Abstract
Despite high remission rates after chemotherapy, only 30–40% of acute myeloid leukemia (AML) patients survive 5 years after diagnosis. This extremely poor prognosis of AML is mainly caused by treatment failure due to chemotherapy resistance. Chemotherapy resistance can be caused by various features including activation of alternative signaling pathways, evasion of cell death or activation of receptor tyrosine kinases such as the insulin growth factor-1 receptor (IGF-1R). Here we have studied the role of the insulin-like growth factor-binding protein-7 (IGFBP7), a tumor suppressor and part of the IGF-1R axis, in AML. We report that IGFBP7 sensitizes AML cells to chemotherapy-induced cell death. Moreover, overexpression of IGFBP7 as well as addition of recombinant human IGFBP7 is able to reduce the survival of AML cells by the induction of a G2 cell cycle arrest and apoptosis. This effect is mainly independent from IGF-1R activation, activated Akt and activated Erk. Importantly, AML patients with high IGFBP7 expression have a better outcome than patients with low IGFBP7 expression, indicating a positive role for IGFBP7 in treatment and outcome of AML. Together, this suggests that the combination of IGFBP7 and chemotherapy might potentially overcome conventional AML drug resistance and thus might improve AML patient survival.
Only 30–40% of acute myeloid leukemia (AML) patients survive 5 years after diagnosis.1 This extremely poor prognosis is mainly caused by treatment failure due to chemotherapy resistance. This resistance is often a multifactorial phenomenon that can include enhanced expression or activation of receptor tyrosine kinases such as the insulin growth factor-1 receptor (IGF-1R).2, 3 The IGF-1R stimulates proliferation, protects cells from apoptosis and has been implicated in the development and maintenance of various cancers.4, 5 Several oncogenes require an intact IGF-1R pathway for their transforming activity6 and moreover, disruption or inhibition of IGF-1R activity has been shown to inhibit the growth and motility of a wide range of cancer cells in vitro and in mouse models.4, 5 IGF-1Rs are membrane receptors and binding of their ligand, the insulin-like growth factor-1 (IGF-1), results in receptor phosphorylation and activation of MAPK and PI3K/Akt signaling.4 Importantly, IGF-1, normally produced by the liver and bone marrow stromal cells, can stimulate the proliferation of cancer cells in vitro and genetic manipulations that reduce IGF-1 signaling can lead to decreased tumor growth.7, 8
In hematological malignancies, a role for IGF-1 signaling has been demonstrated in multiple myeloma (MM) where it stimulates growth and potently mediates survival.9 Several anti-IGF-1R strategies have been shown to inhibit MM growth.10, 11 In AML, expression of the IGF-1R and IGF-1 was detected in AML cell lines and primary AML blasts and stimulation with IGF-1 can promote the growth of AML cells.12, 13, 14 In addition, neutralizing IGF-1R antibodies and the tyrosine kinase inhibitors (TKIs) NVP-AEW541 and NVP-ADW742, have been shown to inhibit proliferation and to induce apoptosis.15, 16
In addition to its mitogenic and anti-apoptotic roles, directly influencing tumor development, IGF-1R appears to be a critical determinant of response to numerous anti-cancer therapies, including TKIs and chemotherapy.2, 3, 17, 18, 19, 20, 21, 22 In AML, activated IGF-1R signaling has been linked to cytarabine resistance, a drug included in every AML treatment schedule.17 Notably, in several cancer cell lines, a small subpopulation of drug-tolerant cancer cells exists that maintains their viability, after treatment with a lethal drug dose, via engagement of the IGF-1R.18
The activity of the IGF-1R is tightly controlled at multiple levels, including their processing, endocytosis, trafficking and availability of its ligands.4 Ligand bioavailability is partly controlled by the family of secreted insulin-like growth factor-binding protein (IGFBP1 to IGFBP6), which can bind to IGFs therewith regulating the interaction of these ligands to their receptors. However, as IGFBPs are able to induce IGF-dependent and IGF-independent effects, the results of several studies on their role in cancer cell survival appeared to be controversial and complex.23, 24 In addition to IGFBPs, various IGFBP-related proteins have been identified.23, 25 One of these is the IGFB-related protein 1, also known as insulin-like growth factor-binding protein-7 (IGFBP7). IGFBP7 has 30% homology to IGFBP1 to IGFBP6 in its N-terminal domain and functions predominantly as a tumor suppressor.23, 24, 25, 26 In contrast to IGFBP1 to IGFBP6, which bind to the IGFs,23 IGFBP7 is a secreted protein that can directly bind to the IGF-1R and thereby inhibits its activity.27 The abundance of IGFBP7 is inversely correlated with tumor progression in hepatocellular carcinoma.28 Importantly, decreased expression of IGFBP7 has been associated with therapy resistance29, 30 and increasing IGFBP7 levels can inhibit melanoma and breast cancer growth.31, 32 IGFBP7 was originally identified as being involved in Raf-mediated apoptosis and senescence33 and also has been shown to induce senescence in mesenchymal stromal cells.34
We established that IGFBP7 induces a cell cycle block and apoptosis in AML cells and cooperates with chemotherapy in the induction of leukemia cell death. AML patients with low IGFBP7 expression have a worse outcome than patients with high IGFBP7 expression, indicating that AML patients might benefit from a combination therapy consisting of chemotherapy and IGFBP7. Our results define IGFBP7 as a focus to enhance chemotherapy efficacy and improve AML patient survival.
Results
Membrane IGF-1Rs are expressed in AML cell lines and inhibition of IGF-1R tyrosine kinase activity compromises AML cell growth
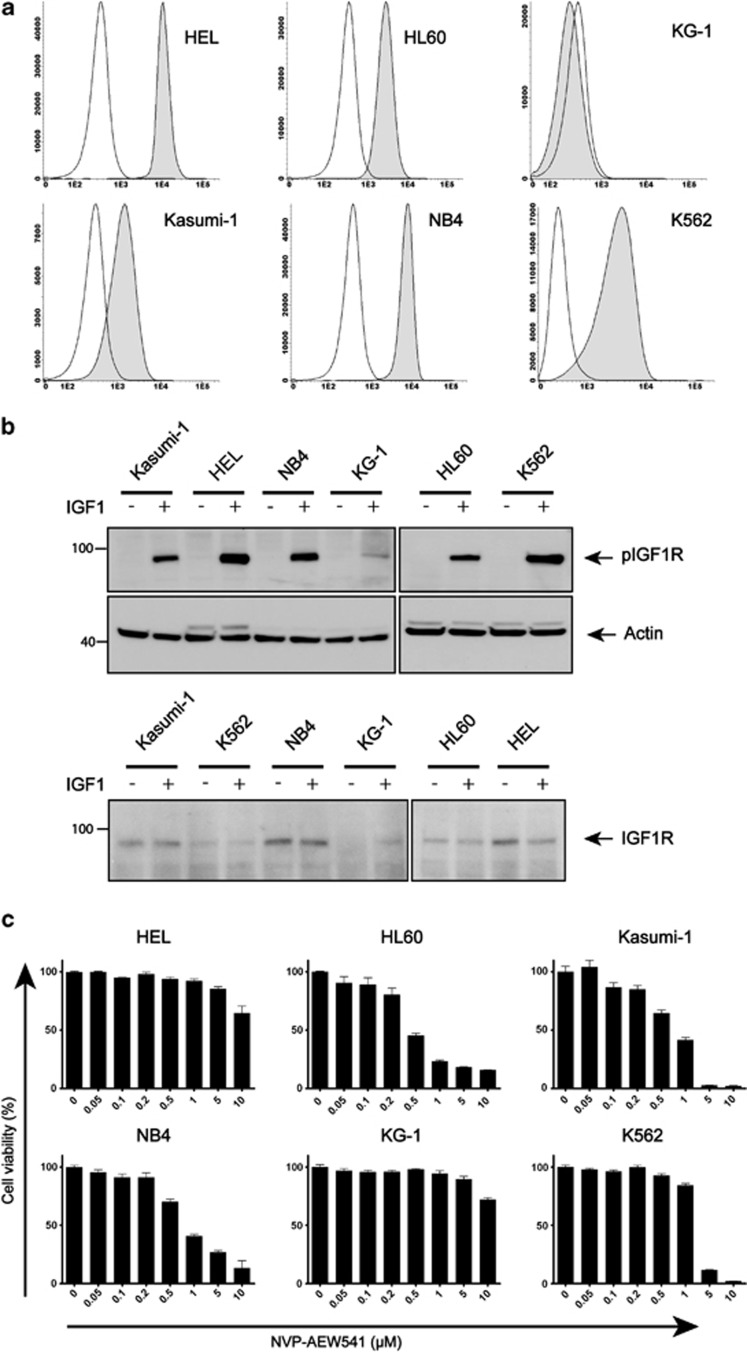
Recently, IGFBP7 has been shown to directly bind to the IGF-1R on the membrane of normal and neoplastic breast epithelial cells.27 To address a potential effect of IGFBP7 on AML cell growth via the IGF-1 axis, we first established the presence of the IGF-1R on human AML cell lines. Analyses using flow cytometry revealed that the IGF-1R was expressed on the cell surface of all AML cell lines tested, except KG-1 (Figure 1a). After stimulation with IGF-1, all AML cell lines showed phosphorylated IGF-1R and/or insulin receptor beta (InsR) (Figure 1b); however, no constitutive IGF-1R/InsR activation, independent from the stimulation with IGF-1, is seen in these AML cells. In general, the expression levels of the IGF-1R, detected using immunoblotting, correspond to expression detected with flow cytometry, suggesting that part, if not all, of the expressed IGF-1R is transported to the cell membrane of AML cells.
