 20 μM cisplatin for 24 h. The cell viability was measured by MTT assay. *,#P<0.05 by one-way ANOVA compared with the control (*) and cisplatin only (#) group, (n=3). OC, organ of Corti; SGN, spiral ganglion neuron; SL, spiral ligament; SLim, spiral limbus; SV, stria vascularis
PARP-1 is a major enzyme that transfers negatively charged ADP-ribose groups (PARylation) from NAD+ to itself or target proteins post-translationally and thus regulates a wide array of cellular processes, including transcription, DNA repair, and mitochondrial function. In particular, PARP-1 is activated in response to DNA damage by various pathophysiological conditions, including oxidative stress and inflammatory injury. The activation of PARP-1 results in PARylation of PARP-1 target proteins, which facilitates the recruitment of DNA repair factors.21, 39 Therefore, to determine the involvement of PARP activation in cisplatin ototoxicity, we assessed the formation of PAR in the cochlea by immunohistochemical (IHC) and western blot analysis. After cisplatin injection, the formation of PAR was markedly increased in a time-dependent manner (Figures 3c and d). In addition, PARP activity was also significantly increased after cisplatin injection (Figure 3e). Furthermore, IHC and western blot analysis revealed that β-Lap obviously blocked cisplatin-induced PAR formation in the cochlear tissue (Figures 3f and g). Similar to the effect of β-Lap on PAR formation, β-Lap also significantly inhibited PARP activity in cisplatin-treated cochlear tissue (Figure 3h). We further evaluated whether PARP-1 expression and its activation influence cisplatin-mediated cytotoxicity. Inhibition of PARP activity with PARP inhibitor DHIQ attenuated cisplatin-mediated HEI-OC1 auditory cell death (Figure 3i). As DHIQ is a nonspecific pharmacological inhibitor of PARP-1 activation, we further examined the specific role of PARP-1 in cisplatin-induced cell death by transfection of PARP1-specific siRNA. Similar to the effect of the PARP inhibitor DHIQ, knockdown of PARP-1 by transfection of siRNA prevented cisplatin-induced cytotoxicity (Figure 3j).
β-Lap regulates the intracellular NAD+/NADH ratio via NQO1 and restores cisplatin-induced decrease in ATP levels by inhibiting PARP-1 activation
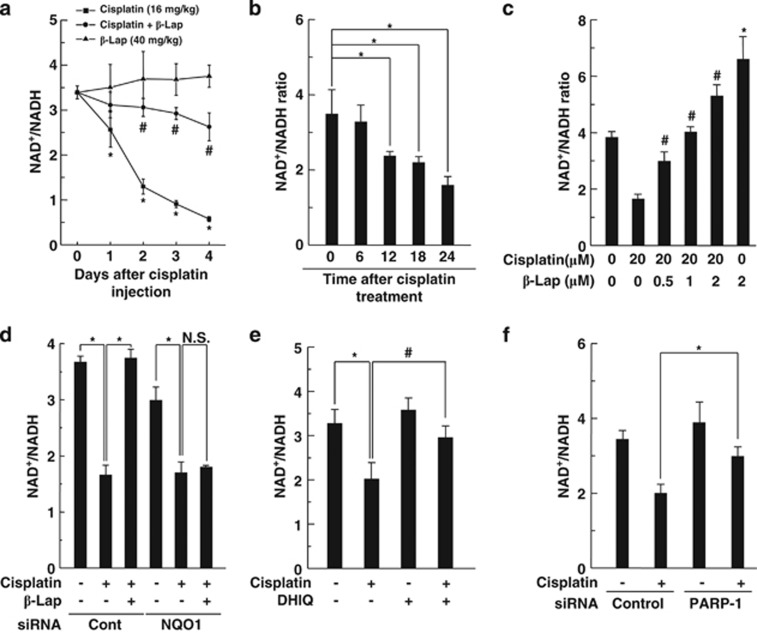
β-Lap is known as a strong substrate of NQO1 for oxidation of NADH to NAD+ and thereby increases the cellular NAD+/NADH ratio.31 In a previous study, we demonstrated that the decrease in the intracellular NAD+/NADH ratio is critically involved in cisplatin-induced renal damage and β-Lap ameliorates cisplatin-induced renal impairment through restoration of cisplatin-induced reduction of cellular NAD+/NADH ratio in renal tissue.9 Therefore, to determine the time-dependent effect of β-Lap on cellular NAD+/NADH ratio, we investigated intracellular NAD+/NADH ratio in cochlear tissues for 4 consecutive days after treatment with cisplatin and/or β-Lap. The intracellular NAD+/NADH ratio in the cochlear tissues of mice was significantly and time dependently decreased by cisplatin exposure. However, such decreases were significantly attenuated by β-Lap treatment (Figure 4a). We also examined the cellular NAD+/NADH ratio in HEI-OC1 auditory cells after treatment with cisplatin. The intracellular NAD+/NADH ratio in HEI-OC1 cells was significantly and time dependently decreased by cisplatin exposure (Figure 4b), whereas β-Lap treatment significantly attenuated the cisplatin-induced decreases of NAD+/NADH ratio with a dose-dependent manner (Figure 4c). The intracellular NAD+ or NADH levels in cisplatin and/or β-Lap-treated HEI-OC1 cells were individually shown in Supplementary Figure 2A, from which the intracellular NAD+/NADH ratio was calculated. Interestingly, the effect of β-Lap on the increase of NAD+/NADH ratio was completely abolished by transfection of NQO1-specific siRNA (Figure 4d). These results indicated that β-Lap, acts as a substrate of NQO1, increased cellular NAD+ levels in a NQO1-dependent manner. As excessive hyperactivation of PARP-1 results in the depletion of intracellular NAD+ and ATP levels, and eventually leads to cell death, we evaluated whether PARP-1 activation influences the decrease in cellular NAD+/NADH ratio. The decrease in the intracellular NAD+/NADH ratio by cisplatin in HEI-OC1 cells was significantly restored by DHIQ, a PARP inhibitor (Figure 4e). Similar to the effect of DHIQ, knockdown of PARP-1 by transfection of siRNA significantly attenuated the decrease in the intracellular NAD+/NADH ratio by cisplatin (Figure 4f). We also examined whether PARP-1 activation contributed to the modulation of ATP levels and β-Lap could restore ATP levels altered by cisplatin treatment. As expected, after a single injection of cisplatin (16 mg/kg body weight), the ATP level in the cochlear tissue rapidly decreased in a time-dependent manner (Supplementary Figure 3A), whereas β-Lap treatment significantly restored the ATP levels (Supplementary Figure 3B). In addition, similar results were observed in cisplatin-treated HEI-OC1 auditory cells (Supplementary Figures 3C and D). β-Lap significantly restored the cisplatin-induced decline in the ATP level in vitro.
Figure 4.
