Abstract
Anemia is a frequent complication in patients with inflammatory bowel disease (IBD), and is associated with decreased quality of life and increased rate of hospitalization. The primary therapeutic targets of IBD-associated anemia are iron deficiency and anemia of chronic disease. An important prognostic parameter of the success or failure of therapy is the outcome of the underlying disease. Iron deficiency should be appropriately managed with iron supplementation. However, the use of oral iron therapy is limited by several problems, the most important being gastrointestinal side effects leading occasionally to disease relapse and poor iron absorption. Intravenous iron preparations are more reliable, with iron sucrose demonstrating the best efficacy and tolerability. Treatment with erythropoietin or darbepoetin has been proven to be effective in patients with anemia, who fail to respond to intravenous iron. Patients with ongoing inflammation have anemia of chronic disease and may require combination therapy comprising of intravenous iron sucrose and erythropoietin. After initiating treatment, careful monitoring of hemoglobin levels and iron parameters is needed in order to avoid recurrence of anemia. In conclusion, anemia in the setting of IBD should be aggressively diagnosed, investigated, and treated. Future studies should define the optimal dose and schedule of intravenous iron supplementation and appropriate erythropoietin therapy in these patients.
Keywords: Anemia, Crohn’s disease, Erythropoiesis, Erythropoietin, Iron, Ulcerative colitis
INTRODUCTION
Anemia is a frequent and serious complication in patients with inflammatory bowel disease (IBD), with reported prevalence varying from 6% to 74%[1,2]. Anemia is associated with a decrease in the quality of life and increased rate of hospitalization[3,4]. In the past, it was common to ascribe anemia as an unavoidable accompaniment of IBD, but recently, correction of anemia is being emphasized as a specific therapeutic objective in these patients[5,6]. Both iron deficiency and anemia of chronic disease contribute to the development of anemia in IBD[3]. The therapeutic targets in the treatment of IBD patients with anemia are iron deficiency and the mechanisms underlying anemia of chronic disease[3].
Anemia is defined as "a reduction in the number of circulating RBCs, hemoglobin concentration, or the volume of packed red cells (hematocrit) in the blood"[7]. The World Health Organization (WHO) specifies the laboratory definition of anemia as hemoglobin below 120 g/L (non-pregnant females), 110 g/L (pregnant women) and 130 g/L (men). A hemoglobin level below 100 g/L is commonly considered as severe anemia and should be aggressively treated. However, in clinical practice, the timing of therapeutic intervention is based not only on the hemoglobin level but also on the relative degree of hemoglobin reduction, the underlying comorbidities, and the presence or absence of symptoms.
Anemia is an important manifestation of IBD and patients with anemia have increased disease severity and reduced quality of life, indicating the need for effective treatment. The underlying cause of anemia in IBD is either iron deficiency, resulting from intestinal bleeding due to mucosal inflammation and ulceration, or the so-called anemia of chronic disease, resulting from inhibition or suppression of erythropoiesis and dysfunction of iron transport, mediated by inflammatory cytokines[3,4]. Thus, the pathogenesis of IBD-associated anemia is complex and represents an example of combined iron deficiency anemia (IDA) and anemia of chronic diseases (ACD). Cobalamin or folate deficiency, drug induced anemia, and other causes of anemia such as haemolysis are seen less frequently.
Most patients with IBD-associated anemia respond to iron therapy. Oral iron supplementation appears to be effective for short periods but drug intolerance leads to discontinuation of therapy in up to 21% of patients[8]. Moreover, the use of oral iron is associated with several limitations[3]. By contrast, intravenous administration of iron sucrose has been found to be an effective treatment in these patients[9,10]. Erythropoietin (EPO) administration is associated with beneficial results in patients who are refractory to oral iron[3]. The combination of iron sucrose and EPO has been proposed as the most efficacious treatment in IBD-associated anemia[6,11]. The aim of the present review is to transfer our knowledge, especially the strategies for stimulating erythropoiesis, into the current clinical practice of treatment of anemia in IBD.
ANEMIA IN IBD
Iron deficiency (ID) is the most common cause of anemia in IBD. Iron, which is present in all mammalian cells, is of pivotal importance not only for oxygen transport and storage but also for many non-hematological functions. In normal subjects, the daily iron loss is 1-2 mg which needs to be replaced from the diet. The causes of iron deficiency are reduced intake, either from dietary deficiency or malabsorption, or increased losses. Chronic intestinal bleeding in IBD may exceed the quantity of iron that is absorbed from the diet, resulting in a negative iron balance[12]. Such an imbalance occurs frequently in IBD, leading to anemia.
When the rate of iron supply to the developing erythroblast is reduced as a result of ID, red cell haemoglobinisation is impaired. The red cells emerging from the bone marrow are microcytic and hypochromic. The increase in EPO response secondary to a drop in haemoglobin levels stimulates erythropoiesis, creating an even greater demand for iron, which is unmet. As a result, there is a high degree of ineffective erythropoiesis[13]. The most appropriate definition of iron deficiency is bone marrow proliferation in response to intravenous iron supplementation, an approach that has not been studied in IBD. Moreover, in addition to a possible iron deficiency in anemia of chronic disease, functional iron deficiency occurs because of intense erythropoiesis[14,15] during therapy with erythropoietic agents, with a decrease in transferrin saturation and serum ferritin. Furthermore, during erythropoietin therapy, the absorption of iron increases by as much as 5 fold[16].
After iron deficiency, anemia of chronic disease is the next important cause of anemia in these patients. There is general correlation between disease activity and the severity of anemia. Abnormal iron metabolism and improper use of iron stores are the typical features of anemia of chronic disease, characterized by low circulating iron concentration in the presence of ample reticuloendothelial iron stores. Thus, anemia of chronic disease can be easily diagnosed by the presence of hypoferraemia and increased serum levels of ferritin.