Figure 1.
Expression of membrane IGF-1R in leukemic cell lines. (a) Flow cytrometric analysis of leukemic cell lines labeled with IGF-1R-phycoerythrin (PE; gray) compared with cells labeled with IgG-PE control antibody (white). (b) Detection of phosphorylated IGF-1R using immunoblotting after IGF-1 ligand stimulation (50 ng/ml). (c) Cell lines were treated with the indicated concentrations of NVP-AEW541 and cell viability was measured using a MTT assay
To study the role of IGFBP7 in inhibition of IGF-1R-mediated cell survival of AML cells, we first investigated whether blocking the activity of the IGF-1R can reduce the viability of AML cells. Incubation of AML cell lines with various concentrations of the IGF-1R TKI NVP-AEW54135, 36 resulted in decreased survival of HL60, Kasumi-1 and NB4 cells at concentrations between 0.5 and 1 μM (Figure 1c). The inhibition of the viability of K562 cells started at a concentration of 1 μM, and viability of both KG-1 and HEL cells could only be decreased with a concentration of 10 μM. As K562, HEL and KG-1 cells do not respond to NVP-AEW541 concentrations inhibiting IGF-1R activity (≤1 μM), the decrease in cell viability is likely due to nonspecific inhibition; for example, the EGFR, PDGFR, c-Met or c-Kit or other kinases known to be inhibited at these concentrations.36
IGFBP7 inhibits the growth of AML cells
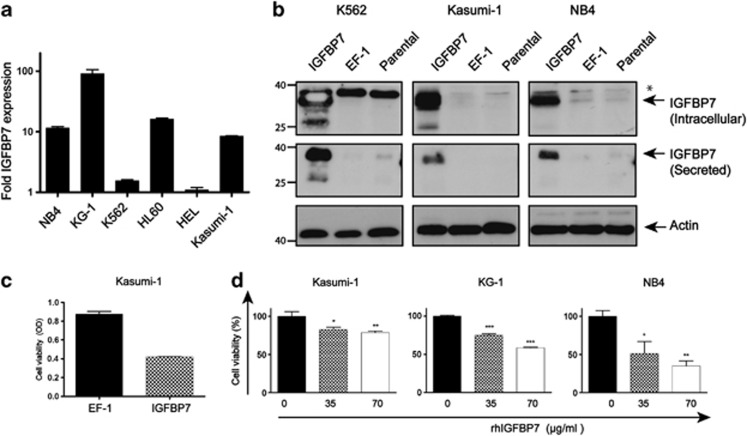
Although it has been shown that IGFBP7 inhibits tumor growth by inducing senescence or apoptosis in solid tumors such as breast cancer and melanoma,24, 26, 27, 31, 32, 33 its function in AML has not been extensively elucidated. IGFBP7 is expressed in all the myeloid leukemia cell lines that we have tested and shows the highest expression in KG-1 cells (Figure 2a). We selected the cell lines Kasumi-1, NB4, and K562 myeloid leukemia cells to overexpress IGFBP7 and show, using immunoblotting of the cell lysates and the medium, that all cell lines have enhanced intracellular IGFBP7 protein, as well as enhanced secretion of IGFBP7 protein (Figure 2b).
Figure 2.
IGFBP7 inhibits the growth of AML cells. (a) Average expression of IGFBP7 measured using qRT-PCR, error bars show the S.D. of a duplicate. (b) Lentivirally transduced leukemic cell lines were analysed for the expression of IGFBP7 by immunoblotting of the lysates (upper panel) and the medium (middle panel). The expression of actin was used as a control for equal loading (lower panel). * indicates an aspecific background band. (c) Cell viability of Kasumi-1 cells transduced with the control (EF-1) or with the IGFBP7 overexpression vector were cultured under low-serum conditions (1%) for 72 h. Bars and error bars show the cell viability and S.D. of a triplicate. (d) AML cells were treated with various concentrations of rhIGFBP7 in Opti-Mem reduced serum medium with 1% FCS for 72 h. Bars represent the average and error bars represent the viability and S.D. of a triplicate. One-way ANOVA analysis was used with a post hoc Tukey test to calculate the P-value *P≤0.05, **P≤0.01, ***P≤0.001
Although in one study it has been shown that IGFBP7 is able to enhance proliferation,37 most studies show that IGFBP7 can reduce cell growth and induce apoptosis.26, 27, 31, 32, 33 To investigate the effect of IGFBP7 on growth of myeloid leukemia cells, we determined the survival of Kasumi-1 cells with enhanced IGFBP7 expression. Kasumi-1 cells overexpressing IGFBP7 did not show a change in growth rate when cultured in the presence of high amounts of growth factors that are present in fetal calf serum (FCS; 15% data not shown). Lowering the amount of FCS (1%) and thereby lowering the inhibitory effect of proteins present in the FCS resulted in reduced viability of Kasumi-1 cells overexpressing IGFBP7 as compared with the control Kasumi-1 cells, either by inhibition of proliferation or induction of apoptosis (Figure 2c). This growth-inhibitory effect might be due to either secreted or intracellular IGFBP7.
To determine whether secreted IGFBP7 could inhibit the growth of AML cells, we generated human recombinant IGFBP7 (rhIGFBP7; Supplementary Figure S1). Treatment of NB4, Kasumi-1 and KG-1 cells with various concentrations of purified rhIGFBP7 resulted as well in the inhibition of cell growth by either decreasing survival or inhibition of proliferation (Figure 2d). Thus, AML cell growth can be exogenously inhibited by rhIGFBP7.