Effect of β-Lap on intracellular NAD+/NADH ratio in cisplatin-treated mice and HEI-OC1 cells. (a) β-Lap (40 mg/kg body weight) was administered orally once a day for 4 consecutive days. Cisplatin (16 mg/kg body weight) was injected once at 12 h after the first β-Lap administration. Cochlear tissue was isolated at the indicated periods. The changes in NAD+/NADH ratio were measured by using the NAD+/NADH assay kit. (b) Cells were treated with 20 μM cisplatin for the indicated times. (c) Cells were treated with various doses of β-Lap for 24 h in the presence or absence of 20 μM cisplatin. (d) Cells were transfected with 100 nM control or p53-specific siRNAs for 24 h, and then further treated with cisplatin (20 μM) and β-Lap (1 μM) for 24 h. (e) Cells were treated with DHIQ (200 μM) and cisplatin (20 μM) for 24 h. (f) Cells were transfected with 100 nM control or PARP-1-specific siRNAs for 24 h, and then further treated with cisplatin (20 μM) for 24 h. NAD+/NADH ratio was measured by using the NAD+/NADH assay kit. *, #P<0.05 by one-way ANOVA compared with the control (*) and cisplatin-only group (#). NS, not significant; n=5
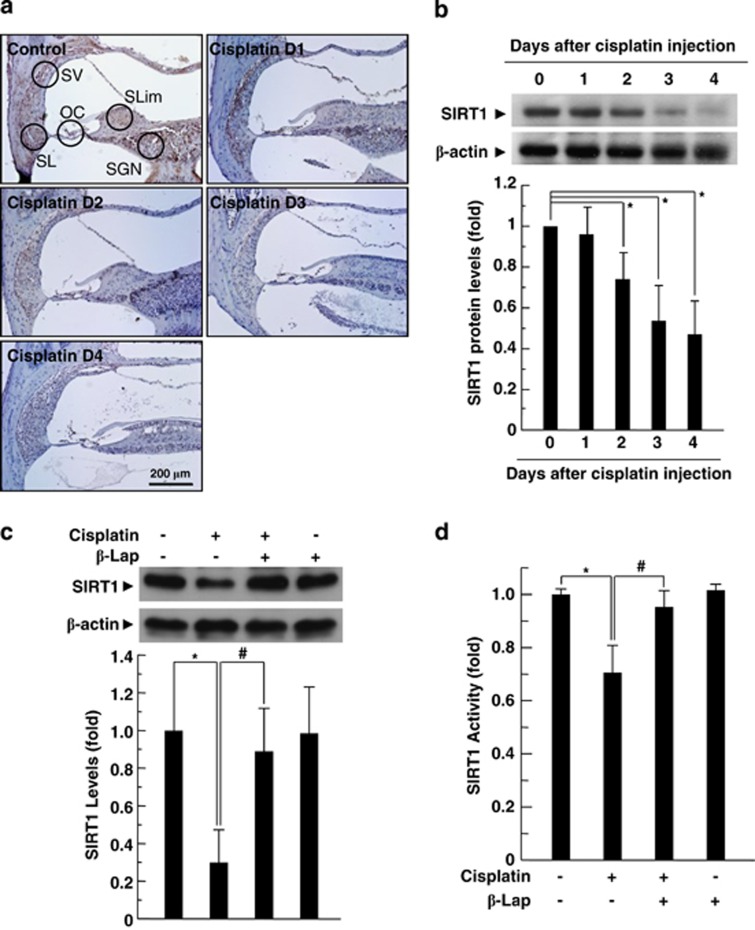
β-Lap restores cisplatin-induced decrease in SIRT1 expression and its enzymatic activity
To evaluate the effects of cisplatin on SIRT1 protein level and its activity, C57BL/6 mice and HEI-OC1 auditory cells were treated with cisplatin for specified time periods. IHC and western blot analysis revealed that in vivo injection of cisplatin reduced the level of SIRT1 expression in the cochlear tissue in a time-dependent manner (Figures 5a and b). In addition, cisplatin treatment to HEI-OC1 auditory cells significantly reduced the level of SIRT1 expression compared with the media control (Supplementary Figure 4A). Twenty-four hours after cisplatin exposure, SIRT1 expression level was approximately 30% of that of the control group. We further examined SIRT1 expression by immunofluorescent staining. As shown in Supplementary Figure 4B, SIRT1 was predominantly localized in the nucleus. However, after exposure to cisplatin, SIRT1 expression in the nucleus was obviously decreased. Consistent with these results, cisplatin treatment significantly decreased SIRT1 deacetylase activity (Supplementary Figure 4C). Next, we evaluated whether β-Lap affected cisplatin-mediated decrease in SIRT1 expression and activity in cochlear tissue or HEI-OC1 cells. Both the protein expression (Figure 5a and Supplementary Figure 4D) and activity (Figure 5b and Supplementary Figure 4E) of SIRT1 decreased after cisplatin treatment, whereas these decreases were obviously attenuated by β-Lap in both cochlear tissue (Figures 5c and d) and HEI-OC1 cells (Supplementary Figures 4d and e).
Figure 5.