Because of differences in the therapeutic response, it is important to distinguish anemia of chronic disease from iron deficiency. Iron parameters need to be checked regularly. When both ID and ACD are present, many of the laboratory measures of iron status become unreliable. Circulating iron concentrations are subnormal in both situations. Measurement of serum ferritin reflects the level of iron in the body stores. When serum ferritin is less than 15 μg/L, iron stores are definitely depleted. When ferritin is less than 30 μg/L there is a strong suggestion of iron deficiency, whereas, a value > 200 μg/L indicates that iron deficiency is unlikely. Moreover, diagnosis of iron deficiency in the setting of IBD is difficult and no single laboratory test is reliable. Undoubtedly, low serum ferritin levels are indicative of iron deficiency. However, in the presence of inflammation, ferritin levels may increase despite iron deficiency. Furthermore, in IBD patients the diagnostic criteria for iron deficiency depend on the level of inflammation, thus in quiescent disease a serum ferritin < 30 μg/L or TfS < 15% indicates iron deficiency, whereas, in active disease a value < 100 μg/L suggests iron deficiency. A combination of true iron deficiency and anemia of chronic disease is possible when the ferritin level is between 30 and 100 g/L. If necessary, a bone marrow aspirate can be examined, which will show absence of macrophage iron in iron-deficient subjects. The ratio of soluble transferrin receptor/log ferritin has also been proposed as a marker for differentiating ID from ACD[17]. A ratio above 2 is suggestive of iron deficiency, whereas a ratio of less than 1 indicates anemia of chronic disease.
IRON TREATMENT
Since iron deficiency is the most prevalent cause of IBD-associated anemia, iron supplementation is the most relevant therapeutic intervention.
Oral iron preparations
Treatment of anemia in IBD with oral iron is limited by poor absorption, drug intolerance, and induction of oxidative stress at the site of bowel inflammation[3]. Moreover, there is evidence that oral iron may increase the disease activity in IBD. Oral iron supplements commonly contain iron in the form of ferrous salts (ferrous sulphate, ferrous gluconate, and ferrous fumarate). All ferrous compounds are oxidized in the gut lumen or within the mucosa with the release of activated hydroxyl radicals, which act on the gut wall and produce a range of gastrointestinal symptoms such as nausea, bloating, diarrhea and upper abdominal pain. Absorption of iron appears to be reduced in IBD. The acute phase protein hepcidin may play a central role in this process as it is over expressed in the liver leading to reduced iron uptake by the duodenum[18]. Moreover, oral iron enhances intestinal inflammation as well as colon carcinogenesis in animal models of colitis[19,20].
Intravenous iron preparations
Patients with IBD and anemia respond well to intravenous iron therapy with an increase in hemoglobin levels[9]. Administration of iron directly into the circulation requires formulations that prevent cellular toxicity of iron salts[21]. Three different products are available: Iron dextran, iron gluconate and iron sucrose. The stability of the dextran complex allows administration of large doses at a single setting. The molecule however may cause dextran-induced anaphylactic reactions. On the other hand, iron gluconate may cause the so-called transient capillary leak syndrome. Non-transferrin bound free ionic iron may induce acute endothelial cell injury, with the development of symptoms such as nausea, hypotension, tachycardia, dyspnoea, and oedema of the hands and feet. Iron sucrose is safer than iron dextran and is well tolerated even by patients who have previously reacted adversely to iron dextran[22]. Single doses of up to 300 mg of iron sucrose are safe[23]. The maximal recommended dose is 600 mg/wk[24]. If the infusion speed is too rapid, or the single total iron dose is too high, non-transferrin bound free iron may cause transient hypotension, tachycardia and dyspnoea, as described with iron gluconate.
Recently, a new intravenous iron preparation, ferric carboxymaltose (FeCarb) has been introduced. FeCarb can be administered intravenously in single or multiple doses of up to 1000 mg within 15 min. A recent report suggests that administration of FeCarb in IBD-associated anemia provides faster hemoglobin response, a greater increase in iron stores and better patient tolerance[25]. Intravenous iron therapy for IBD-associated anemia has been recommended since the 1970s[26], but clinical trials were only performed in the early 1990s[6]. During the last several years, experience in the use of intravenous iron sucrose in IBD has evolved greatly. Gasche et al demonstrated the efficacy of such form of iron supplementation[6,27,28]. Moreover, intravenous iron sucrose has a good safety profile in IBD-associated anemia, with a 65%-75% response rate in 4-8 wk, associated with improvement in the quality of life[6]. In IBD patients, high levels of serum transferrin, soluble transferring receptor, and serum EPO predict the response to intravenous iron supplementation, while low levels indicate the need for concomitant EPO therapy[9]. Two comparative trials (oral vs intravenous iron) have recently been published[29,30], indicating better tolerability of iv iron sucrose therapy.
The current management of IBD-associated anemia with iron sucrose is based on infusing the total iron dose by multiple small infusions, since a single dose should not exceed 500 mg (or 7 mg/kg body weight). Depending on the body weight, the usual dose for single infusions varies from 200 mg to 300 mg of iron sucrose in 100 mL sodium chloride. The total iron infusion may take between 4 to 10 wk. After this period, about 25% patients will fail to show a significant hemoglobin increase, defined as an increase in hemoglobin concentration of ≥ 20 g/L at the end of the wk 4.
One limitation of intravenous iron in patients not receiving EPO therapy is that much of the administered iron is transported into the reticuloendothelial system as storage iron, where it is less readily available for erythropoiesis.
Safety issues
Intravenous iron therapy has been carefully analyzed for risks and adverse events. Iron is an essential nutrient for proliferating microorganisms, and the sequestration of iron from microorganisms into the reticuloendothelial system is a potentially effective defense strategy against pathogens[31]. In addition, iron therapy in the setting of long-term immune activation promotes the formation of highly toxic hydroxyl radicals that can cause tissue damage and endothelial dysfunction, with increased risk of acute cardiovascular events[31-33]. Thus, intravenous iron therapy may predispose patients to infections and unfavorable coronary outcomes.
Iron therapy is currently not recommended for patients with anemia of chronic disease who have high or normal ferritin levels (above 100 g/L), because of the risk of possible adverse outcome.
ERYTHROPOIETIN AND ANEMIA IN IBD
Erythropoietin, a glycoprotein hormone secreted by the kidney, is the primary growth factor regulating erythropoiesis[34]. Erythropoiesis must maintain a steady state level of circulating RBCs and in addition respond to acute challenges. The bone marrow is a highly dynamic organ that produces two to three million red cells every second. These red cells contain hemoglobin and are replaced after 75-150 d[3]. Any imbalance in the rates of red-cell loss and production due to inadequate release of EPO, absence of co-factors required for red-cell formation (in particular iron), or an impaired ability of the erythroid progenitor cells to respond to EPO, results in anemia[35]. Erythropoiesis is controlled by the hypoxia sensing mechanism in the kidney which responds by modulating the output of EPO[36].
EPO acts on committed erythroid progenitor cells in the bone marrow to regulate their proliferation, to promote their differentiation and to maintain their viability as they differentiate. Thus, EPO is the major regulator of erythropoiesis[37]. Under normal physiological conditions, EPO expression is inversely related to tissue oxygenation and hemoglobin levels, and there is a semilogarithmic relationship between the EPO response (log) and the degree of anemia (linear)[38]. Moreover, the concentration of EPO that is normally between 5 to 29 U/L, can increase 100-fold in the presence of severe anemia. However, in some patients, EPO concentrations fail to increase despite significant anemia[39].