IGFBP7 suppresses tumor growth in AML xenografts
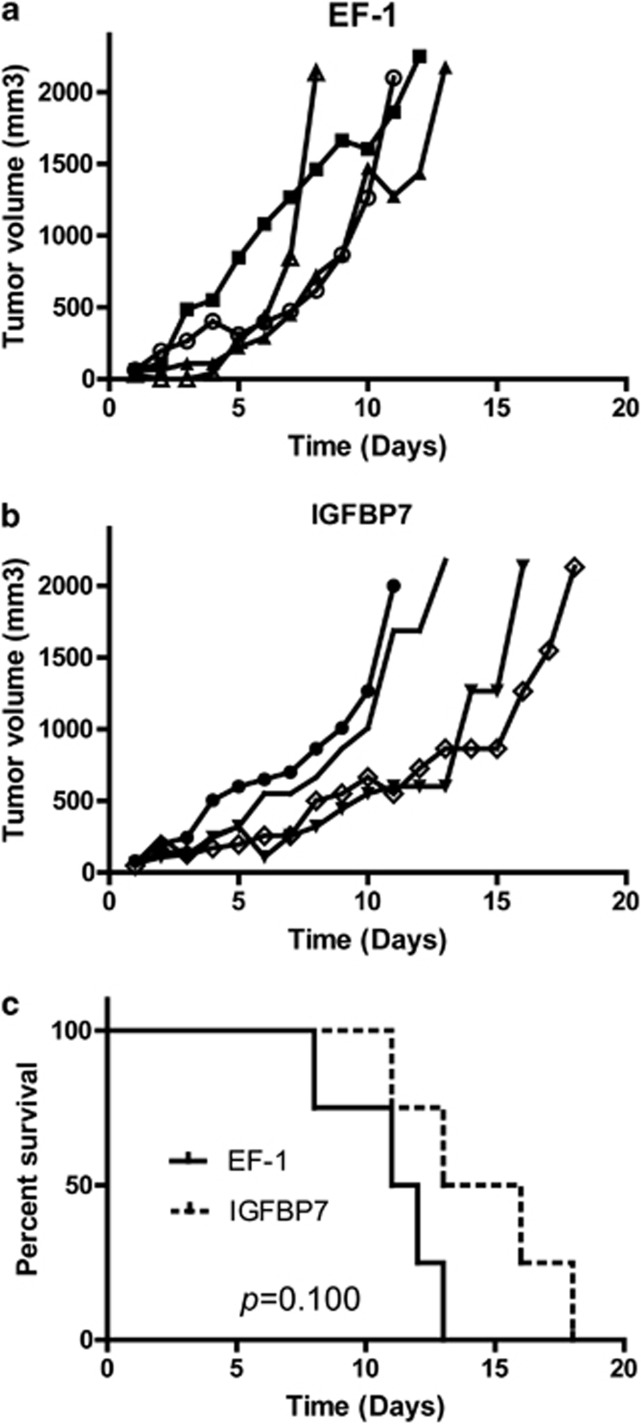
As IGFBP7 can reduce AML cell growth in vitro (Figure 2), we sought to determine its effect on growth inhibition in an in vivo AML xenograft mouse model. Kasumi-1 cells overexpressing IGFBP7 were subcutaneously injected into the NOD/SCID-IL2g−/− (NSG) mice and tumor size was measured over time. Subcutaneously growing tumors derived from cells overexpressing IGFBP7 show a decreased growth rate compared to tumors derived from control cells (Figures 3a and b). Subsequently, survival analysis (Figure 3c) of mice injected with IGFBP7-overexpressing Kasumi-1 cells and those injected with control Kasumi-1 cells showed that mice injected with Kasumi-1 cells overexpressing IGFBP7 have a trend towards a better survival (P=0.1). Mice injected with Kasumi-1 control cells have a median survival of 11.5 days as compared with 14.5 days of mice injected with Kasumi-1 cells overexpressing IGFBP7.
Figure 3.
Decreased tumor growth of AML cells overexpressing IGFBP7 in vivo. (a and b) NOD/SCID-IL2g−/− (NSG) mice were subcutaneously injected with 3 × 106 Kasumi-1 cells transduced with an empty vector (a) or with IGFBP7 overexpression (b). Tumor size was measured every day and shown from the day that tumors reached a size of 50 mm3. (c) Kaplan–Meier, survival analysis of indicated NSG mice. Mice were killed when tumors reached 2000 mm3. Log-rank (Mantel–Cox) was used to calculate the P-value
IGFBP7 inhibits AML cell growth, at least partly, by an IGF-1/IGF-1R-independent mechanism
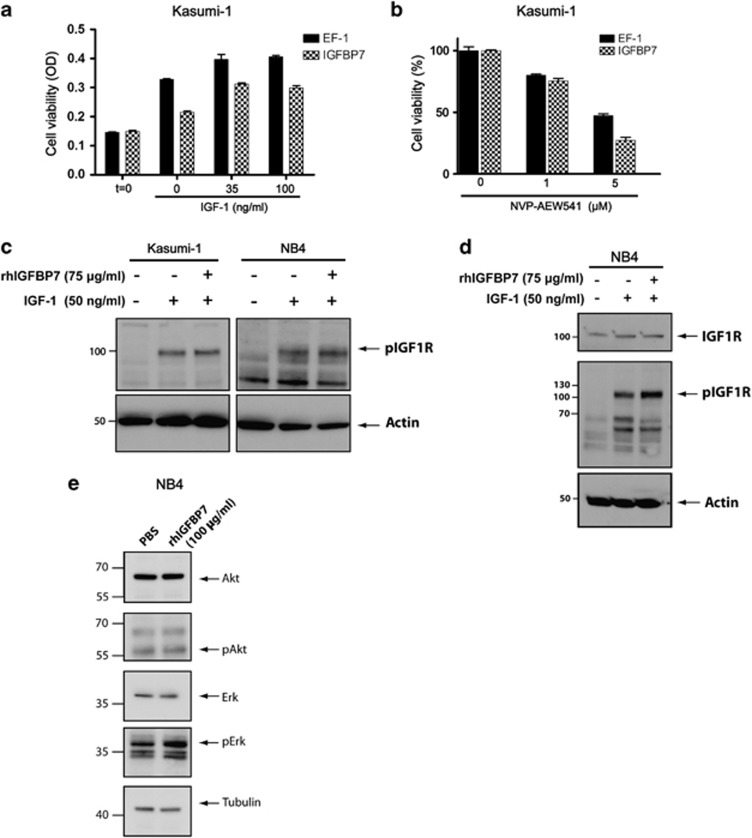
IGFBP7 interacts with both IGF-1 and insulin with very low affinity37 and has been shown to directly interact with the IGF-1R.27 Although in several cancer settings IGFBP7 influences proliferation in an IGF-dependent manner, we observed that KG-1 cells, lacking membrane IGF-1R expression, can be growth-inhibited by rhIGFBP7. This suggests that IGFBP7 can function via IGF-1R-independent signaling, which has also been suggested for other IGFBPs.23, 25 To elucidate whether inhibition of AML growth by IGFBP7 is dependent on IGF-1 signaling, we cultured Kasumi-1 cells overexpressing IGFBP7 in the presence of various concentrations of IGF-1 (Figure 4a). Presence of 35 or 100 ng/ml IGF-1 enhanced the growth of Kasumi-1 cells, for both IGFBP7 overexpression as well as control cells. However, in the presence of IGF-1, Kasumi-1 cells overexpressing IGFBP7 are still growth-inhibited and even saturating amounts of IGF-1 (100 ng/ml) do not abolish this effect caused by IGFBP7, indicating that IGFBP7-induced growth inhibition is at least partly independent from IGF-1.
Figure 4.
IGFBP7 inhibits the growth of AML cells, at least partly, in an IGF-1- and IGF-1R-independent manner. (a) Control or IGFBP7-overexpressing Kasumi-1 cells were grown under low-serum conditions in the presence of various amounts of rhIGF-1. Bars represent the cell viability (OD) measured by using a MTT assay. Bars represent the average viability of a triplicate and error bars show the S.D. (b) Kasumi-1, cells with or without the overexpression of IGFBP7, were treated with NVP-AEW541. Bars represent the average cell viability of a triplicate and error bars show the S.D. (c) Kasumi-1 and NB4 cells were incubated±rhIGFBP7 for 4 h before induction with rhIGF-1 or PBS. The activity of the IGF-1R was determined using immunoblotting with an antibody against the phosphorylated IGF-1R. (d) rhIGFBP7 was pre-incubated with IGF-1 for 4 h before the induction of NB4 cells and activation of the IGF-1R was determined by detection of phosphorylated IGF-1R using immunoblotting. (e) NB4 cells treated with rhIGFBP7 or PBS were subjected to immunoblotting. All experiments regarding this figure were performed under low-serum conditions
To divide the growth-inhibitory effect of IGFBP7 in an IGF-1R dependent and -independent effect, we incubated Kasumi-1 cells overexpressing IGFBP7 with the TKI NVP-AEW541 (Figure 4b). On top of the growth-inhibitory effect of the TKI, there is additional inhibition by IGFBP7, suggesting that IGFBP7 exerts its effect partly in an IGF-1R-independent manner. Thus, IGFBP7 can inhibit the growth of AML cells independent from IGF-1R activation or IGF-1 stimulation.