Effect of β-Lap on cisplatin-induced decrease in SIRT1 expression and its enzymatic activity. β-Lap (40 mg/kg body weight) was administered orally once a day for 4 consecutive days. Cisplatin (16 mg/kg body weight) was injected once at 12 h after the first β-Lap administration. Cochlear tissue was isolated at indicated times (a and b) and 4 days (c and d) after treatment with cisplatin (16 mg/kg body weight) or β-Lap (40 mg/kg body weight). Level of SIRT1 protein was analyzed by IHC (a) and western blotting (b and c) in the cochlear tissue. Densitometric analysis is presented as the fold induction of SIRT1 relative to β-actin. SIRT1 activity was measured using SIRT1 assay kit in the cochlear tissues (d). *, #P<0.05 by one-way ANOVA compared with the control (*) and cisplatin only (#) group, n=5. OC: organ of Corti; SGN: spiral ganglion neuron; SL: spiral ligament; SLim: spiral limbus; SV: stria vascularis
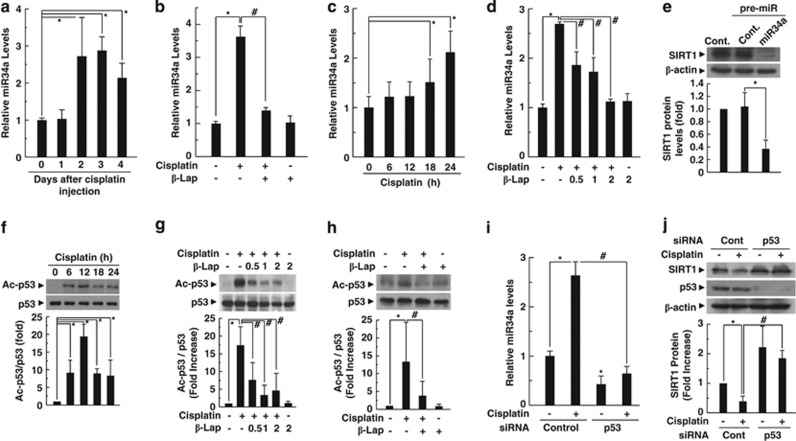
β-Lap restores SIRT1 expression through the modulation of acetylated p53 and its transcriptional target miR-34a expression
Recent reports indicate that miR-34a is a negative regulator of SIRT1 and downstream effector of p53.40, 41 As SIRT1 expression is decreased by cisplatin, we next investigated the expression level of miR-34a in the cochlear tissue of animals in the experimental groups and in HEI-OC1 auditory cells. After cisplatin exposure, the expression level of miR-34a was significantly increased in the cochlear tissue (Figure 6a) and HEI-OC1 cells (Figure 6c), whereas the increase was completely attenuated to control levels by treatment with β-Lap (Figures 6b and d). To explore the effect of miR-34a on SIRT1 protein expression, we examined the effect of miR-34a overexpression on SIRT1 expression level by western blot analysis. As shown in Figure 6e, miR-34a overexpression by transfection of miR-34 precursor markedly suppressed the SIRT1 expression in HEI-OC1 cells. The activation of p53, especially acetylated p53, has been described as a key mediator of cisplatin toxicity and upstream regulator of miR-34a. Therefore, we evaluated whether β-Lap decreased cisplatin-induced acetylation of p53 in HEI-OC1 cells. Western blot analyses indicated that cisplatin treatment significantly increased acetylation of p53 (Figure 6f) without change in total p53 protein level, whereas β-Lap completely suppressed p53 acetylation in HEI-OC1 cells (Figure 6g). In addition, β-Lap significantly attenuated cisplatin-induced p53 acetylation in cochlear tissues of mice (Figure 6h). To further confirm actual activation of p53 by its acetylation after cisplatin treatment, we examined the downstream targets of p53 including Bax and p21. Treatment with cisplatin to HEI-OC1 cells significantly increased the levels of Bax and p21 protein expression in a time-dependent manner (Supplementary Figure 5A). However, such increases were significantly attenuated by β-Lap in a dose-dependent manner (Supplementary Figure 5B). Therefore, these results strongly suggest that cisplatin induces p53 activation by its acetylation, but not by the increase of p53 protein expression. To clarify the role of p53 on miR-34a and SIRT1 expression, we performed knockdown of p53 by siRNA transfection. The knockdown of p53 markedly attenuated the increase in miR-34a expression and restored the SIRT1 expression levels in cisplatin-treated HEI-OC1 cells (Figures 6i and j).
Figure 6.
β-Lap attenuates cisplatin-induced decrease in SIRT1 expression through deacetylation of p53. (a–d) Levels of miR-34a expression were analyzed by quantitative RT-PCR in the cochlear tissue (a and b) and HEI-OC1 cells (c and d). (e) HEI-OC1 cells were transfected with pre-miR-34a and control miR for 36 h. Level of SIRT1 protein was analyzed by western blotting. (f–h) Acetylated p53 and total p53 levels were determined by western blotting using anti-acetylated p53 and anti-p53 antibodies in the HEI-OC1 cells and cochlear tissues. *, #P<0.05 by one-way ANOVA compared with the control (*) and cisplatin only group (#). (i and j) Cells were transfected with 100 nM control or p53-specific siRNAs, and then incubated for 24 h. The cells were further treated with cisplatin (20 μM) for 24 h. *, #P<0.05 by one-way ANOVA compared with the control (*) and control-siRNA transfected cells (#), n=3
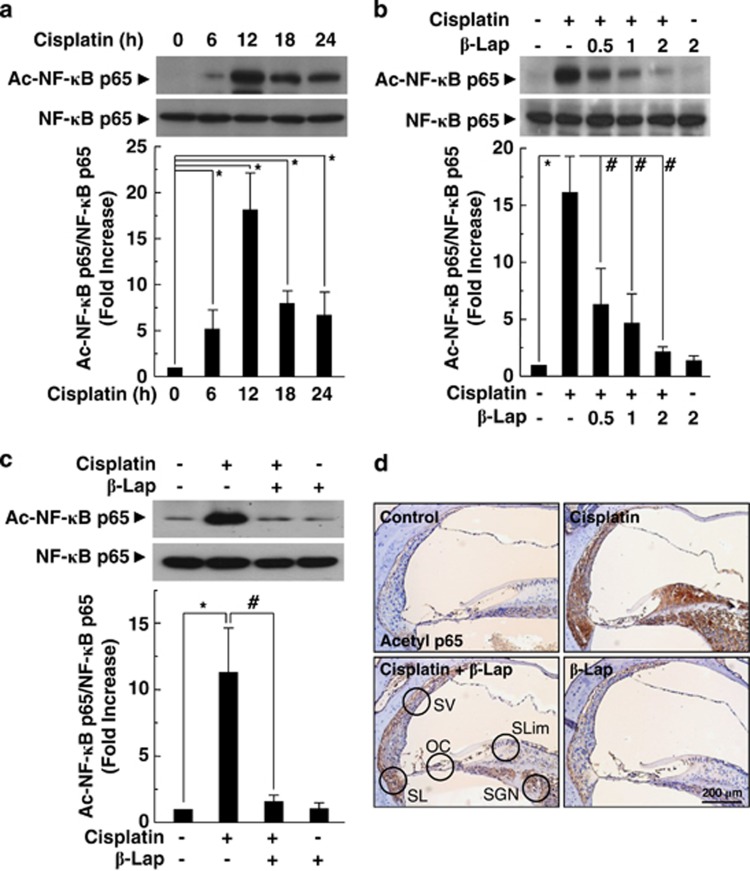
β-Lap inhibits the acetylation of NF-κB subunit p65 and production of pro-inflammatory cytokines
As SIRT1 activation decreases acetylation of NF-κB subunit p65 at lysine-310,19 we next examined whether acetylation of NF-κB subunit p65 is decreased by β-Lap. Western blot analysis indicated that cisplatin significantly increased acetylation of NF-κB p65, and the maximum increase was observed 12 h after cisplatin treatment in HEI-OC1 cells (Figure 7a). To explore the effect of β-Lap on cisplatin-induced acetylation of NF-κB, we examined the changes in NF-κB p65 acetylation by western blot and IHC analysis. We found that β-Lap suppressed cisplatin-induced acetylation of NF-κB p65 in HEI-OC1 cells and cochlear tissue (Figures 7b–d). Consistent with these results, β-Lap significantly attenuated cisplatin-induced TNF-α protein expression (Supplementary Figure 6A) and mRNA levels of pro-inflammatory cytokine genes in the cochlear tissue (Supplementary Figures 6B–D). Taken together, these results suggested that the induction of the cellular NAD+ levels using β-Lap, whose intracellular target is NQO-1, ameliorated cisplatin-ototoxicity by the regulation of PARP-1 and SIRT1 activity.
