Abstract
AIM: To investigate that both the neuronal function of the contractile system and structural apparatus of the gastrointestinal tract are affected in patients with longstanding diabetes and auto mic neuropathy.
METHODS: The evoked esophageal and duodenal contractile activity to standardized bag distension was assessed using a specialized ultrasound-based probe. Twelve type-1 diabetic patients with autonomic neuropathy and severe gastrointestinal symptoms and 12 healthy controls were studied. The geometry and biomechanical parameters (strain, tension/stress, and stiffness) were assessed.
RESULTS: The diabetic patients had increased frequency of distension-induced contractions (6.0 ± 0.6 vs 3.3 ± 0.5, P < 0.001). This increased reactivity was correlated with the duration of the disease (P = 0.009). Impaired coordination of the contractile activity in diabetic patients was demonstrated as imbalance between the time required to evoke the first contraction at the distension site and proximal to it (1.5 ± 0.6 vs 0.5 ± 0.1, P = 0.03). The esophageal wall and especially the mucosa-submucosa layer had increased thickness in the patients (P < 0.001), and the longitudinal and radial compressive stretch was less in diabetics (P < 0.001). The esophageal and duodenal wall stiffness and circumferential deformation induced by the distensions were not affected in the patients (all P > 0.14).
CONCLUSION: The impaired contractile activity with an imbalance in the distension-induced contractions likely reflects neuronal abnormalities due to autonomic neuropathy. However, structural changes and remodeling of the gastrointestinal tract are also evident and may add to the neuronal changes. This may contribute to the pathophysiology of diabetic gut dysfunction and impact on future management of diabetic patients with gastrointestinal symptoms.
Keywords: Diabetes, Autonomic Neuropathy, Bio-mechanics, Contractility, Ultrasound, Esophagus, Duodenum, Deformation, Stress
INTRODUCTION
Gastrointestinal (GI) symptoms (nausea, vomiting, bloating, abdominal pain, diarrhea, etc.) are frequent in patients with diabetes mellitus[1-4]. The symptoms are often severe and substantially compromise quality of life. Abnormal GI function in diabetic patients has been demonstrated with methods such as manometry, scintigraphy, radiography, and breath tests. For example, the esophagus is characterized by dysmotility with fewer contractions having decreased amplitudes and abnormal wave forms[5]. The pathogenesis of the GI symptoms in diabetes is complex in nature, multi-factorial and not well-understood[5]. Dysmotility and delayed emptying of the stomach have been demonstrated, and in the small and large intestine dysmotility, delayed transit, and bacterial overgrowth have been observed[5]. The GI dysfunction and symptoms may be caused by autonomic neuropathy being one of the most prevalent complications affecting up to 40% of patients with long-standing diabetes[6,7], and several clinical studies have demonstrated neuropathy of the autonomic nervous system (especially vagal but also sympathetic), as well of enteric nerves[8]. Impaired visceral sensory function, glycemic control and psychological factors may also be contributing factors[9-13]. Finally, mechanical factors may also contribute to the symptoms. Hence, studies in animals with experimental diabetes have shown structural remodeling and protein cross-linking in the GI wall layers compared to control animals[14-18]. Structural remodeling caused by diabetes in animals is known to cause changes in the biomechanical properties, resulting in increase of both stiffness and thickness of the GI wall[19-22].
The impact of the structural changes in the human GI wall on the function and on biomechanical properties has not been studied in detail due to inaccessibility of the organs and lack of suitable methodology. Such studies are needed since it is still generally assumed that the contractile (and structural) apparatus of the GI tract is normal, and that the disordered function and abnormal contractile activity predominantly reflects neuronal abnormalities[23,24]. Better ways of studying the GI tract may impact on the future management of diabetic patients with GI symptoms. Cross-sectional ultrasound imaging have recently been developed to study the biomechanical properties of the GI tract during distension in animals[25,26] and humans[27-29]. The deformation pattern, the radial distributions of strain, stress and stiffness, and the distension-induced sensation have been assessed in the human esophagus[30]. The technique has also been applied to assess the biomechanical properties of the human duodenum[31]. Ultrasound has no known short or long term hazards and provides excellent soft tissue imaging with a good temporal and spatial resolution and is a valuable tool for studying GI function in vivo.
The hypotheses in the present study were that (1) the biomechanical properties of the esophageal and duodenal wall were changed due to diabetes-induced tissue remodeling and that (2) the contractile activity of the esophagus and duodenum was affected by the neuronal dysfunction related to diabetic autonomic neuropathy. Hence, the aims were to apply the new ultrasound based testing approach to (1) assess the distension-induced contractile activity in the human upper gut in healthy controls and in patients affected by gastrointestinal dysfunction due to diabetic autonomic neuropathy, and (2) to look into the mechanism of the findings by assessing the GI remodeling including the wall thickness and distension-induced deformation patterns.