Next, we investigated whether IGFBP7 is able to reduce IGF-1R activity, as has been shown in breast cancer cells.27 To that end, we analyzed IGF-1-induced phosphorylation of the IGF-1R in Kasumi-1 and K562 cells overexpressing IGFBP7 as compared with the control cells. In contrast to the effect of rhIGFBP7 on breast cancer cells,27 we did not observe a decrease in IGF-1R phosphorylation in cells overexpressing IGFBP7, whereas these cells were inhibited in growth (data not shown). Moreover, pre-incubation of rhIGFBP7 with Kasumi-1 and NB4 cells and subsequent stimulation with IGF-1 did not result in decreased IGF-1R phosphorylation (Figure 4c). RhIGFBP7 pre-incubated with IGF-1 and subsequent stimulation of NB4 cells could also not decrease the activation of the IGF-1R (Figure 4d). In general, receptor tyrosine kinases activate both the Erk and Akt signaling pathways. To study whether rhIGFBP7 can inhibit Erk or Akt activation we cultured NB4 cells in the presence of rhIGFBP7 and established that the levels of phosphorylated Akt and Erk are not affected while the cells are inhibited in their growth (Figure 4e). Overall, these results suggest that IGFBP7 functions independently from inhibition of IGF-1R phosphorylation in reducing AML cell growth.
IGFBP7 induces a cell cycle block and apoptosis in AML cells
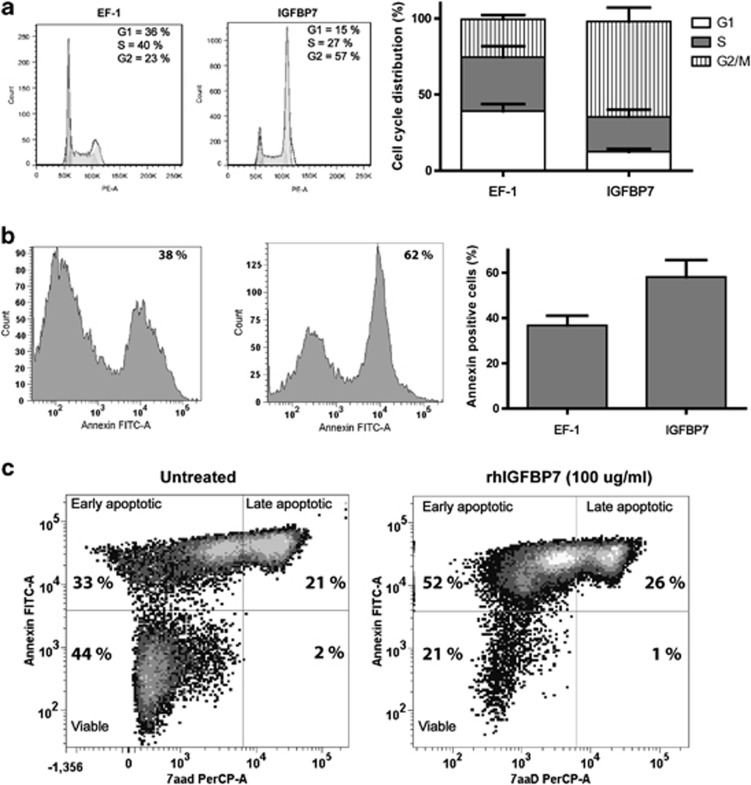
The inhibition of AML cell growth by IGFBP7 might be due to induction of differentiation, inhibition of proliferation and/or induction of a cell cycle block subsequently followed by the induction of apoptosis. We analyzed Kasumi-1 cells overexpressing IGFBP7 for cell-cycle phase distribution and induction of apoptosis. Cells with enhanced IGFBP7 expression that were grown for 2 days under low-serum conditions have an increase in the fraction of cells in the G2/M phase (23% in the control cells (left panel) versus 57% in cells with enhanced IGFBP7 (right panel)) with a corresponding decrease in the fraction of cells in G1 and S phases (Figure 5a). Kasumi-1 cells with enhanced expression of IGFBP7 have an increased number of Annexin-V-positive cells (38% in the control cells versus 62% in cells with enhanced IGFBP7 (Figure 5b) indicating induction of apoptosis by IGFBP7. To determine whether soluble secreted IGFBP7 could induce apoptosis of AML cells, we incubated NB4 cells with rhIGFBP7 and measured the induction of apoptosis (Figure 5c). Treatment with rhIGFBP7 resulted in a loss of viable cells (44–21%) and an increase in early (from 33 to 52% Annexin-V-positive) and late apoptotic (from 21 to 26% Annexin-V- and 7-AAD-positive) cells, indicating that rhIGFBP7 can reduce the viability of AML cells by induction of apoptosis.
Figure 5.
IGFBP7 induces a G2/M cell cycle block and apoptosis in AML cells. (a) Cell cycle distribution analysis (left panel) of Kasumi-1 control cells or with overexpression of IGFBP7. The frequency of cells in the G1, S and G2 phases of the cell cycle was determined (right panel). (b) Kasumi-1 cells with or without the overexpression of IGFBP7 were cultured for 72 h under low-serum conditions and Annexin-V positivity was measured using flow cytometry (left panel). Annexin-V positivity after overexpression of IGFBP7 in Kasumi-1 of n=2 (right panel). (c) NB4 cells were cultured in Opti-Mem reduced serum medium with 1% FSC in the presence of rhIGFBP7. Apoptosis was detected by establishing Annexin-V and 7-AAD positivity using flow cytometry
IGFBP7 cooperates with doxorubicin, etoposide and cytarabine in promoting AML cell death
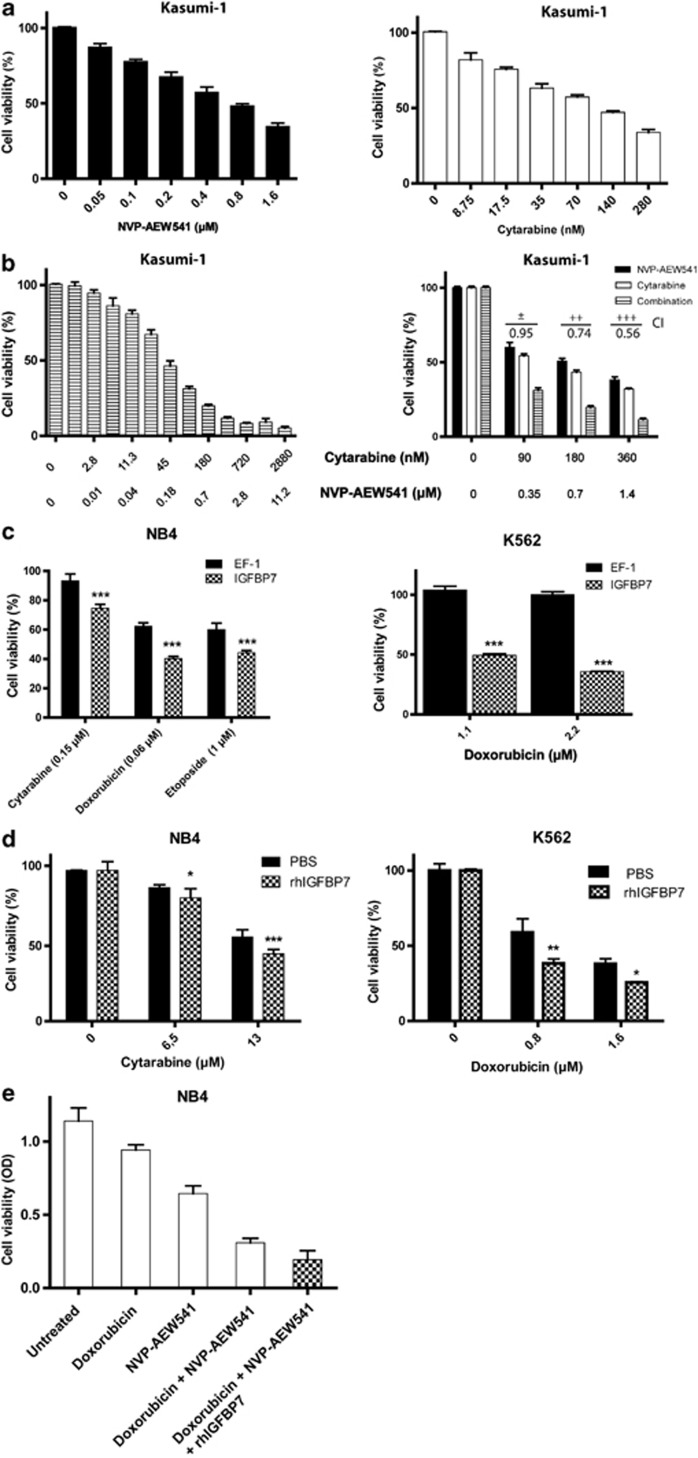
As treatment failure in AML is mainly caused by chemotherapy resistance or tolerance,1 we studied whether IGFBP7 or the inhibition of IGF-1R activity can sensitize for chemotherapy-induced AML cell death. Treatment of Kasumi-1 cells with cytarabine or NVP-AEW541 as a single agent shows decreased cell viability (Figure 6a); however, the combination of both (concentrations cytarabine >90 nM and NVP-AEW541 >0.35 nM) works synergistically (CI is <0.99; Figure 6b), indicating that combining both drugs has a more than additive effect.38 The effect of NVP-AEW541 on enhancement of chemotherapy efficacy is most likely due to inhibition of IGF-1R activity, since at these concentrations this TKI mainly inhibits IGF-1R activity. Although the growth-inhibitory