Figure 7.
β-Lap inhibits cisplatin-induced acetylation of NF-κB p65 and production of pro-inflammatory cytokines. (a–c) Acetylated NF-κB p65 and total NF-κB p65 levels were determined by western blotting using anti-acetylated NF-κB p65 (K310) and anti-NF-κB p65 in the HEI-OC1 cells (a and b) and cochlear tissues (c). Acetylated NF-κB p65 was localized by IHC in the cochlear tissue (d). Control, PBS-treated group; Cisplatin, 16 mg/kg cisplatin only group; Cisplatin+β-Lap, cisplatin and 40 mg/kg β-Lap combined group; β-Lap, 40 mg/kg β-Lap only group
Discussion
Cisplatin is a widely used chemotherapeutic drug for the treatment of various solid tumors. However, adverse effects including nephrotoxicity and ototoxicity limit its therapeutic efficacy. Cisplatin-induced nephrotoxicity can be attenuated by saline hydration as well as mannitol diuresis, whereas there are no clinically proven protective remedies for cisplatin ototoxicity. Although the incidence and severity of hearing loss after cisplatin treatment vary considerably, 40–80% of patients have an elevated hearing threshold following cisplatin treatment.42, 43, 44 Thus, it is an imperative to develop treatments that will ameliorate cisplatin ototoxicity.
NAD+ and NADH act as metabolic cofactors and rate-limiting co-substrates for many enzymes involved in various biological processes, including energy metabolism, stress adaptation and cellular homeostasis. Many studies have demonstrated that maintaining intracellular NAD+ is important for cell survival in various diseases, including axonal degeneration, cerebral ischemia, and cardiac hypertrophy. NAD+ biosynthesis is mainly accomplished through either the de novo pathway from tryptophan or the salvage pathway from nicotinamide and nicotinic acid. NAD+ can be also converted from NADH by NQO1, which catalyzes two-electron reduction of natural substrates such as Coenzyme Q-10 or vitamin E, but this reaction rate is very slow. Recently, many studies have reported that the conversion of NADH to NAD+ by NQO1 and β-Lap, a strong NQO1 substrate, elicits beneficial effects on features of metabolic syndromes, including aging, obesity, hypertension, arterial restenosis, and salt- or cisplatin-induced renal injury,34, 35, 36, 37, 38 raising the possibility that intracellular NAD+ increase through the NQO1 enzymatic action might be a potential therapeutic approach for treating various diseases. β-Lap is mainly identified as an antitumor agent because of its selective killing of cancer cells in an NQO1-dependent manner. However, research interest on β-Lap has been renewed because of its therapeutic role in various metabolic diseases, because it increases SIRT1 activity by boosting intracellular NAD+ level.
In this study, we demonstrated the impact of NAD+ metabolism in cisplatin ototoxicity. Our results are the first to show that cisplatin-induced cochlear damage and hearing loss are mediated by the significant decline in SIRT1 activity by a decrease in intracellular NAD+ level because of the hyperactivation of PARP-1 and the decrease of SIRT1 expression regulated by the p53-miR-34a pathway. Moreover, the decrease in SIRT1 activity could not deacetylase its downstream targets such as p53 and NF-κB, which were highly activated by acetylation after cisplatin exposure, and thereby aggravated cisplatin-mediated ototoxicity through inflammation and apoptosis. However, β-Lap administration attenuated cisplatin-induced hearing loss and deleterious effects on cochlear tissue. In addition, β-Lap reduced cytotoxicity by consistently decreasing ROS production and subsequent DNA damage induced by cisplatin. These in turn restored intracellular NAD+ levels through inactivation of PARP-1. β-Lap, by itself, increased cellular NAD+ levels in a NQO1-dependent manner. Consistently, β-Lap attenuated the cisplatin-induced decrease in both SIRT1 expression and activity, and facilitated the deacetylation of p53 and NF-κB.
Previous studies have demonstrated that cisplatin accumulates in the target tissues such as the cochlea to produce ROS, which may deplete cellular defense mechanisms against oxidative stress and DNA damage. Cisplatin also directly binds to DNA, resulting in the disruption of the synthesis of key proteins and leading to cell injury and cell death. Supporting this notion, our data indicated that the levels of DNA damage in the cochlear tissue were significantly increased by cisplatin treatment consistent with the increase in intracellular ROS levels. Accumulation of DNA damage can lead to cell cycle arrest or genomic instability. The removal of oxidative DNA damage through repair of DNA single-strand breaks is facilitated by PARPs. PARP-1 is the most critical protein-modifying nuclear enzyme involved in DNA repair. PARP-1 is a major NAD+ consumer in this cellular processes, in which the ADP-ribose moiety is transferred to PARP-1 itself or other acceptor proteins in order to build poly(ADP-ribose) polymer (PAR).45 PARP-1 is strongly activated by DNA damage and oxidative stress. Under physiological conditions, mild activation of PARP-1 can regulate several cellular processes, including DNA repair, cell cycle progression, cell survival, chromatin remodeling, and genomic stability. However, hyperactivation of PARP-1 upon severe oxidative damage causes rapid depletion of intracellular NAD+ levels and ATP, and eventually leads to cell death and related pathological conditions.46, 47 In this study, hyperactivation of PARP-1 was observed in cisplatin-treated cochlea, and this hyperactivation led to a decline in the intracellular NAD+ levels and SIRT1 activity. Interestingly, it is well established that PARP-1 and SIRT1 activity are inter-dependent as they compete for a limited pool of cellular NAD+. SIRT1 has a Km for NAD+ that lies within the range of the physiological changes in intracellular NAD+ content. However, Km of PARP-1 for NAD+ is 2–10 times lower than SIRT1.48 Thus, PAPR-1 activation may critically influence SIRT1 activity by reducing NAD+ bioavailability. This is further supported by recent studies where genetic depletion of PARP-1 or pharmacological inhibition of PARP-1 activity increases intracellular NAD+ level and subsequent SIRT1 activity. Consistent with these results, we demonstrated that cisplatin-mediated PARP-1 activation critically influenced cytotoxicity and ATP levels, as well as SIRT1 activity, through the decrease in cellular NAD+/NADH. Furthermore, recent observations have demonstrated that SIRT1 has a pivotal role in cisplatin toxicity.8, 49 In our previous study, we reported that SIRT1 expression was increased in cisplatin-treated kidney tissues, whereas SIRT1 activity was decreased in cisplatin-treated kidney tissue because of the decrease in intracellular NAD+ level.9 Interestingly, in this study, we observed simultaneous decreases in SIRT1 activity and protein expression in cisplatin-treated cochlear tissues and HEI-OC1 auditory cells. These results suggest that SIRT1 expression might be differentially regulated by cisplatin depending on the tissue types. It has to be elucidated how cisplatin affects the SIRT1 expression levels. In general, the expression of SIRT1 can be regulated