MATERIALS AND METHODS
Study subjects
Data were obtained from 15 diabetic patients recruited at the Department of Endocrinology M, Aarhus University Hospital (13 males, 2 females, mean age 43 years, range 25-62 years) and 12 healthy controls (7 males, 5 females, mean age 37 years, range 29-50 years) recruited among the hospital staff and at the university. The local Ethical Committee approved the study protocol (VN 2003/120mch) which also conforms to the Declaration of Helsinki. Oral and written informed consent was obtained from all subjects.
All of the 12 patients who completed the study (Table 1) had type 1 diabetes lasting 12 to 46 years (average 23 years) and all suffered from debilitating symptomatic diabetic autonomic neuropathy (shown by a minimum of two symptoms from different organ systems) and verified by abnormal cardiovascular reflexes (heart rate variability and blood pressure changes during deep breathing and going from lying to standing). All suffered from peripheral neuropathy as demonstrated by absent or diminished patellar reflexes and abnormal biotensiometry values. The patients underwent examinations that were justified by their symptoms to exclude any organic diseases affecting the GI tract. Clinical data from the 12 patients who completed the study are presented below and in Table 1. All patients had severe GI symptoms: nausea (12 of 12), vomiting (10 of 12), abdominal pain (4 of 12), diarrhea (9 of 12), and constipation (2 of 12). Five of the 12 patients were taking medication known to affect gastrointestinal function (erythromycin, metoclopramide and proton pump inhibitors) while the rest were not treated because of previous insufficient response to various drugs. Four patients suffered from neuropathic pain and were treated with analgesics (oxycodone, gabapentin, pregabalin and paracetamol). None of the patients had prior abdominal surgery or suffered from psychiatric diseases or had any suspicion of psychological abnormalities. The control subjects did not take medications, had no prior abdominal surgery and did not suffer from any GI symptoms or pain-related diseases. They all had normal physical examination and blood tests.
Table 1.
Clinical data describing the 12 type-1 diabetic patients that completed the study
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Gender | M | M | F | M | M | M | M | M | M | M | M | F |
| Age (yr) | 30 | 47 | 25 | 32 | 40 | 46 | 42 | 39 | 41 | 62 | 53 | 33 |
| Height (cm) | 176 | 180 | 165 | 176 | 169 | 182 | 180 | 185 | 168 | 174 | 175 | 170 |
| Weight (kg) | 64 | 86 | 65 | 60 | 90 | 78 | 75 | 85 | 66 | 79 | 95 | 61 |
| Diabetes duration (yr) | 18 | 37 | 16 | 12 | 32 | 27 | 32 | 14 | 13 | 46 | 20 | 14 |
| Autonomic neuropathy | S,T | S,T | S,T | S,T | S,T | S | S,T | S | S | S | S | S,T |
| Peripheral neuropathy | S,T | S,T | S | S,T | S,T | S | S,T | S | S | S | S | S,T |
| Neuropathic pain | - | + | - | - | - | - | + | + | - | - | - | + |
| Retinopathy | + | + | + | + | + | + | + | + | + | + | + | + |
| Nephropathy | - | + | + | - | + | + | + | - | - | + | + | + |
| Bladder paresis | - | - | - | - | + | - | - | - | - | - | - | + |
| Gastroparesis1 | + | + | + | + | + | - | - | - | - | + | - | + |
| Sexual dysfunction | - | - | - | - | - | - | - | - | + | - | - | + |
| HbA1c (%) | 11.1 | 8.6 | 14.1 | 10.5 | 7.7 | 10.7 | 9.2 | 9.3 | 10.4 | 10.2 | 9.2 | 7.3 |
| Creatinine (mmol/L) | 75 | 101 | 111 | 63 | 73 | 87 | 123 | 78 | 96 | 108 | 118 | 52 |
| Nausea | + | + | + | + | + | + | + | + | + | + | + | + |
| Vomiting | + | + | + | + | + | - | + | + | + | + | + | - |
| Abdominal pain | + | - | + | - | + | - | - | - | - | + | - | - |
| Diarrhea | + | - | + | + | + | + | + | + | + | - | - | + |
| Constipation | - | - | - | - | + | - | - | + | - | - | - | - |
| Gastroscopy | N | N | N | N | N | N | N | N | N | N | N | N |
| Colonoscopy | ND | ND | N | ND | N | ND | ND | N | N | ND | ND | N |
| Small bowel radiology | ND | ND | N | N | N | N | ND | ND | N | N | ND | N |
| Breath test2 | N | ND | N | N | N | N | ND | ND | ND | N | ND | N |
| Insulin treatment | inj | pump | inj | Inj | inj | inj | inj | inj | inj | inj | inj | pump |
| GI medication | - | - | + | - | - | - | + | - | + | + | - | + |
| Analgetics | - | + | - | - | - | - | + | + | - | - | - | + |
| Smoking | + | - | + | + | - | + | - | + | - | + | - | - |
M: male, and F: female. S: verified by classic symptoms, and T = verified by tests. “-“: not present, and “+”: present. N: normal examination, and ND: not done. “inj”: injection by insulin pen. “pump”: njection by pump system.
Assessed by scintigraphy.
To exclude bacterial overgrowth. Normal range of HbA1c is < 6% and Creatinine < 125 mmol/L (M) or < 115 mmol/L (F).