effect of IGFBP7 is for a major part independent from IGF-1R activity, a cooperative effect of IGFBP7 with chemotherapy might still be present. Therefore, we determined whether IGFBP7 can cooperate with chemotherapy in induction of AML cell death. Indeed, NB4 cells overexpressing IGFBP7 are sensitized for the induction of cell death by doxorubicin, cytarabine and etoposide (Figure 6c). Of all myeloid cell lines tested, we observed the largest effect of IGFBP7 on doxorubicin-induced cell death in the K562 cell line. At doxorubicin concentrations that do not induce cell death of myeloid leukemia cells, the combination with IGFBP7 does (Figure 6c, right panel).
Figure 6.
Inhibition of IGF-1 R activity or IGFBP7 cooperates with chemotherapy in the inhibition of AML cell viability. (a) Cell viability of Kasumi-1 cells incubated with various concentrations of NVP-AEW541 (left panel) and cytarabine (right panel). (b) Kasumi-1 cells incubated with a combination of NVP-AEW541 and cytarabine. The combination index (CI) of the indicated drug combinations (right panel) were calculated using the Calcusyn software.38 Defined CI values: ±(0.90–1.10), ++ (0.70–0.85), +++ (0.3–0.7). (c) Cell viability of NB4 cells with or without the overexpression of IGFBP7 and incubated with doxorubicin, cytarabine and etoposide (left panel) and K562 treated with doxorubicin (right panel). (d) Cell viability of NB4 and K562 cells in the presence of rhIGFBP7 (20 μg/ml in the case of NB4 and 300 μg/ml in the case of K562) with various concentrations of cytarabine (left panel) or doxorubicin (right panel) measured by the MTT assay. All bars represent the average cell viability and error bars show the S.D. of a triplicate. One-way ANOVA analysis was used in combination with a post hoc Tukey test to calculate the P-value *P≤0.05, **P≤0.01, ***P≤0.001. (e) NB4 cells were cultured in Opti-Mem reduced serum medium with 1% FSC in the presence of 0.04 μM doxorubicin, 0.5 μM NVP-AEW541 or the combination of doxorubicin and NVP-AEW541 or the combination of doxorubicin, NVP-AEW541 and 20 μg/ml rhIGFBP7
Sensitization of chemotherapy by overexpression of IGFBP7 might be either due to intracellular or secreted soluble IGFBP7. Therefore, we investigated whether IGFBP7 can exogenously sensitize for chemotherapy-induced cell death in myeloid leukemia cells. Similar to the overexpression of IGFBP7, rhIGFBP7 cooperates with chemotherapy (doxorubicin and cytarabine) in decreasing cell viability of the myeloid leukemia cell lines NB4 and K562 (Figure 6d). Altogether, these results indicate that elevated IGFBP7 levels can result in enhanced chemotherapy-induced AML cell death. As the effect of IGFBP7 might be for a major part independent from inhibition of IGF-1R activity, we investigated whether rhIGFBP7 can be additive to the inhibitory effect of NVP-AEW541 in combination with chemotherapy. Combining rhIGFBP7 with doxorubicin and NVP-AEW541 results in reduced cell survival as compared with the combination alone (Figure 6e).
The prognostic value of IGFBP7 expression in AML
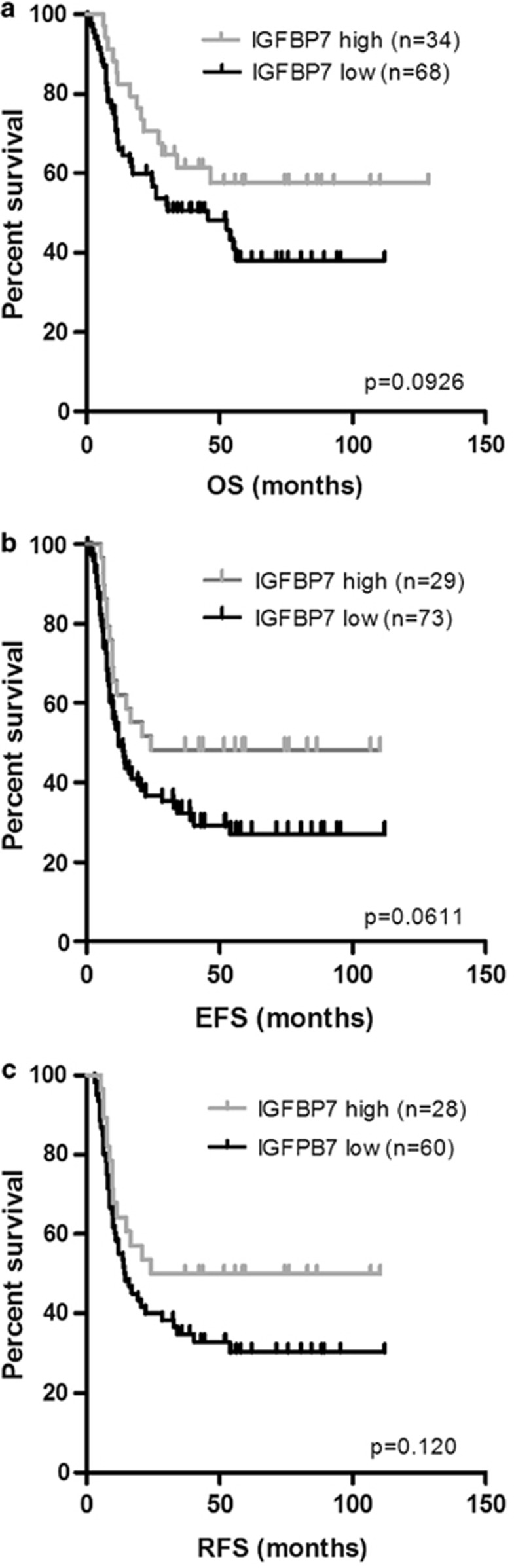
As IGFBP7 can induce apoptosis and enhance chemotherapy-induced cell death, higher expression of IGFBP7 in the AML patient's bone marrow might result in a better response to treatment. To investigate whether IGFBP7 expression levels are associated with AML patients' prognosis, we correlated results from mRNA sequencing data with clinical outcome of 102 AML patients.39 We excluded patients that are older than 60 years, as they belong to a group of patients with an extremely poor prognosis and also excluded patients with a PML-RAR translocation (M3 Fab classification), since these patients are treated differently from the rest of the group (with all-trans retinoic acid). The top 33% of AML cases with the highest IGFBP7 expression (n=34) were compared with the rest of the AML cohort (n=68). Patients with low IGFBP7 expression have a worse overall survival (OS; Figure 7a), event-free survival (EFS; Figure 7b) and relapse-free survival (RFS; Figure 7c) compared with patients with high IGFBP7 expression. In addition, we studied whether expression of the IGF-1R or IGF-1 were associated with OS, EFS and RFS. In this cohort, neither IGF-1 nor the IGF-1R was correlated with the outcome of AML patients (Supplementary Figures 2A–D).