by PARP-2. PARP-2 acts as a transcriptional repressor of SIRT1 by direct binding with SIRT1 promoter. The expression of SIRT1 can be also regulated by microRNAs (miRNAs), such as miR-9, miR-22, miR-34, miR-181, and miR-200. Among them, miR-34a is the first identified miRNA to regulate SIRT1.41 Recently, miR-34a has been reported to bind directly to 3'-UTR of SIRT1 and regulate cell death and senescence by repressing SIRT1 expression in various cells, suggesting that miR-34a may be a negative regulator of SIRT1.50 Therefore, we investigated whether PARP-2 and miR-34a affect SIRT1 expression. Our results showed a significant increase in miR-34a expression in the cisplatin-treated cochlear tissue and HEI-OC1 auditory cells. In addition, our gain-of-function approaches to miR-34a demonstrated that transfection of miR-34a precursor into HEI-OC1 auditory cells resulted in the downregulation of SIRT1 expression. However, neither PARP-2 induction (Supplementary Figure 7A) nor direct binding between PARP-2 and SIRT1 promoter (Supplementary Figure 7B) could be observed in cisplatin-treated HEI-OC1 cells. These observations indicate that miR-34a is mainly involved in the downregulation of SIRT1 expression after cisplatin treatment. In addition, miR-34a is also known as a downstream effecter of p53. It is well known that tumor suppressor p53 is one of the main players in cisplatin-mediated cytotoxicity. Previous studies demonstrated that downregulation of p53 attenuated cisplatin-induced cytotoxicity.51 p53 is subjected to post-translational modifications that have critical effects on its stability and function, including phosphorylation, acetylation, sumoylation, neddylation, and methylation.52 Activation of p53 is an important cellular response to DNA damage. It is noteworthy that recent studies have reported that p53 is activated by its acetylation on lysine residues, including Lys 370, 372, 382, and 386 in the carboxy-terminal region53 and acetylated p53 is critically involved in cisplatin toxicity.8, 9 As miR-34a negatively regulates SIRT1 expression level, we examined whether p53 is influenced in miR-34a-mediated repression of SIRT1. As expected, we confirmed the cisplatin-induced p53 activation by measuring its enhanced acetylation level. In addition, downregulation of p53 protein expression by transfection of p53-specific siRNA markedly restored SIRT1 expression to normal control levels via decrease in miR-34a in the cisplatin-treated HEI-OC1 cells. Interestingly, SIRT1 regulates p53 function through direct interaction and subsequent deacetylation of p53.18, 54 In the nucleus, acetylated p53 stimulates its sequence-specific DNA binding and subsequent recruitment of other transcription cofactors to promoter regions, and thereby enhances the transcription of target genes, such as the p53-upregulated modulator of apoptosis, NADPH activator A, and p53-induced gene 3, that are involved in ROS production through mitochondrial dysfunction or apoptosis. Nuclear-localized SIRT1 was shown to deacetylate p53 and represses p53-mediated cell growth arrest and apoptosis in response to DNA damage and oxidative stress.55 Thus, our results suggest that cisplatin-mediated reduction of SIRT1 expression and activity can accelerate the p53-miR-34a pathway as a mutual ‘cause and effect' feedback loop.
NF-κB transcription factor is one of the key regulators of inflammation. In our previous study, we demonstrated that pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, have a critical role in cisplatin-induced cochlear injury through activation of NF-κB.7 NF-κB activation is achieved by either an IκB-dependent pathway through IκB phosphorylation and subsequent degradation or an IκB-independent pathway through post-translational modifications of Rels, including acetylation of the NF-κB p65 subunit. NF-κB p65 can be acetylated at five specific lysine residues (Lys-122, -123, -218, -221, and -310). In particular, acetylation of Lys-310 is required for the transcriptional activity of NF-κB, whereas the other acetylation sites are involved in DNA binding.56 A large body of recent evidence indicates that SIRT1 regulates the inflammatory responses through NF-κB p65 deacetylation. SIRT1 knockdown leads to the activation of the inflammatory pathway with increased inflammatory gene expression, whereas SIRT1 activation produces anti-inflammatory effects.57 SIRT1 physically interacts with the nuclear translocated NF-κB p65 and deacetylates NF-κB p65 at Lys-310, thereby inhibiting the transcriptional activity of NF-κB.19 In addition, SIRT1 could also inactivate p300/CBP, critical co-activators of NF-κB, by deacetylation and facilitate the downregulation of NF-κB-dependent expression of inflammatory genes.58 Interestingly, it has been reported that the overexpression of SIRT1 or treatment with resveratrol, an indirect activator of SIRT1, decreased the acetylation of NF-κB p65 and cytotoxicity induced by cisplatin in the human proximal tubule cells.59 In this study, we also observed that the acetylation of NF-κB p65 was increased in cisplatin-treated cochlear tissue and HEI-OC1 auditory cells facilitated by the decreased SIRT1 activity and expression caused by the decrease in intracellular NAD+ levels and the increased miR-34a, respectively. However, β-Lap treatment downregulated the acetylation of NF-κB p65 as well as the production of pro-inflammatory cytokines through activation of SIRT1 in the cochlea.
In conclusion, we have demonstrated for the first time that both the protein expression level and the activity of SIRT1 were suppressed by the reduction of intracellular NAD+ levels in cisplatin-treated cochlear tissue. We also found that the decrease in SIRT1 protein expression and its activity after cisplatin exposure were mediated by the increase in transcriptional activity of p53 for miR-34a expression and PARP-1 activation causing NAD+ depletion, respectively. However, the increase in cellular NAD+ levels by the NQO1 enzymatic action using β-Lap as a substrate prevented mice from cisplatin-induced cochlear damage and hearing impairment through the modulation of PARP-1, SIRT1, p53, and NF-κB. Considering that β-Lap itself did not attenuate the tumoricidal effect of cisplatin,9 these results suggest that the direct modulation of the cellular NAD+ level by pharmacological agents could be a promising therapeutic strategy for enhancing the efficacy of cisplatin chemotherapy without its adverse effects.
Materials and Methods
Reagents
β-Lap was chemically synthesized by KT&G Life Science (Suwon, Korea) and micronized as particles coated with calcium silicate to enhance oral bioavailability.34, 35 Cisplatin and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma Chemical Co (Sigma, St Louis, MO, USA). Dicoumarol was purchased from Calbiochem (San Diego, CA, USA). Antibodies to γ-H2AX, H2AX, acetyl-NF-κB p65, and acetyl-p53 were purchased from Cell Signaling Inc. (Beverly, MA, USA). Antibodies against NF-κB p65, SIRT1, p53, Bax, p21, and β-actin were purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA, USA). DMEM, FBS, and other tissue culture reagents were obtained from Life Technologies Inc. (Gaithersburg, MD, USA).