Measurement of serum EPO levels is useful only in anemic patients with hemoglobin levels less than 100 g/L, since EPO levels at higher hemoglobin concentrations remain well within the normal range[40]. Serum EPO levels that are inappropriately low for the degree of anemia, indicates blunted EPO response, and is encountered in anemia of chronic disease[41].
Higher serum EPO levels have been reported in IBD patients compared to the normal population[39,42,43]. The EPO levels increase with the degree of anemia[27]. Interestingly, it has been shown that in IBD-associated anemia, EPO production is inadequate in relation to the degree of anemia[5,9]. This finding may be of help when considering EPO therapy[9].
Although anemia is a frequent complication of many diseases, its clinical relevance and the importance of its correction have long been neglected. Anemia was found to have a marked effect not only on the quality of life, but also on various physiological functions[44-46]. Before the mid-1980s there was no effective therapeutic means of stimulating erythropoiesis. The clinical use of EPO began in 1986 with treatment of patients with chronic kidney disease[47,48]. Since then, indications for the use of EPO therapy to boost erythropoiesis have broadened considerably.
ERYTHROPOIETIC AGENTS
Three erythropoietic agents are currently available in routine clinical practice: epoetin alfa, epoetin beta, and darbepoietin alfa, which differ in terms of their pharmacologic modifications, receptor-binding affinity, and serum half-life, thus allowing for alternative dosing and scheduling strategies[49].
Recombinant Human Erythropoietin
Epoetin-α and Epoetin-β are the two available brands of recombinant human erythropoietin (rHu EPO). In 1977, small amounts of human EPO were obtained from the urine of patients with aplastic anemia[50]. Based on limited peptide sequence information of this purified material, the gene of human EPO was isolated and cloned in 1983[51], and the use of genetic engineering techniques allowed large-scale production of recombinant human EPO in a suitable mammalian cell line. The clinical success of this product has resulted in the use of epoetin in millions of anemic patients.
Like the endogenous hormone, epoetin binds to the dimerised EPO receptor on the surface of erythroid progenitor cells, inducing a conformational change in the receptor. This change induces phosphorylation of tyrosine residues by JAK-2 kinase on several intracellular molecules, including STAT-5, which is the major signal transducer and activator of transcription, causing gene activation in the cell nucleus[52]. Activation of these pathways stimulates proliferation and inhibits apoptosis of the erythroid progenitor cells[53].
Epoetin is highly effective in stimulating erythro-poiesis[5,6,54,55]. In addition there is increasing experimental evidence that EPO has a range of non-erythropoietic, pleiotropic effects, including tissue protection of the nervous system, myocardium, kidneys, intestines and the joints[56].
Darbepoetin alfa
Despite the undoubted therapeutic efficacy of epoetin, a major limitation of this treatment is that it has to be administered parenterally two or three times a week. Much effort has therefore been directed at producing longer-acting EPO analogues that would retain their biological activity, but require less frequent dosing. The first to be synthesized was darbepoetin (DPO) alfa[57]. DPO alfa has five N-linked glycosylation chains compared with the endogenous and recombinant EPO, both of which have three. All three molecules have, additionally, a single O-linked glycosylation chain[58]. The molecular weight of DPO alfa is 37.1 kDa, compared with 30.4 kDa for EPO, and its elimination half-life in humans after an intravenous injection is about three-fold longer (25.3 h vs 8.5 h for epoetin alfa)[59]. This allows less frequent dosing, with most patients receiving injections once weekly or once every 2-3-4 wk[60].
Continuous erythropoietin receptor activator
Continuous EPO receptor activator (CERA) is the another erythropoietic agent, that has recently completed phase III of its clinical development program[61,62]. CERA was developed by the integration of a single 30 kDa polymer chain into the EPO molecule, thus, increasing the molecular weight to twice that of epoetin to about 60 kDa, and considerably increasing the elimination half-life in humans to about 130 h. The hypothesis being tested in phase III clinical studies is whether CERA can be administered safely and effectively every 3-4 wk. The preliminary data suggests that this is perhaps the case[63,64].
Next generation erythropoietic analogues
Synthetic erythropoiesis protein, EPO fusion protein and EPO-mimetic peptides are the next generation of erythropoietic agents that exploit recent advances in drug development for stimulating erythropoiesis. These agents stimulate erythropoiesis through the activation of EPO receptors[65-67].
ROLE OF ERYTHROPOIETIN IN IBD-ASSOCIATED ANEMIA
Hemoglobin increase
Several studies have examined the efficacy and the safety of EPO in the treatment of refractory anemia in patients with IBD[5,6,11,68,69]. Horina et al first tested tEPO therapy in three patients with a long-standing history of IBD and refractory chronic anemia (Hb < 100 gr/L, plasma EPO < 100 U/L). A marked increase in hemoglobin values was noted in all three patients[68]. Gasche et al[27] reported that after 5 wk of treatment with intravenous iron alone or in combination with EPO, all anemic patients had a marked increase in hemoglobin levels. However, the mean increase in EPO-treated patients was higher compared to patients receiving iron therapy alone (50 g/L vs 20 gr/L respectively). Subsequently, recombinant human EPO was found to be effective in a controlled trial of patients with anemia refractory to oral iron supplementation[5]. In this double-blind study, the superiority of combination therapy (iron + rHEPO) over iron alone was again demonstrated. In another placebo-controlled study in a patient with Crohn’s disease-associated anemia that was refractory to oral iron, intravenous iron, and EPO resulted in greater and quicker hemoglobin response compared with intravenous iron alone[6]. Concomitant EPO therapy was associated with a more rapid incline in hemoglobin levels. EPO treatment has also been shown to be safe and effective in children with iron refractory IBD-associated anemia[11].
In a recent study[70], Darbepoetin-alpha (DPO) was tested in patients with IBD-associated refractory anemia. This study, the first to assess DPO alpha in IBD-associated refractory anemia, demonstrated that the administration of DPO in combination with intravenous iron sucrose raised hemoglobin levels. Furthermore, the longer half-life of DPO compared to EPO allowed less frequent administration and was more convenient to the patients. Thus, all the trials have demonstrated a significant beneficial effect of erythropoietin agents in this patient population.