Figure 7.
Survival analysis of AML patients with high and low levels of IGFBP7. (a) The OS of AML patients with low and high IGFBP7 levels. (b) The EFS of AML patients with low and high IGFBP7 levels (c) RFS of AML patients with low and high IGFBP7 levels. Expression data were derived from the Cancer Genome Atlas Research Network39 and we compared the top 33% of patients with high IGFBP7 expression to the rest of patients
Discussion
In the present study, we show that AML patients with low IGFBP7 expression have a worse OS than patients with high expression. This suggests that decreased expression of IGFBP7 might result in a poorer treatment response and enhanced survival of AML cells after chemotherapy. Thus, low IGFBP7 expression in AML cells might be, at least partly, responsible for reduced chemotherapy sensitivity and consequently enhancing IGFBP7, by overexpression or addition of rhIGFBP7, might increase the efficacy of chemotherapy and/or induce AML cell death. Indeed, our results show that a combination of IGFBP7 with doxorubicin or cytarabine results in increased sensitivity of AML cells for chemotherapy-induced cell death. The extreme poor prognosis of AML patients is mainly due to survival of chemotherapy-resistant leukemic cells. These cells are responsible for the return of the leukemia, the relapse, which is very hard to treat and the main reason for the low survival chances for AML patients.1 As resistance to chemotherapy is the major problem that AML patients currently face, identification of additional novel therapies such as IGFBP7, enhancing chemotherapy efficacy, is crucial for improvement of AML outcome. Mechanisms underlying this resistance to 'classical' cytotoxic chemotherapeutics include many features, for example, alterations in the drug target, activation of prosurvival pathways and ineffective induction of cell death. Our results show that an increase in IGFBP7 results in enhanced sensitivity to chemotherapy-induced cell death, likely due to elimination of chemotherapy resistance mechanisms in AML cells. This suggests that combining IGFBP7 with chemotherapy might improve AML patients outcome. The increase in chemotherapy sensitivity by IGFBP7 might hold true for the bulk of the AML, however, also for small subpopulations of resistant leukemic cells. Combinations including rhIGFBP7 and chemotherapy might therefore be beneficial for patients with refractory AML as well as with AML relapse. Besides cooperation with chemotherapy, we show that IGFBP7, either by an intracellular function or a function at the outside of the cell, can directly inhibit AML cell growth by the induction of a G2 cell cycle block and apoptosis. The effect of IGFBP7 might be even more enhanced when AML cells reside in their natural microenvironment, for example, in in vivo mouse models or on stroma; since then both autocrine- and paracrine-secreted IGFBP7 might block the interactions of the AML cells with cytokines and/or receptors expressed in this microenvironment. In contrast to our results, Hu et al.40 showed that downregulation of IGFBP7 results in decreased adhesion and survival of U937 cells. This discrepancy might be due to a context-dependent function of IGFBP7, as we also observed that IGFBP7 does not induce apoptosis in all the AML cell lines that we tested. Relapse-initiating cells that survive treatment can be highly resistant to conventional chemotherapy due to activation of alternative signaling pathways such as activation of the IGF-1R.41 The IGF-1/IGF-1R axis has an important role in a wide variety of cancers, including AML, whereby active signaling can induce cell proliferation and survival but also chemotherapy resistance.2, 3, 4, 5, 12, 13, 14, 17, 18, 19, 20, 21, 22 We show that the IGF-1R is expressed in most AML cell lines and can be activated upon stimulation with IGF-1; however, no constitutive activation of IGF-1R activity is seen in AML cell lines. In addition, we could not establish an association between AML patient outcome and expression of the IGF-1R or IGF-1, which might indicate that, in agreement with earlier findings in breast cancer, not the total IGF-1R but the phosphorylated (activated) form of IGF-1R is an indicator of poor prognosis.42, 43
Although in breast cancer it has been shown that IGFBP7 directly binds to the IGF-1R,27 we show that IGFBP7-mediated induction of apoptosis in AML cells is for a major part independent from activation of the IGF-1R/IGF-1 axis. This suggests that IGFBP7 is involved in activation or inhibition of, thus far, unknown signaling pathways. Indeed, the family of IGFBPs is known to modulate cellular proliferation or apoptosis by both IGF-dependent and -independent mechanisms.25 For example, the N-terminal 95-amino-acid IGFBP-3 domain, which shows ∼30% homology with IGFBP7, inhibits proliferation probably by interacting with an unknown cell surface receptor or by acting as a nuclear transcription factor.44, 45 As a major part of the effect of IGFBP7 on AML cell apoptosis is IGF-1R/IGF-1-independent, the effect of IGFBP7 on chemotherapy sensitization might be as well via a, thus far, unknown mechanism, different from inhibition of IGF-1R activity. In contrast to our results, re-expression of IGFBP7 in Smarcb1-deficient tumor cells reduced the activity of Akt.46 This discrepancy might be explained by the culture system we are using in which we culture cells under low-serum conditions, having low basal Akt activity. This might result in an undetectable small decrease in phosphorylated Akt after IGFBP7 exposure. In addition, reduction in activated Akt levels might be a fast response while the inhibition of growth is only detectable after a few days of culturing with IGFBP7.
In summary, we show that IGFBP7 results in the induction of apoptosis in AML cells. Most importantly, IGFBP7 can sensitize AML cells to chemotherapy, indicating a potential manner to eradicate the bulk or subpopulations of therapy-resistant leukemic cells, thereby improving the outcome of AML.
Materials and Methods
Construction of plasmids
Lentiviral plasmid encoding human IGFBP7 was generated by ligation of the full-length IGFBP7 cDNA clone (SC119176, Origene, Rockville, MD, USA) into the pCDH-CMV-EF-1-puro plasmid (CD510B-1, System Biosciences, San Francisco, CA, USA). The plasmid containing human IGFBP7 (rhIGFBP7) with a histidine tag and a myc tag was generated by ligation of the full-length cDNA of IGFBP7 into pCDNA3.1 (-A)-his-myc (no. V855-20, Invitrogen, Carlsbad, CA, USA).
Cell lines and virus production
All cell lines were derived from the American Type Culture Collection (ATCC, Manassas, VA, USA). HEL, NB4, HL60 and K562 cell lines were cultured in Roswell Park Memorial Institute-1640 (RPMI-1640) medium with 10% FCS. KG-1 and Kasumi-1 were cultured in RPMI-1640 containing 15% FCS. Cells cultured under low-serum conditions were either cultured in RPMI-1640 with 1% FCS or in Opti-Mem Reduced-Serum Medium (used in the experiments illustrated in Figures 2d, 5c and 6e; Life Technologies, Bleiswijk, The Netherlands) with 1% FCS.
IGFBP7-overexpressing AML cell lines were generated by lentiviral transduction with pCDH-CMV-EF-1-puro-IGFBP7 (System Biosciences). HEK293T cells were seeded and grown in Dulbecco's Modified Eagle Medium/10% FCS. Plasmid DNA mix – pCDH-CMV-EF-1-puro or pCDH-CMV-EF-1-puro-IGFBP7, PMD29, PRRK and PRSV-REV (containing pol, gag and rev) – was mixed with Hepes-buffered saline and 200 mM CaCl2 and was added to the HEK293T cells. The medium was harvested after 72 h and added to AML cell lines cultured on retronectin (Takara Bio Inc, Otsu, Japan). Cells were selected using 1-2 μg/ml puromycin (Sigma Aldrich, St. Louis, MO, USA).