Cell culture
The establishment and characterization of conditionally immortalized HEI-OC1 auditory cells have been described in our previous report.7 Expression of OHC-specific markers such as Math1 and Myosin 7a suggests that HEI-OC1 cells represent OHC precursors. For the experiments, HEI-OC1 cells were cultured under the following permissive conditions: 33 oC and 5% CO2 in high-glucose DMEM supplemented with 10% FBS. HEI-OC1 cells were incubated with cisplatin (20 μM) in the presence of varying concentrations of β-Lap. To determine cell viability, MTT (0.25 mg) was added to 1 ml of cell suspension for 4 h. After three washes of cells with PBS (pH 7.4), the insoluble formazan product was dissolved in DMSO. Then, the optical density of each culture well was measured using a microplate reader (Titertek Multiskan, Flow Laboratories, McLean, VA, USA) at 590 nm. The optical density in control cells was taken as 100% of viability.
Animal experiments and measurement of hearing function
Male C57BL/6 mice were purchased from the Central Laboratory Animal Inc. (Seoul, Korea). NQO1 knockout mice on a C57BL/6 background were kindly provided by Dr. CH Lee (Animal Model Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea). All mice were fed a standard commercial diet while housed at an ambient temperature of 20–22 °C with a relative humidity of 50%±5% under 12-h/12-h light–dark cycle in a specific pathogen-free facility. The experimental mice were 8 weeks old and divided into four groups: control (n=5), cisplatin (16 mg/kg; Sigma; n=5), β-Lap plus cisplatin (n=5), and β-Lap alone (40 mg/kg; n=5). β-Lap was administered orally once a day for 4 consecutive days. A single dose of cisplatin was administered intraperitoneally 12 h after the first β-Lap administration. For analysis of the auditory threshold, ABR was recorded at 1 day before and 4 days after the cisplatin treatment with tone bursts of 4, 8, 16, and 32 kHz (1-ms rise/fall time, 2-ms plateau) using an ABR workstation (Tucker-Davis Technologies, Alachua, FL, USA). Mice were anesthetized using a cocktail of ketamine (40 mg/kg) and xylazine (10 mg/kg) and kept warm with a heating pad during ABR recording. A subdermal (active) needle electrode was inserted at the vertex, while ground and reference electrodes were inserted subdermally in the loose skin beneath the pinnae of opposite ears. The ABR waveforms were averaged over a 10-ms time window, using the Tucker-Davis ABR workstation software. The sound intensity was varied at 10-dB intervals near the hearing threshold. The ABR thresholds between pre-treatment and post-treatment conditions were then compared. Judgment of the threshold was made off-line by two independent, experimentally blinded observers on the basis of the ABR records. In addition, the mice were killed at specified time points for IHC and biochemical analysis. The experimental protocol was approved by the Animal Care and Use Committee at Wonkwang University.
Transfection with siRNA constructs
Predesigned siRNAs against mouse NQO1 (sc-37140), PARP-1 (sc-29438), p53 (sc-29436), and control scrambled siRNA were purchased from Santa Cruz Biotechnology. For the siRNA knockdown experiments, HEI-OC1 cells were transiently transfected with 100 nM siRNA constructs using X-tremeGENE siRNA transfection reagent (Roche Applied Science, Penzberg, Germany), following the manufacturer's protocol. The interference in expression was confirmed by immunoblot analysis.
Measurement of SIRT1 activity
The effects of cisplatin and β-Lap on SIRT1 activity were determined using a fluorescent SIRT1 assay kit (Enzo Life Sciences International Inc., Plymouth Meeting, PA, USA) following the manufacturer's instructions. Briefly, the SIRT1 activity assays were performed using Fluor de Lys-SIRT1, NAD+, and SIRT1 in SIRT1 assay buffer (25 mM Tris-Cl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, and 1 mg/ml bovine serum albumin) in a 96-well plate. Reactions were initiated by adding each substrate solution. After incubation at 37 oC for 1 h, the plate was further incubated with developing solution for 5 min. The deacetylation of the substrate was measured using CytoFluor series 4000 fluorometer (PerSeptive Biosystems Inc., Framingham, MA, USA) with excitation wavelength set to 360 nm and emission wavelength set to 460 nm.
Measurement of PARP-1 activity
PARP activity was assayed using the Trevigen Universal chemiluminescent PARP assay kit (Trevigen, Gaithersburg, MD, USA) following the manufacturer's instructions. The lysate (30 μg/well) was added in duplicates to the wells containing PARP buffer and PARP cocktail, followed by incubation at room temperature for 1 h. The wells were washed thrice with PBS plus 0.1% Triton X-100 (PBST), followed by incubation with a 1:1000 dilution of streptavidin-horseradish peroxidase in strep diluent buffer for 1 h. After three washes with PBST, chemiluminescent detection was performed. The background reading was subtracted from the readings of the samples, and PARP activity was calculated using the standard curve obtained from the readings of standards.
Determination of ROS production
ROS production was determined by the modified method from Gang et al.60 Briefly, to determine total ROS, cochlear tissue extracts (100 μl) from each experiment group were incubated with 20 μM H2-DCFDA (Invitrogen, San Diego, CA, USA) for 60 min at 37 °C. Fluorescence intensity was recorded using the CytoFluor series 4000 fluorometer (PerSeptive Biosystems Inc.) and normalized to protein content.
Real-time PCR for miR-34a and pro-inflammatory cytokines
For evaluation of miR-34a expression, total RNA including the small RNA fraction was extracted from isolated cochlea and HEI-OC1 cells using a mirVana miRNA isolation kit (Ambion, Austin, TX, USA) following the manufacturer's instructions. Extracted RNA was reverse transcribed by using Taqman miRNA Reverse Transcription Kit and then amplified in a 20 μl PCR and primer set for amplification of miR-34a (Assay ID: 000426) and U6 (Assay ID: 001093) using TaqMan miR assays following the manufacturer's recommended protocol. The amplification step consisted of an initial denaturation at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s and then annealing at 60 °C for 1 min. All reactions were carried out on the LightCycler PCR system (Roche Applied Science) using the TaqMan Universal PCR master Mix. Relative levels were determined using the ΔΔCt (threshold cycle value) method with U6 as an endogenous control, and the fold changes were calculated for each sample. For determination of the levels of pro-inflammatory cytokines, total RNA was isolated from cells using TRIzol (Invitrogen) following the manufacturer's protocol. Three micrograms of RNA were used for cDNA conversion using the First Strand cDNA Synthesis Superscript kit (Invitrogen) following the manufacturer's protocol. Quantitative real-time PCR was performed using SYBR Green Mastermix (Invitrogen). Reactions were performed in triplicates, and specificity was monitored using melting curve analysis after cycling. Primers used were as follows: TNF-α, 5′-CTGAGGTCAATCTGCCCAAGTAC-3′ and 5′-CTTCACAGAGCAATGACTCCAAAG-3′ IL-1β, 5′-TCTTTGAAGTTGACGGACCC-3′ and 5′-TGAGTGATACTGCCTGCCTG-3′ IL-6, 5′-TCGTGGAAATGAGAAAAGAGTTG-3′ and 5′-AGTGCATCATCGTTGTTCATACA-3′ GAPDH, 5′-TCCCACTCTTCCACCTTCGA-3′ and 5′-AGTTGGGATAGGGCCTCTCTTG-3′. Relative mRNA expression levels were quantified using the ΔΔCt method and GAPDH was used as an internal control. Results are expressed as fold changes relative to controls.