Improvement in quality of life
Most studies have shown that successful treatment of anemia with EPO, is accompanied with improvement in the energy and activity level and the overall quality of life[71]. Surprisingly, in the hemoglobin range of 80-140 g/L, the largest improvement in quality of life occurred when hemoglobin levels increased from 110 to 130 g/L[72]. Alterations in the quality of life were also examined in anemic patients with Crohn’s disease treated with iron sucrose and EPO[6]. The sense of well being, and improvement in mood, physical ability, and social activity accounted for most of the improvement in quality of life. Moreover, avoidance of blood transfusions enhanced the improvement in quality of life[15]. Thus, the quality of life is significantly improved in patients with IBD-associated anemia with erythropoietin therapy.
MECHANISMS OF ACTION
The therapeutic effect of EPO involves counteracting the antiproliferative effect of cytokines, along with stimulation of iron uptake and heme biosynthesis in erythroid progenitor cells. Accordingly, a poor response to treatment with erythropoietic agents is associated with increased levels of proinflammatory cytokines, and poor iron availability.
There is little data on the possible effects of therapy with erythropoietic agents and the correction of anemia, on the course of the underlying disease, particularly since epoetin can exert additional biologic effects, including interference with the signal transduction cascade of cytokines. Treatment of rats with EPO was found to reduce the degree of colitis caused by DNBS suggesting that EPO may be useful in IBD treatment[73]. Furthermore, EPO may enhance the healing of colonic anastomosis after colonic surgery by increasing the number of fibroblasts and accelerating the maturation of new blood vessels[74].
PREDICTIVE FACTORS OF RESPONSE TO ERYTHROPOIETIC THERAPY
The response (erythropoiesis) to a dose of EPO is not related to the patient’s gender or age, suggesting that patient-specific factors such the underlying chronic disease, iron-restricted erythropoiesis, and other factors that normally result in a wide distribution of hemoglobin responses, account for the variability in erythropoietin response to EPO in different individuals[15].
There are no baseline indices of response to erythropoietin that can be used in routine clinical practice. If functional iron deficiency and vitamin deficiency are excluded, a low serum EPO level appears to be the only established predictive factor of some importance[75]. In IBD patients, high levels of serum transferrin, soluble transferrin receptor, and serum EPO predict the response to intravenous iron supplementation, while low levels indicate a need for concomitant EPO therapy[9].
Further studies are needed to investigate the value of hepcidin, C-reactive protein and other measures as predictive factors. The iron-regulatory hormone hepcidin is of particular interest as it is believed to be the primary factor in anemia of chronic disease. Cytokine-mediated induction of hepcidin in inflammatory or infectious conditions[76] decreases duodenal absorption of iron and induces iron retention by macrophages. Likewise, CRP is also increased in inflammatory conditions, and high CRP levels are associated with reduced Hb levels and resistance to EPO treatment. Therefore, pre-treatment levels of hepcidin and CRP may provide important information on the response to erythropoietic proteins in IBD patients.
INDICATIONS FOR ERYTHROPOIETIC THERAPY IN IBD-ASSOCIATED ANEMIA
It is difficult to determine as to which patient with IBD-associated refractory anemia will require combination therapy with erythropoietic agents in view of the absence of any long-term data. However, EPO should be reserved for symptomatic patients who may otherwise require blood transfusions, who have not responded to intravenous iron, and in whom aggressive management of IBD (including immunosuppressive therapy) has not suppressed the mucosal inflammation. EPO is an adjunct and not an alternative to appropriate treatment of IBD[77]. Several issues remain to be resolved including the use of EPO for prevention of anemia, the target hemoglobin levels, and the immunologic role of EPO in the setting of chronic bowel inflammation.
SIDE EFFECTS AND CONCERNS ABOUT EPO THERAPY
Erythropoietic therapy is highly effective in stimulating erythropoiesis and has an excellent safety profile. Apart from the rare induction of antibodies, all adverse effects of epoetin appear to be directly related to its pharmacodynamic properties i.e., the increase in red-cell number. In patients with kidney disease, such an increase may lead to a moderate rise in the blood pressure. In addition, there is a risk of thromboembolic complications with higher hemoglobin levels. Clinical trials in patients with cancer[78] and chronic kidney disease[79], designed to assess the effect of increasing the haemoglobin concentration to the normal range, compared with a subnormal target, have suggested no overall benefit and even the risk of potential harm. Moreover, it has recently been observed that “on the basis of available data, the maintenance of haemoglobin concentrations above 130 g/L appears to be unsafe in patients with chronic renal failure” [80].
The success of epoetin has been clouded by the discovery in a few patients of the development of neutralizing antibodies against recombinant proteins that cross-react with native EPO, and cause pure red-cell aplasia[81]. This problem is likely caused by the use of a new buffer (polysorbate 80), which replaced human serum albumin, and which induces the release of organic compounds with adjuvant properties from the rubber stoppers of prefilled syringes[82].
Another issue of concern is the presence of EPO receptors on several malignant cell lines, including mammary, ovarian, uterine, prostate, hepatocellular, and renal carcinomas, as well as on the myeloid cell lines[83]. There are contradictory reports concerning the effect of epoetin on such cells. Although the use of EPO caused tumor regression in a murine model of myeloma[84], administration to EPO-receptor-expressing human renal carcinoma cells in vitro stimulated their proliferation[85]. Large amounts of EPO receptors were found in 90% of biopsy specimens of human breast carcinoma[86]. The production of EPO receptors by cancer cells appears to be regulated by hypoxia, and in clinical cancer specimens the highest levels of EPO receptors were associated with neoangiogenesis, tumor hypoxia, and presence of infiltrating tumors. One potential adverse effect may be the induction of neoangiogenesis, since EPO increases inflammation and ischemia-induced neovascularization by enhancing the mobilization of endothelial progenitor cells[87]. In a study in nude mice, implantation of EPO-receptor expressing cell lines and subsequent inhibition of EPO-receptor signaling resulted in inhibition of angiogenesis and destruction of the tumor mass[83].
The effects of erythropoiesis-stimulating agents are still not fully understood. Not only do they increase haemoglobin concentration, but they may also act via alternative dose-dependent pathways that are harmful. Careful studies on the potential harmful effects of EPO therapy in patients with different forms of anemia of chronic disease remain to be carried out.