Immunoblotting
Cells were lysed in 1% NP40 lysis buffer (50 mM Tris, Ph7.5, 150 mM NaCl, 1% NP40, 5 mM EDTA pH 8) containing protease inhibitors and phosphostop (Roche, no. 04906845001, Basel, Switzerland). Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, no. 500-00001, Hercules, CA, USA). Cell lysates were boiled in 4 × reduced sample buffer (Bio-Rad) and proteins were separated by 4–16% precast SDS-PAGE (Bio-Rad) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) in Tris/glycine/SDS buffer containing 20% volume/volume methanol. Membranes were blocked using 5% (w/v) non-fatty dried milk powder (Millipore), or with 5% bovine serum albumin (Millipore) in case of blotting with antibodies directed to phospho-proteins, for at least 30 min in PBS/0.1% Tween (PBS/T), and incubated with the following antibodies: anti-IGFBP7 (R&D Systems, Minneapolis, MN, USA, no. MAB1334), anti-IGF-1R (Santa Cruz Biotechnology, Santa Cruz, CA, USA, no. 81464), anti-pIGF-1R Y1135/1136/Insulin receptor beta Y1150/1151 (Cell Signaling, Danvers, MA, USA; no. 3024S), anti-actin (Santa Cruz Biotechnology, no. MAB1501R) and anti-c-terminal-Myc (company, no. SC40). Anti-Akt (Cell Signaling no. 9272), anti-pAkt (s473; Cell Signaling no. 193H12), anti-Erk1/2 (Cell Signaling no. 137F5) and pErk (Cell Signaling no. 197G2, p-p44/42, T202/Y204). After washing with PBS/T, membranes were incubated with anti-mouse horse radish peroxidase (HRP; Dako, Heverlee, Belgium, no. p0260) or anti-rabbit-HRP (SC, no. SC2004). Enhanced bioluminescence for HRP (ECL) was from GE Healthcare (Waukesha, WI, USA).
Flow cytometric analysis of IGF-1R expression
AML cell lines were incubated with anti-CD221-PE (IGF-1R) or IgG-PE isotype control (BD Biosciences, San Diego, CA, USA) for 15–30 min. Cells were washed with PBS/0.1% human serum albumin (PBS/HSA) and measured by flow cytometry with a FACSCanto flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Analysis was performed using the BD-FACSDiva software (Becton Dickinson).
Cell viability assays (MTT)
A total of 10 × 104 cells were seeded in 96-well plates and cultured for 72–96 h. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; thiazolyl blue; Sigma Aldrich) was added and incubated with the cells for 4 h. MTT crystals were dissolved in isopropanol-HCl. Color conversion was measured at 570 nm and corrected for background at 690 nm. Each experiment was performed in triplicate and the values are represented as optical density (OD) or percentages. One-way ANOVA analysis was used with a post hoc Tukey test to calculate the P-value *P≤0.05, **P≤0.01, ***P≤0.001. Doxorubicin and etoposide were from Pharmachemie (Haarlem, The Netherlands). Cytarabine from Onco Tain (Brussels, Belgium). For cell viability assays regarding IGF-1R inhibition, we used NVP-AEW541 (Selleckchem, Houston, TX, USA, no. S1034).
Apoptosis detection
A total of 1 × 106 cells were cultured with RMPI-1640 containing 1% FCS and labeled with 7-AAD and Annexin-V (TAU technologies, Albuquerque, NM, USA) for 15–30 min. Cells were washed with PBS/0.1% HSA, fixed with 1% PFA and measured using flow cytometry with a FACSCanto flow cytometer (Becton Dickinson). Analysis was performed using the BD-FACS Diva software (Becton Dickinson).
Cell cycle distribution
A total of 1 × 106 cells were cultured in RMPI-1640 containing 1% FCS, fixed in 70% ethanol and incubated for 30 min with RNAse (100 μg/ml, Sigma Aldrich) at 37 °C. Subsequently, cells were stained with propidium iodide (PI) (50 μg/ml, Sigma). PI was visualized using a FACSCanto flow cytometer. Analysis was carried out using the Flow Jo 6.3 software (Treestar, Ashland, OR, USA).
Recombinant protein purification
For production of rhIGFBP7, we transfected pcDNA3.1(-A)-IGFBP7-his-myc into Cos-7 cells by using lipofectamine (Invitrogen, Darmstadt Germany). Cells were selected using G418 sulfate (Invitrogen) and limiting dilution was subsequently performed to select for cells with high IGFBP7 expression. Conditioned medium was harvested, concentrated using centrifugal filters (Amicon Ultra-15, 10 k, Millipore) and loaded onto a 5 ml Ni2+ column (HiTrap Chelating sepharose, (GE Healthcare). The column was washed with 25 ml 20 mM Tris, pH 8.0, containing 200 mM NaCl and 20 mM imidazole. rhIGFBP7 was eluted with 20 mM Tris pH 8.0, 100 mM NaCl, 150 mM imidazol. Fractions containing rhIGFBP7 were pooled and loaded onto a 1 ml Sepharose column (HiTrap HP, GE Healthcare). The column was washed with 7.5 ml 20 mM Tris, pH 8.0, 200 mM NaCl and the protein was eluted with 20 mM Tris, pH 8.0, 500 mM NaCl.
AML xenograft assay
Non-obese diabetic/severe combined immunodeficiency IL2 gamma chain knockout mice (NOD/SCID-IL2g−/−, NSG; Jackson Laboratory, Bar Harbor, MA, USA), both male as well as female, 7–8 weeks of age, were subcutaneously injected with 3 × 106 Kasumi-1 cells. Tumor size was measured and the volume was calculated using the formula (W2 × L)/2), width (W) and length (L). Mice were killed when reaching a tumor size ≥2000 mm3.
Survival analysis
mRNA sequencing results together with clinical data from ∼200 AML patients39 were downloaded from https://tcga-data.nci.nih.gov/docs/publications/aml_2012. For survival analysis the OS, EFS and RFS of patients ≤60 years of age with AML (excluded are the patients belonging to Fab classification M3) was correlated with the level of IGFBP7 expression in the leukemia. The top third highest IGFBP7-expressing AML patients in each group were compared with the rest of the AML cases. All statistical analyses were performed using the SPSS 21.0 package (IBM SPSS Statistics for Windows, Version 20.0, Armonk, NY, USA), with significance set at P≤0.05.