Determination of NAD+/NADH ratio
NAD+ and NADH were measured using a fluorescent NAD+/NADH detection kit (Cell Technology Inc., Mountain View, CA, USA) following the manufacturer's instruction. Briefly, cells were homogenized in 200 μl acid extraction buffer to measure total cellular NAD+ concentration, or 200 μl alkali extraction to determine total cellular NADH concentration. Homogenates were then neutralized, and after an enzymatic cycling reaction, the concentration of NAD+/NADH was measured using a fluorescence microplate reader.
Western blot analysis
Twenty micrograms of protein were subjected to electrophoresis on 10% SDS-polyacrylamide gels for 3 h at 20 mA, and then the protein was transferred to a nitrocellulose membrane. The membrane was then incubated in 5% (wt/vol) dried milk protein in PBS containing 0.05% (vol/vol) Tween-20 (PBST) for 1 h, washed in PBST, and then further reacted with primary antibody (1:1,000) for 1 h. Next, the membrane was extensively washed with PBST and incubated with appropriate secondary antibodies for 1 h at room temperature. After extensive washes, protein bands on the membrane were visualized using chemiluminescent reagents according to the manufacturer's instructions (Supersignal Substrate; Pierce, Rockford, IL, USA).
IHC staining and TUNEL staining
The removed temporal bone was fixed in 4% paraformaldehyde for 16 h and then decalcified with 10% EDTA in PBS for 2 weeks, after which it was dehydrated and embedded in paraffin wax. Next, 5-μm-thick sections were deparaffinized in xylene and rehydrated through graded concentrations of ethanol. For the immunohistochemistry study, an immunohistochemistry kit (DAKO LSAB Universal K680, Carpinteria, CA, USA) was used following the manufacturer's instructions. The endogenous peroxidase was blocked with 3% hydrogen peroxide for 5 min at room temperature. After the sections were washed in PBS, nonspecific binding was blocked with 1% BSA for 1 h. Primary antibodies were then added to the slides, after which the incubation was continued for 1 h. After repeated washes with PBS, the sections were incubated with biotinylated secondary antibody for 30 min and then covered with streptavidin-peroxidase for 15 min. Finally, the sections were stained in a freshly prepared substrate solution (3 mg of 3-amino-9-ethylcarbazole in 10 ml of sodium acetate buffer (pH 4.9), 500 μl of dimethylformamide, and 0.03% hydrogen peroxide) for 5 min. The nuclei of the immunostained cells were then counterstained with Mayer's hematoxylin (Sigma). After washes with PBS, the specimens were examined by fluorescence microscopy. Apoptotic cells were detected in situ using the TUNEL assay (TUNEL POD kit, Roche Molec Biochemic, Mannheim, Germany). Briefly, a section was deparaffinized and rehydrated. After incubation with 20 μg/ml proteinase K (Boehringer Mannheim, Mannheim, Germany), the endogenous peroxidase was blocked by incubating the samples in 2% H2O2 in methanol for 30 min at room temperature. Next, the tissue sections were washed in PBS and incubated with labeling solution for 1 h at 37 °C. The nuclei were then counterstained with propidium iodide (0.5 μg/ml, Molecular Probes) for 10 min at room temperature. After washes with PBS, the specimens were examined by fluorescence microscopy.
Measurement of ATP concentrations
Intracellular ATP levels were measured using EnzyLight ATP assay (BioAssay Systems, Hayward, CA, USA). Luminescence was measured on a luminometer AutoLumat LB953 (EG and G Berthold, Bad Wildbad, Germany) and quantitated relative to ATP standards.
Statistical analyses
Each experiment was performed at least three times, and all values reported represent the means±S.D. of triplicate analyses. Statistical multivariate analysis was performed by analysis of variance and Duncan tests, using the SPSS 11 (Chicago, IL, USA) statistical software. Two-way ANOVA and/or one-way ANOVA were used to determine the significance of the results. The statistical results were reviewed by a masters-level biostatistician, and P<0.05 was considered as statistically significant.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government [MISP]: [No. 2011-0028866], [No. 2011-0030130], and [NRF-2012R1A1A2044261].