STRATEGIES FOR STIMULATING ERYTHROPOIESIS IN IBD
Anemia is a common complication of IBD and poses a serious therapeutic challenge. The optimal management of IBD-associated anemia is to promote an increase in hemoglobin levels accompanied with an improvement in the quality of life. Therapy is targeted at the mechanisms underlying anemia of chronic disease and iron deficiency. The most important approach is to treat the underlying disease (Figure 1). In previous studies assessing EPO in anemic IBD patients, current or recent immunosuppressive therapy was a specific exclusion criterion[5,6], suggesting that the patients may not be receiving optimal anti-inflammatory therapy. Moreover, as the IBD disease activity correlates with the degree of anemia, treatment of IBD should lower its incidence. Thus, as effective therapy (including immunosuppressants and anti TNF-α) induces mucosal healing[88,89], one would expect a lower incidence of anemia.
Figure 1.
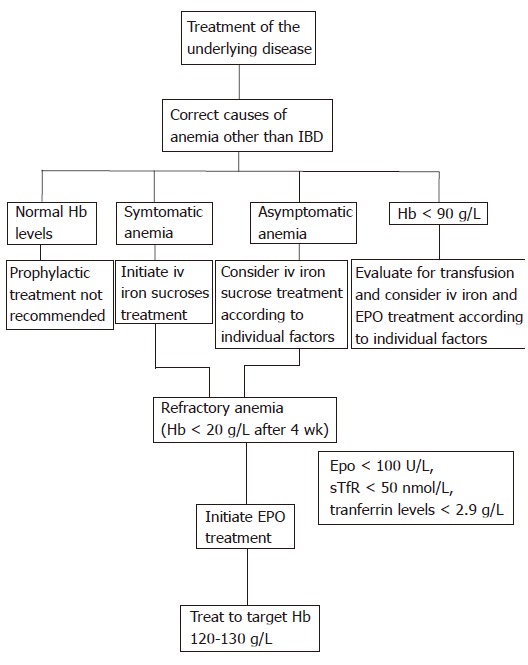
IBD-associated anemia. Suggested treatment algorithm for stimulating erythropoiesis in patients with IBD-associated anemia. Hb: Hemoglobin; iv: Intravenous; EPO: Erythropoietin.
Additionally, causes of anemia other than IBD (nutritional defects, bleeding, haemolysis, chronic kidney disease, cancer, metabolic disorders and cardiovascular disease) should be evaluated and treated. Asymptomatic anemia warrants correction, especially in patients older than 65 years of age, those with additional risk factors (such as coronary artery disease, pulmonary disease, and chronic kidney disease), or a combination of these factors.
A stepwise approach for stimulating erythropoiesis should be followed in patients with IBD. First, iron deficiency which is the most common cause of anemia should be corrected. Recent studies have demonstrated the efficacy and the safety of intravenous iron supplementation with iron sucrose. About two-thirds of patients respond to this treatment within 4-10 wk. The remaining subjects who fail to improve likely have anemia of chronic disease. Gasche et al suggest that serum levels of EPO, soluble transferring receptor, and transferrin predict a positive response to iron infusion, and may identify those patients who will benefit from early treatment with EPO[9].
EPO administration has been shown to be of benefit in patients who do not respond to iron supplementation. Several studies have demonstrated the efficacy and the safety of EPO in IBD patients with refractory anemia. EPO treatment results in a marked increase in hemoglobin values and improvement in quality of life. However, there is controversy as to whether EPO is cost-effective in treating anemia in IBD. Many physicians have employed erythropoietic agents which are not currently approved by the FDA. Thus, clinicians will have to make treatment decisions based on limited data currently available. These agents are effective in increasing hemoglobin level, and improving the quality of life for the duration they are used. However, the length of treatment, optimal dose and target hemoglobin level remain to be established. The disadvantage of these agents is that they add another drug to the patient’s treatment regimen, thereby increasing the costs, inconvenience, and potential side effects. Several studies in patients with renal failure and cancer indicate that the target hemoglobin level with EPO should be 120-130 g/L. However, a normal haematocrit value may not be optimal.
IBD-associated anemia can also be managed with blood transfusions which are widely used for rapid and effective intervention. Transfusions are particularly helpful in the context of severe anemia (Hb < 90 g/L) and life-threatening anemia (Hb < 70 g/L). However, the optimal hemoglobin threshold for red-cell transfusion in IBD patients is unknown. Recent evidence[90,91] does not support the unrestricted use of blood transfusions, because of the risks associated with this procedure such as iron overload and sensitization of the immune system. Thus, the timing of blood transfusion in IBD-associated anemia must take into consider not only the hemoglobin level but also the relative degree of hemoglobin decrease, any underlying comorbidities and the presence of anemia-related symptoms. It is also important that the use of blood transfusions should be followed by treatment with iron supplementation, with or without EPO.
In our clinical practice, most patients with IBD-associated anemia respond well to intravenous iron alone. It is important that the bowel inflammation is treated adequately and that sufficient iron is given. In anemic patients with active IBD, we usually start treatment with corticosteroids, in order to alleviate the clinical symptoms, followed by intravenous iron sucrose to replenish the iron stores. This strategy helps to stimulate impaired erythropoiesis and thus reduce the need for concomitant EPO injections. However, patients with ongoing inflammation have anemia of chronic disease and may require combination therapy with intravenous iron sucrose and erythropoietic agents. It has been suggested that about 25% of patients with IBD-associated anemia require combined treatment with iron sucrose and EPO[8]. Recently, we examined the use of DPO to determine whether it is effective in refractory anemia in IBD[70]. The administration of DPO in combination with intravenous iron sucrose was effective in these patients. Careful monitoring of hemoglobin levels and iron parameters is required to avoid recurrence of anemia in IBD patients. Additional clinical trials are warranted to establish the optimal dose and schedule of intravenous iron supplementation, and erythropoietic therapy in IBD-associated anemia.
CONCLUSION
Anemia is a frequent complication of IBD. Patients with anemia usually have greater disease severity and lower quality of life, demanding aggressive diagnosis and treatment. Iron deficiency is managed most reliably by intravenous preparations. Iron sucrose demonstrates the best efficacy and tolerability. Difficulties arise in patients with refractory anemia, who do not respond to intravenous iron. EPO administration has proven to be effective in these patients. EPO has additional beneficial effects on hemoglobin concentration. However, EPO is expensive and not without potential side effects. It is difficult to determine as to which patient with refractory anemia will require combination therapy with erythropoietic agents. The responsible use of medical resources, as well as the absence of data on long-term safety of EPO in patients with IBD, suggests that iron sucrose should be considered as the first-line therapy in IBD-associated anemia. Therefore, EPO has a secondary role, in patients who do not respond to intravenous iron alone. The long-term outcome of alleviating anemia depends on whether the bowel inflammation can be adequately treated.