Glossary
- AML
acute myeloid leukemia
- TCGA
The Cancer Genome Atlas
- CI
Combination Index
- EFS
event-free survival
- FAB
French-American-British
- IGF-1
insulin growth factor-1
- IGF-1R
insulin growth factor-1 receptor
- IGFBP
insulin-like growth factor-binding protein
- InsR
insulin receptor
- MM
multiple myeloma
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- NSG
non-obese diabetic/severe combined immunodeficiency IL2 gamma chain knockout (NOD/SCID-IL2g-/-)
- OS
overall survival
- pIGF-1R
phospo-IGF-1R
- RFS
relapse-free survival
- rhIGFBP7
recombinant human IGFBP7
- TKI
tyrosine kinase inhibitor
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Raschellà
Supplementary Material
References
- 1Löwenberg B. Acute myeloid leukemia: the challenge of capturing disease variety. Hematology Am Soc Hematol Educ Program 2008: 1–11. [DOI] [PubMed]
- 2Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci 2008; 13: 3273–3287. [DOI] [PubMed] [Google Scholar]
- 3He Y, Zhang J, Zheng J, Du W, Xiao H, Liu W et al. The insulin-like growth factor-1 receptor kinase inhibitor, NVP-ADW742, suppresses survival and resistance to chemotherapy in acute myeloid leukemia cells. Oncol Res 2010; 19: 35–43. [DOI] [PubMed] [Google Scholar]
- 4Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012; 12: 159–167. [DOI] [PubMed] [Google Scholar]
- 5Khandwala HM, Mc Cutcheon IE, Flyvbjerg A, Friend KE. The effect of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 2000; 21: 215–244. [DOI] [PubMed] [Google Scholar]
- 6Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, De Angelis T et al. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol 1994; 7: 4588–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Wu Y, Cui K, Miyoshi K, Henninghausen L, Green JE, Setser J et al. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res 2003; 63: 4384–4388. [PubMed] [Google Scholar]
- 8Werner H, Le Roith D. The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit Rev Oncog 1997; 8: 71–92. [DOI] [PubMed] [Google Scholar]
- 9Ge NL, Rudikoff S. Insulin-like growth factor I is a dual effector of multiple myeloma cell growth. Blood 2000; 96: 2856–2861. [PubMed] [Google Scholar]
- 10Maiso P, Ocio EM, Garayoa M, Montero JC, Hofmann F, García-Echeverría C et al. The insulin-like growth factor-I receptor inhibitor NVP-AEW541 provokes cell cycle arrest and apoptosis in multiple myeloma cells. Br J Haematol 2008; 141: 470–482. [DOI] [PubMed] [Google Scholar]
- 11Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004; 5: 221–230. [DOI] [PubMed] [Google Scholar]
- 12Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia 2007; 21: 1921–1930. [DOI] [PubMed] [Google Scholar]
- 13Estrov Z, Meir R, Barak Y, Zaizov R, Zadik Z. Human growth hormone and insulin-like growth factor-1 enhance the proliferation of human leukemic blasts. J Clin Oncol 1991; 9: 394–399. [DOI] [PubMed] [Google Scholar]
- 14Frostad S, Bruserud O. In vitro effects of insulin-like growth factor-1 (IGF-1) on proliferation and constitutive cytokine secretion by acute myelogenous leukemia blasts. Eur J Haematol 1999; 62: 191–198. [DOI] [PubMed] [Google Scholar]
- 15Tazzari PL, Tabellini G, Bortul R, Papa V, Evangelisti C, Grafone T et al. The insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine insulin-like growth factor-I secretion. Leukemia 2007; 21: 886–896. [DOI] [PubMed] [Google Scholar]
- 16Chapuis N, Tamburini J, Cornillet-Lefebvre P, Gillot L, Bardet V, Willems L et al. Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: therapeutic value of neutralizing anti-IGF-1R antibody. Haematologica 2012; 95: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Abe S, Funato T, Takahashi S, Yokoyama H, Yamamoto J, Tomiya Y et al. Increased expression of insulin-like growth factor is associated with Ara-C resistance in leukemia. Exp Med 2006; 209: 217–228. [DOI] [PubMed] [Google Scholar]
- 18Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010; 141: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res 2008; 68: 8322–8332. [DOI] [PubMed] [Google Scholar]
- 20Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res 2002; 62: 200–207. [PubMed] [Google Scholar]
- 21Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G 2nd et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res 2009; 69: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Eckstein N, Servan K, Hildebrandt B, Pölitz A, von Jonquières G, Wolf-Kümmeth S et al. Hyperactivation of the insulin-like growth factor receptor I signaling pathway is an essential event for cisplatin resistance of ovarian cancer cells. Cancer Res 2009; 69: 2996–3003. [DOI] [PubMed] [Google Scholar]
- 23Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor-binding proteins. Endocr Rev 2002; 23: 824–854. [DOI] [PubMed] [Google Scholar]
- 24Burger AM, Leyland-Jones B, Banerjee K, Spyropoulus DD, Seth AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer 2005; 41: 1515–1527. [DOI] [PubMed] [Google Scholar]
- 25Hwa V, Oh Y, Rosenfeld G. The insulin-like growth factor binding protein (IGFBP) superfamily. Endocr Rev 1999; 20: 761–787. [DOI] [PubMed] [Google Scholar]
- 26Sato Y, Chen Z, Miyazaki K. Strong suppression of tumor growth by insulin-like growth factor-binding protein-related protein 1/tumor-derived cell adhesion factor/mac25. Cancer Sci 2007; 98: 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M et al. IGFBP7 binds to the IGF-1 receptor and blocks its activation by Insulin-like growth factors. Sci Signal 2012; 5: 1–11. [DOI] [PubMed] [Google Scholar]
- 28Tomimaru Y, Eguchi H, Wada H, Kobayashi S, Marubashi S, Tanemura M et al. IGFBP7 downregulation is associated with tumor progression and clinical outcome in hepatocellular carcinoma. Int J Cancer 2012; 130: 319–327. [DOI] [PubMed] [Google Scholar]
- 29Okamura J, Huang Y, Moon D, Brait M, Chang X, Kim MS. Downregulation of insulin-like growth factor-binding protein 7 in cisplatin-resistant non-small cell lung cancer. Cancer Biol Ther 2012; 13: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012; 483: 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Benatar T, Yang W, Amemiya Y, Evdokimova H, Kahn C, Holloway A et al. IGFBP7 reduces breast tumor growth by induction of senescence and apoptosis pathways. 2012. Breast Cancer Res Treat 2012; 133: 563–573. [DOI] [PubMed] [Google Scholar]
- 32Chen RY, Chen HX, Jian P, Xu L, Li J, Fan YM et al. Intratumoral injection of pEGFC1-IGFBP7 inhibits malignant melanoma growth in C57BL/6J mice by inducing apoptosis and down-regulating VEGF expression. Oncol Rep 2010; 4: 981–988. [DOI] [PubMed] [Google Scholar]
- 33Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Role for IGFBP7 in senescence induction by BRAF. Cell 2010; 141: 746–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G et al. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 2013; 4: e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35García-Echeverría C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J et al. In vivo antitumor activity of NVP-AEW541; A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell 2004; 5: 231–239. [DOI] [PubMed] [Google Scholar]
- 36García-Echeverría C, Zimmermann J, Pandiella A, San Miguel JF. The insulin-like growth factor-I receptor inhibitor NVP-AEW541 provokes cell cycle arrest and apoptosis in multiple myeloma cells. Br J Haematol 2008; 141: 470–482. [DOI] [PubMed] [Google Scholar]
- 37Akaogi K, Sato J, Okabe Y, Sakamoto Y, Yasumitsu H, Miyazaki K. Synergistic growth stimulation of mouse fibroblasts by tumor-derived adhesion factor with insulin-like growth factors and insulin. Cell Growth Differ 1996; 7: 1671–1677. [PubMed] [Google Scholar]
- 38Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 2010; 70: 440–446. [DOI] [PubMed] [Google Scholar]
- 39Ley D, Guzman CX, Adolfsson KH, Scott AM, Braunschweig AB et al. Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Hu S, Chen R, Man X, Feng X, Cen J et al. Function and expression of insulin-like growth factor-binding protein 7 (IGFBP7) gene in childhood acute myeloidleukemia. Pediatr Hematol Oncol 2011; 28: 279–287. [DOI] [PubMed] [Google Scholar]
- 41Holohan C, Schaeybroeck van S, Longley DB, Johnson PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013; 13: 714–726. [DOI] [PubMed] [Google Scholar]
- 42Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res 2008; 68: 10238–10246. [DOI] [PubMed] [Google Scholar]
- 43Yerushalmi R, Gelmon KA, Leung S, Gao D, Cheang M, Pollak M et al. Insulin-like growth factor receptor (IGF-1R) in breast cancer subtypes. Breast Cancer Res Treat 2012; 132: 131–142. [DOI] [PubMed] [Google Scholar]
- 44Leal SM, Huang SS, Huang JS. Interactions of high affinity insulin-like growth factor-binding proteins with the type V transforming growth factor-beta receptor in mink lung epithelial cells. J Biol Chem 1999; 274: 6711–6717. [DOI] [PubMed] [Google Scholar]
- 45Schedlich LJ, Young TF, Firth SM, Baxter RC. Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J Biol Chem 1998; 273: 18347–18352. [DOI] [PubMed] [Google Scholar]
- 46Darr J, Klochendler A, Isaac S, Eden A. Loss of IGFBP7 expression and persistent Akt activation contribute to SMARCCB1/Snf5-mediated tumorigenesis. Oncogene 2013; 261 doi:10.1038/onc.2013.261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.