Glossary
- Cisplatin
cis-diamminedichloroplatinum (II)
- OHCs
outer hair cells
- ROS
reactive oxygen species
- NAD+
nicotinamide adenine dinucleotide
- SIRTs
sirtuins
- PARPs
poly(ADP-ribose) transferases
- PAR
poly(ADP-ribose) polymer
- NQO1
NADH:quinone oxidoreductase 1
- β-Lap
β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione)
- ABR
auditory brainstem response
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by C Munoz-Pinedo
Supplementary Material
References
- 1Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 2005; 4: 307–320. [DOI] [PubMed] [Google Scholar]
- 2Rybak LP, Somani S. Ototoxicity Amelioration by protective agents. Ann N Y Acad Sci 1999; 884: 143–151. [PubMed] [Google Scholar]
- 3Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. J Toxicol 2012; 2012: 645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 2008; 73: 994–1007. [DOI] [PubMed] [Google Scholar]
- 5Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci 2010; 30: 3933–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Jiang M, Yi X, Hsu S, Wang CY, Dong Z. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 2004; 287: F1140–F1147. [DOI] [PubMed] [Google Scholar]
- 7So H, Kim H, Lee JH, Park C, Kim Y, Kim E et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol 2007; 8: 338–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Kim DH, Jung YJ, Lee JE, Lee AS, Kang KP, Lee S et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am J Physiol Renal Physiol 2011; 301: F427–F435. [DOI] [PubMed] [Google Scholar]
- 9Oh GS, Kim HJ, Choi JH, Shen A, Choe SK, Karna A et al. Pharmacological activation of NQO1 increases NAD levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int 2014; 85: 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Oka S, Hsu CP, Sadoshima J. Regulation of cell survival and death by pyridine nucleotides. Circ Res 2012; 111: 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Abdellatif M. Sirtuins and pyridine nucleotides. Circ Res 2012; 111: 642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Imai S, Kiess W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front Biosci (Landmark Ed) 2009; 14: 2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci 2007; 32: 12–19. [DOI] [PubMed] [Google Scholar]
- 14Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 2008; 14: 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 2007; 26: 3169–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 2007; 404: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W et al. Mammalian SIRT1 represses forkhead transcription factors. Cell 2004; 116: 551–563. [DOI] [PubMed] [Google Scholar]
- 18Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001; 107: 149–159. [DOI] [PubMed] [Google Scholar]
- 19Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004; 23: 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem 2005; 280: 43121–43130. [DOI] [PubMed] [Google Scholar]
- 21Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 2002; 54: 375–429. [DOI] [PubMed] [Google Scholar]
- 22Mukhopadhyay P, Horvath B, Kechrid M, Tanchian G, Rajesh M, Naura AS et al. Poly(ADP-ribose) polymerase-1 is a key mediator of cisplatin-induced kidney inflammation and injury. Free Radic Biol Med 2011; 51: 1774–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Shino Y, Itoh Y, Kubota T, Yano T, Sendo T, Oishi R. Role of poly(ADP-ribose)polymerase in cisplatin-induced injury in LLC-PK1 cells. Free Radic Biol Med 2003; 35: 966–977. [DOI] [PubMed] [Google Scholar]
- 24Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 2000; 129: 77–97. [DOI] [PubMed] [Google Scholar]
- 25Gaikwad A, Long DJII, Stringer JL, Jaiswal AK. In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J Biol Chem 2001; 276: 22559–22564. [DOI] [PubMed] [Google Scholar]
- 26Pazdro R, Burgess JR. The antioxidant 3H-1,2-dithiole-3-thione potentiates advanced glycation end-product-induced oxidative stress in SH-SY5Y cells. Exp Diabetes Res 2012; 2012: 137607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Jones CIIII, Zhu H, Martin SF, Han Z, Li Y, Alevriadou BR. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann Biomed Eng 2007; 35: 683–693. [DOI] [PubMed] [Google Scholar]
- 28Moscovitz O, Tsvetkov P, Hazan N, Michaelevski I, Keisar H, Ben-Nissan G et al. A mutually inhibitory feedback loop between the 20S proteasome and its regulator. NQO1. Mol Cell 2012; 47: 76–86. [DOI] [PubMed] [Google Scholar]
- 29Haefeli RH, Erb M, Gemperli AC, Robay D, Courdier Fruh I, Anklin C et al. NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy levels. PLoS One 2011; 6: e17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Huang X, Dong Y, Bey EA, Kilgore JA, Bair JS, Li LS et al. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res 2012; 72: 3038–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Pardee AB, Li YZ, Li CJ. Cancer therapy with beta-lapachone. Curr Cancer Drug Targets 2002; 2: 227–242. [DOI] [PubMed] [Google Scholar]
- 32Li LS, Bey EA, Dong Y, Meng J, Patra B, Yan J et al. Modulating endogenous NQO1 levels identifies key regulatory mechanisms of action of beta-lapachone for pancreatic cancer therapy. Clin Cancer Res 2011; 17: 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Planchon SM, Wuerzberger S, Frydman B, Witiak DT, Hutson P, Church DR et al. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: a p53-independent response. Cancer Res 1995; 55: 3706–3711. [PMC free article] [PubMed] [Google Scholar]
- 34Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes 2009; 58: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Kim SY, Jeoung NH, Oh CJ, Choi YK, Lee HJ, Kim HJ et al. Activation of NAD(P)H:quinone oxidoreductase 1 prevents arterial restenosis by suppressing vascular smooth muscle cell proliferation. Circ Res 2009; 104: 842–850. [DOI] [PubMed] [Google Scholar]
- 36Kim YH, Hwang JH, Noh JR, Gang GT, Kim do H, Son HY et al. Activation of NAD(P)H:quinone oxidoreductase ameliorates spontaneous hypertension in an animal model via modulation of eNOS activity. Cardiovasc Res 2011; 91: 519–527. [DOI] [PubMed] [Google Scholar]
- 37Kim YH, Hwang JH, Noh JR, Gang GT, Tadi S, Yim YH et al. Prevention of salt-induced renal injury by activation of NAD(P)H:quinone oxidoreductase 1, associated with NADPH oxidase. Free. Radic Biol Med 2012; 52: 880–888. [DOI] [PubMed] [Google Scholar]
- 38Lee JS, Park AH, Lee SH, Lee SH, Kim JH, Yang SJ et al. Beta-lapachone, a modulator of NAD metabolism, prevents health declines in aged mice. PLoS One 2012; 7: e47122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39David KK, Andrabi SA, Dawson TM, Dawson VL. Parthanatos, a messenger of death. Front Biosci (Landmark Ed) 2009; 14: 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ 2010; 17: 193–199. [DOI] [PubMed] [Google Scholar]
- 41Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 2008; 105: 13421–13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42de Jongh FE, van Veen RN, Veltman SJ, de Wit R, van der Burg ME, van den Bent MJ et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer 2003; 88: 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer 1998; 77: 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol 2005; 23: 8588–8596. [DOI] [PubMed] [Google Scholar]
- 45Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell 2010; 39: 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Goodwin PM, Lewis PJ, Davies MI, Skidmore CJ, Shall S. The effect of gamma radiation and neocarzinostatin on NAD and ATP levels in mouse leukaemia cells. Biochim Biophys Acta 1978; 543: 576–582. [DOI] [PubMed] [Google Scholar]
- 47Skidmore CJ, Davies MI, Goodwin PM, Halldorsson H, Lewis PJ, Shall S et al. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem 1979; 101: 135–142. [DOI] [PubMed] [Google Scholar]
- 48Canto C, Auwerx J. NAD+ as a signaling molecule modulating metabolism. Cold Spring Harb Symp Quant Biol 2012; 76: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem 2010; 285: 13045–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Revollo JR, Li X. The ways and means that fine tune Sirt1 activity. Trends Biochem Sci 2013; 38: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 2009; 20: 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Kruse JP, Gu W. Modes of p53 regulation. Cell 2009; 137: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell 2008; 133: 612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001; 107: 137–148. [DOI] [PubMed] [Google Scholar]
- 55Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell 2011; 2: 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J 2007; 21: 2642–2654. [DOI] [PubMed] [Google Scholar]
- 57Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 2009; 29: 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J Mol Med (Berl) 2003; 81: 549–557. [DOI] [PubMed] [Google Scholar]
- 59Jung YJ, Lee JE, Lee AS, Kang KP, Lee S, Park SK et al. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-kappaB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem Biophys Res Commun 2012; 419: 206–210. [DOI] [PubMed] [Google Scholar]
- 60Gang GT, Kim YH, Noh JR, Kim KS, Jung JY, Shong M et al. Protective role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in cisplatin-induced nephrotoxicity. Toxicol Lett 2013; 221: 165–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.