Footnotes
S- Editor Ma N L- Editor Anand BS E- Editor Yin DH
References
- 1.Ebinger M, Leidl R, Thomas S, Von Tirpitz C, Reinshagen M, Adler G, Konig HH. Cost of outpatient care in patients with inflammatory bowel disease in a German University Hospital. J Gastroenterol Hepatol. 2004;19:192–199. doi: 10.1111/j.1440-1746.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- 2.Werlin SL, Grand RJ. Severe colitis in children and adolescents: diagnosis. Course, and treatment. Gastroenterology. 1977;73:828–832. [PubMed] [Google Scholar]
- 3.Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–1197. doi: 10.1136/gut.2003.035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:44S–49S. doi: 10.1016/j.amjmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber S, Howaldt S, Schnoor M, Nikolaus S, Bauditz J, Gasché C, Lochs H, Raedler A. Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease. N Engl J Med. 1996;334:619–623. doi: 10.1056/NEJM199603073341002. [DOI] [PubMed] [Google Scholar]
- 6.Gasché C, Dejaco C, Waldhoer T, Tillinger W, Reinisch W, Fueger GF, Gangl A, Lochs H. Intravenous iron and erythropoietin for anemia associated with Crohn disease. A randomized, controlled trial. Ann Intern Med. 1997;126:782–787. doi: 10.7326/0003-4819-126-10-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Anemia: A hidden epidemic. Available from: http: //www.anemia.org/professional/monograph. Accessed June 12 2006.
- 8.Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther. 2006;24:1507–1523. doi: 10.1111/j.1365-2036.2006.03146.x. [DOI] [PubMed] [Google Scholar]
- 9.Gasche C, Waldhoer T, Feichtenschlager T, Male C, Mayer A, Mittermaier C, Petritsch W. Prediction of response to iron sucrose in inflammatory bowel disease-associated anemia. Am J Gastroenterol. 2001;96:2382–2387. doi: 10.1111/j.1572-0241.2001.04094.x. [DOI] [PubMed] [Google Scholar]
- 10.Bodemar G, Kechagias S, Almer S, Danielson BG. Treatment of anaemia in inflammatory bowel disease with iron sucrose. Scand J Gastroenterol. 2004;39:454–458. doi: 10.1080/00365520310008818-1. [DOI] [PubMed] [Google Scholar]
- 11.Dohil R, Hassall E, Wadsworth LD, Israel DM. Recombinant human erythropoietin for treatment of anemia of chronic disease in children with Crohn's disease. J Pediatr. 1998;132:155–159. doi: 10.1016/s0022-3476(98)70503-7. [DOI] [PubMed] [Google Scholar]
- 12.Child JA, Brozović B, Dyer NH, Mollin DL, Dawson AM. The diagnosis of iron deficiency in patients with Crohn's disease. Gut. 1973;14:642–648. doi: 10.1136/gut.14.8.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavill I. Erythropoiesis and iron. Best Pract Res Clin Haematol. 2002;15:399–409. doi: 10.1016/s1521-6926(02)90004-6. [DOI] [PubMed] [Google Scholar]
- 14.Brugnara C. Iron deficiency and erythropoiesis: new diagnostic approaches. Clin Chem. 2003;49:1573–1578. doi: 10.1373/49.10.1573. [DOI] [PubMed] [Google Scholar]
- 15.Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96:823–833. [PubMed] [Google Scholar]
- 16.Skikne BS, Cook JD. Effect of enhanced erythropoiesis on iron absorption. J Lab Clin Med. 1992;120:746–751. [PubMed] [Google Scholar]
- 17.Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–1057. [PubMed] [Google Scholar]
- 18.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 19.Carrier JC, Aghdassi E, Jeejeebhoy K, Allard JP. Exacerbation of dextran sulfate sodium-induced colitis by dietary iron supplementation: role of NF-kappaB. Int J Colorectal Dis. 2006;21:381–387. doi: 10.1007/s00384-005-0011-7. [DOI] [PubMed] [Google Scholar]
- 20.Seril DN, Liao J, Ho KL, Warsi A, Yang CS, Yang GY. Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci. 2002;47:1266–1278. doi: 10.1023/a:1015362228659. [DOI] [PubMed] [Google Scholar]
- 21.Geisser P, Baer M, Schaub E. Structure/histotoxicity relationship of parenteral iron preparations. Arzneimittelforschung. 1992;42:1439–1452. [PubMed] [Google Scholar]
- 22.Van Wyck DB, Cavallo G, Spinowitz BS, Adhikarla R, Gagnon S, Charytan C, Levin N. Safety and efficacy of iron sucrose in patients sensitive to iron dextran: North American clinical trial. Am J Kidney Dis. 2000;36:88–97. doi: 10.1053/ajkd.2000.8276. [DOI] [PubMed] [Google Scholar]
- 23.Yee J, Besarab A. Iron sucrose: the oldest iron therapy becomes new. Am J Kidney Dis. 2002;40:1111–1121. doi: 10.1053/ajkd.2002.36853. [DOI] [PubMed] [Google Scholar]
- 24.Chandler G, Harchowal J, Macdougall IC. Intravenous iron sucrose: establishing a safe dose. Am J Kidney Dis. 2001;38:988–991. doi: 10.1053/ajkd.2001.28587. [DOI] [PubMed] [Google Scholar]
- 25.Kulnigg S, Rumyantsev V, Stoinov S, Kulnigg S, Rumyantsev V, Stoinov S, Simanenkov V, Levchenko E, Karnafel W, Garcia LC, et al. A novel intravenous iron formulation for treatment of anemia in IBD: The Ferinject randomized, controlled trial. Gastroenterology. 2007;132 suppl 2:S501–S502. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 26.Bartels U, Pedersen NS, Jarnum S. Iron absorption and serum ferritin in chronic inflammatory bowel disease. Scand J Gastroenterol. 1978;13:649–656. doi: 10.3109/00365527809181777. [DOI] [PubMed] [Google Scholar]
- 27.Gasché C, Reinisch W, Lochs H, Parsaei B, Bakos S, Wyatt J, Fueger GF, Gangl A. Anemia in Crohn's disease. Importance of inadequate erythropoietin production and iron deficiency. Dig Dis Sci. 1994;39:1930–1934. doi: 10.1007/BF02088127. [DOI] [PubMed] [Google Scholar]
- 28.Gasche C, Dejaco C, Reinisch W, Tillinger W, Waldhoer T, Fueger GF, Lochs H, Gangl A. Sequential treatment of anemia in ulcerative colitis with intravenous iron and erythropoietin. Digestion. 1999;60:262–267. doi: 10.1159/000007667. [DOI] [PubMed] [Google Scholar]
- 29.Schröder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge U, Schrott M, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503–2509. doi: 10.1111/j.1572-0241.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 30.Erichsen K, Ulvik RJ, Nysaeter G, Johansen J, Ostborg J, Berstad A, Berge RK, Hausken T. Oral ferrous fumarate or intravenous iron sucrose for patients with inflammatory bowel disease. Scand J Gastroenterol. 2005;40:1058–1065. doi: 10.1080/00365520510023198. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg ED. Iron loading and disease surveillance. Emerg Infect Dis. 1999;5:346–352. doi: 10.3201/eid0503.990305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kletzmayr J, Sunder-Plassmann G, Hörl WH. High dose intravenous iron: a note of caution. Nephrol Dial Transplant. 2002;17:962–965. doi: 10.1093/ndt/17.6.962. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan JL. Iron therapy and cardiovascular disease. Kidney Int Suppl. 1999;69:S135–S137. doi: 10.1046/j.1523-1755.1999.055suppl.69135.x. [DOI] [PubMed] [Google Scholar]
- 34.Eschbach JW, Adamson JW. Guidelines for recombinant human erythropoietin therapy. Am J Kidney Dis. 1989;14:2–8. [PubMed] [Google Scholar]
- 35.Macdougall IC, Eckardt KU. Novel strategies for stimulating erythropoiesis and potential new treatments for anaemia. Lancet. 2006;368:947–953. doi: 10.1016/S0140-6736(06)69120-4. [DOI] [PubMed] [Google Scholar]
- 36.Tan CC, Eckardt KU, Firth JD, Ratcliffe PJ. Feedback modulation of renal and hepatic erythropoietin mRNA in response to graded anemia and hypoxia. Am J Physiol. 1992;263:F474–F481. doi: 10.1152/ajprenal.1992.263.3.F474. [DOI] [PubMed] [Google Scholar]
- 37.Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 38.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 39.Tsitsika A, Stamoulakatou A, Kafritsa Y, Paleologos G, Panayotou I, Premetis E, Roma E, Papassotiriou I. Erythropoietin levels in children and adolescents with inflammatory bowel disease. J Pediatr Hematol Oncol. 2005;27:93–96. doi: 10.1097/01.mph.0000153441.34407.d9. [DOI] [PubMed] [Google Scholar]
- 40.Miller CB, Jones RJ, Piantadosi S, Abeloff MD, Spivak JL. Decreased erythropoietin response in patients with the anemia of cancer. N Engl J Med. 1990;322:1689–1692. doi: 10.1056/NEJM199006143222401. [DOI] [PubMed] [Google Scholar]
- 41.Barosi G. Inadequate erythropoietin response to anemia: definition and clinical relevance. Ann Hematol. 1994;68:215–223. doi: 10.1007/BF01737420. [DOI] [PubMed] [Google Scholar]
- 42.Demirtürk L, Hülagü S, Yaylaci M, Altin M, Ozel M. Serum erythropoietin levels in patients with severe anemia secondary to inflammatory bowel disease and the use of recombinant human erythropoietin in patients with anemia refractory to treatment. Dis Colon Rectum. 1995;38:896–897. doi: 10.1007/BF02049850. [DOI] [PubMed] [Google Scholar]
- 43.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, Koutroubakis IE, Kouroumalis EA. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96:776–781. doi: 10.1111/j.1572-0241.2001.03621.x. [DOI] [PubMed] [Google Scholar]
- 44.Macdougall IC. Quality of life and anemia: the nephrology experience. Semin Oncol. 1998;25:39–42. [PubMed] [Google Scholar]
- 45.Daneryd P, Svanberg E, Körner U, Lindholm E, Sandström R, Brevinge H, Pettersson C, Bosaeus I, Lundholm K. Protection of metabolic and exercise capacity in unselected weight-losing cancer patients following treatment with recombinant erythropoietin: a randomized prospective study. Cancer Res. 1998;58:5374–5379. [PubMed] [Google Scholar]
- 46.Ludwig H, Strasser K. Symptomatology of anemia. Semin Oncol. 2001;28:7–14. doi: 10.1016/s0093-7754(01)90206-4. [DOI] [PubMed] [Google Scholar]
- 47.Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2:1175–1178. doi: 10.1016/s0140-6736(86)92192-6. [DOI] [PubMed] [Google Scholar]
- 48.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 49.Cella D, Dobrez D, Glaspy J. Control of cancer-related anemia with erythropoietic agents: a review of evidence for improved quality of life and clinical outcomes. Ann Oncol. 2003;14:511–519. doi: 10.1093/annonc/mdg167. [DOI] [PubMed] [Google Scholar]
- 50.Miyake T, Kung CK, Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252:5558–5564. [PubMed] [Google Scholar]
- 51.Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacombe C, Mayeux P. The molecular biology of erythropoietin. Nephrol Dial Transplant. 1999;14 Suppl 2:22–28. doi: 10.1093/ndt/14.suppl_2.22. [DOI] [PubMed] [Google Scholar]
- 53.Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 54.Chang J, Couture F, Young S, McWatters KL, Lau CY. Weekly epoetin alfa maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapy. J Clin Oncol. 2005;23:2597–2605. doi: 10.1200/JCO.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 55.Witzig TE, Silberstein PT, Loprinzi CL, Sloan JA, Novotny PJ, Mailliard JA, Rowland KM, Alberts SR, Krook JE, Levitt R, et al. Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol. 2005;23:2606–2617. doi: 10.1200/JCO.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Kaltwasser JP, Kessler U, Gottschalk R, Stucki G, Möller B. Effect of recombinant human erythropoietin and intravenous iron on anemia and disease activity in rheumatoid arthritis. J Rheumatol. 2001;28:2430–2436. [PubMed] [Google Scholar]
- 57.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Br J Cancer. 2001;84 Suppl 1:3–10. doi: 10.1054/bjoc.2001.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, Egrie J. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–2395. doi: 10.1681/ASN.V10112392. [DOI] [PubMed] [Google Scholar]
- 59.Vanrenterghem Y, Bárány P, Mann JF, Kerr PG, Wilson J, Baker NF, Gray SJ. Randomized trial of darbepoetin alfa for treatment of renal anemia at a reduced dose frequency compared with rHuEPO in dialysis patients. Kidney Int. 2002;62:2167–2175. doi: 10.1046/j.1523-1755.2002.00657.x. [DOI] [PubMed] [Google Scholar]
- 60.Nissenson AR, Swan SK, Lindberg JS, Soroka SD, Beatey R, Wang C, Picarello N, McDermott-Vitak A, Maroni BJ. Randomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patients. Am J Kidney Dis. 2002;40:110–118. doi: 10.1053/ajkd.2002.33919. [DOI] [PubMed] [Google Scholar]
- 61.Haselbeck A, Bailon P, Pahlke W. The discovery and chara-cterization of CERA, an innovative agent for the treatment of anemia. Blood. 2002;100:227A (Abstract 857). [Google Scholar]
- 62.Macdougall IC. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436–440. [PubMed] [Google Scholar]
- 63.Provenzano R, Besarab A, Macdougall IC, Dougherty FC, Beyer U on behalf of the BA16528 study group. CERA (Continuous Erythropoietin Receptor Activator) administered up to once every 3 weeks corrects anemia in patients with chronic kidney disease not on dialysis (abstract) J Am Soc Nephrol. 2004;15:544A. [Google Scholar]
- 64.Locatelli F, Villa G, Arias M, Marchesi D, Dougherty FC, Beyer U on behalf of the BA16286 study group. CERA (Continuous Erythropoietin Receptor Activator) maintains hemoglobin levels in dialysis patients when administered subcutaneously up to once every 4 weeks (abstract) J Am Soc Nephrol. 2004;15:543A. [Google Scholar]
- 65.Kochendoerfer GG, Chen SY, Mao F, Cressman S, Traviglia S, Shao H, Hunter CL, Low DW, Cagle EN, Carnevali M, et al. Design and chemical synthesis of a homogeneous polymer-modified erythropoiesis protein. Science. 2003;299:884–887. doi: 10.1126/science.1079085. [DOI] [PubMed] [Google Scholar]
- 66.Sytkowski AJ, Lunn ED, Risinger MA, Davis KL. An erythropoietin fusion protein comprised of identical repeating domains exhibits enhanced biological properties. J Biol Chem. 1999;274:24773–24778. doi: 10.1074/jbc.274.35.24773. [DOI] [PubMed] [Google Scholar]
- 67.Wrighton NC, Farrell FX, Chang R, Kashyap AK, Barbone FP, Mulcahy LS, Johnson DL, Barrett RW, Jolliffe LK, Dower WJ. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273:458–464. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 68.Horina JH, Petritsch W, Schmid CR, Reicht G, Wenzl H, Silly H, Krejs GJ. Treatment of anemia in inflammatory bowel disease with recombinant human erythropoietin: results in three patients. Gastroenterology. 1993;104:1828–1831. doi: 10.1016/0016-5085(93)90666-z. [DOI] [PubMed] [Google Scholar]
- 69.Christodoulou DK, Tsianos EV. Anemia in inflammatory bowel disease - the role of recombinant human erythropoietin. Eur J Intern Med. 2000;11:222–227. doi: 10.1016/s0953-6205(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 70.Koutroubakis IE, Karmiris K, Makreas S, Xidakis C, Niniraki M, Kouroumalis EA. Effectiveness of darbepoetin-alfa in combination with intravenous iron sucrose in patients with inflammatory bowel disease and refractory anaemia: a pilot study. Eur J Gastroenterol Hepatol. 2006;18:421–425. doi: 10.1097/00042737-200604000-00017. [DOI] [PubMed] [Google Scholar]
- 71.Demetri GD, Kris M, Wade J, Degos L, Cella D. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol. 1998;16:3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- 72.Crawford J, Cella D, Cleeland CS, Cremieux PY, Demetri GD, Sarokhan BJ, Slavin MB, Glaspy JA. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95:888–895. doi: 10.1002/cncr.10763. [DOI] [PubMed] [Google Scholar]
- 73.Cuzzocrea S, Mazzon E, Di Paola R, Patel NS, Genovese T, Muià C, De Sarro A, Thiemermann C. Erythropoietin reduces the development of experimental inflammatory bowel disease. J Pharmacol Exp Ther. 2004;311:1272–1280. doi: 10.1124/jpet.104.073197. [DOI] [PubMed] [Google Scholar]
- 74.Fatouros MS, Vekinis G, Bourantas KL, Mylonakis EP, Scopelitou AS, Malamou-Mitsis VD, Kappas AM. Influence of growth factors erythropoietin and granulocyte macrophage colony stimulating factor on mechanical strength and healing of colonic anastomoses in rats. Eur J Surg. 1999;165:986–992. doi: 10.1080/110241599750008143. [DOI] [PubMed] [Google Scholar]
- 75.Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, Repetto L, Soubeyran P. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer. 2007;43:258–270. doi: 10.1016/j.ejca.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cronin CC, Shanahan F. Anemia in patients with chronic inflammatory bowel disease. Am J Gastroenterol. 2001;96:2296–2298. doi: 10.1111/j.1572-0241.2001.04032.x. [DOI] [PubMed] [Google Scholar]
- 78.Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 79.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 80.Steinbrook R. Haemoglobin concentrations in chronic kidney disease. Lancet. 2006;368:2191–2193. doi: 10.1016/S0140-6736(06)69707-9. [DOI] [PubMed] [Google Scholar]
- 81.Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, Michaud P, Papo T, Ugo V, Teyssandier I, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346:469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- 82.Boven K, Stryker S, Knight J, Thomas A, van Regenmortel M, Kemeny DM, Power D, Rossert J, Casadevall N. The increased incidence of pure red cell aplasia with an Eprex formulation in uncoated rubber stopper syringes. Kidney Int. 2005;67:2346–2353. doi: 10.1111/j.1523-1755.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 83.Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, Onozaki M, Hashimoto M, Musha T, Ogawa K, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24:1021–1029. doi: 10.1093/carcin/bgg060. [DOI] [PubMed] [Google Scholar]
- 84.Mittelman M, Neumann D, Peled A, Kanter P, Haran-Ghera N. Erythropoietin induces tumor regression and antitumor immune responses in murine myeloma models. Proc Natl Acad Sci USA. 2001;98:5181–5186. doi: 10.1073/pnas.081275298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Westenfelder C, Baranowski RL. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000;58:647–657. doi: 10.1046/j.1523-1755.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 86.Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- 87.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 88.D'Haens G, Geboes K, Rutgeerts P. Endoscopic and histologic healing of Crohn's (ileo-) colitis with azathioprine. Gastrointest Endosc. 1999;50:667–671. doi: 10.1016/s0016-5107(99)80017-0. [DOI] [PubMed] [Google Scholar]
- 89.D'haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999;116:1029–1034. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 90.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 91.Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]