Experimental probe design
The probe consisted of a 120 cm catheter (Ditens A/S, Aalborg, Denmark) with a 6.2 mm outer diameter and eight lumens of different sizes (Figure 1 top)[30]. A 50 μm thick polyurethane bag (Ditens A/S, Aalborg, Denmark) was attached to the catheter with 5 cm between the attachment points and with its centre positioned corresponding to the crystal of the ultrasound probe. The bag could be inflated to a maximum diameter of 50 mm (cross-sectional area (CSA) of 2000 mm2) with a constant bag length without stretching the bag wall. The size of the bag was chosen on the basis of previous studies of the duodenum where the CSA never exceeded 2000 mm2 when the bag was inflated to the point where moderate pain was reported[32].
Figure 1.
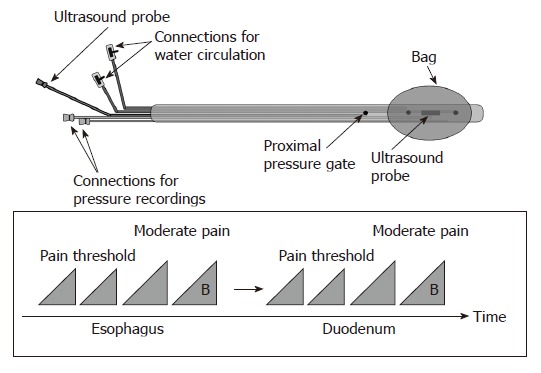
The probe design allows bag distension of the esophagus and duodenum together with cross-sectional ultrasound imaging and recording of the bag and proximal pressures (top). The distension protocol is shown at the bottom. The first distensions to the pain threshold were preconditioning stimuli also used for learning. They were followed by a distension (15 mL/min in the esophagus and 25 mL/min in the duodenum) to the perception of moderate pain. Finally, a bolus of 20 mg of butylscopolamine (B) was given intravenously in order to diminish distension-evoked smooth muscle contractions and a final distension to the perception of moderate pain was done.
The largest lumen in the probe contained a 20 MHz endoscopic 360 degrees ultrasound probe (UM-3R, Olympus Corporation, Tokyo, Japan). The signal from the endoscopic ultrasound unit (EU-M30, Olympus Corporation, Tokyo, Japan) was directly captured and stored digitally (AVI MPEG4 format) by frame grabber software (Studio 8, Pinnacle Systems Inc., CA, USA) for later analysis. Another large lumen was for infusion and withdrawal of fluid to the bag. The lumen was connected to a roller pump (Type 110, Ole Dich, Hvidovre, Denmark) for inflation and deflation of the bag with 37°C sterile water at a constant rate[30,33].
Two small lumens ( < 1 mm in diameter) were used for recording of pressures inside the bag and 6 cm proximal to the center of the bag. The channels were continuously perfused at a rate of 0.1 mL/min with sterile water by a low-compliance perfusion system. The pressure channels were attached to external pressure transducers (Baxter, Deerfield, IL, United States). The signals were amplified, analogue-to-digital converted and stored on a computer for later analysis (Openlab, Ditens A/S, Aalborg, Denmark).
Study protocol
The patients were fasting for 12 h prior to the experiment due to the well known delayed gastric emptying in this patient group (Table 1). During the fasting period the blood glucose concentrations were monitored every hour and adjusted to approximate the normal range (below 6 mmol/L) using intravenous glucose infusions and subcutaneous injection of fast-acting insulin (Actrapid, Novo, Bagsværd, Denmark). The healthy controls were fasting for six hours (Figure 1, bottom).
The probe was swallowed and the subject was positioned supine with the upper part of the body 30 degrees tilted. The lower esophageal sphincter was identified guided by the pressure recordings and the ultrasound image. The bag was positioned 10 cm above the lower esophageal sphincter. Distension was done by inflating the bag at a rate of 15 mL/min. Several preconditioning distensions to the pain threshold were done prior to experimental distensions to the perception of moderate pain[34,35]. The preconditioning distensions were repeated until the obtained data were reproducible. The bag was deflated between distensions at a rate of 15 mL/min. Then 20 mg of butylscopolamine (Buscopan, Boehringer, Ingelheim, Germany) was administrated intravenously to abolish esophageal contractility followed by one distension to the perception of moderate pain.
The probe was then advanced into the horizontal part of the duodenum guided by transabdominal ultrasound (SonoSite 180, SonoSite Inc, Bothell, WA, USA), endoscopic ultrasound and by the motility pattern observed. Approximately 30 min after the esophageal testing, and when duodenal phase II activity was observed, duodenal distensions were performed before and after administration of butylscopolamine as described above but at an inflation rate of 25 mL/min.
The total examination time was approximately two hours and the blood glucose concentrations in all patients and controls were measured before, after one hour, and at the end of the study. The blood glucose level was adjusted to approximate the normal range if it deviated during the study.
Analysis of the distension-induced contractile activity
Contractions were defined as having amplitude ≥ 10 cm H2O and duration ≥ 3 s. Contractile activity at the distension site and proximal to it were analyzed for the filling phase of all distensions. The time from start of the distension to induction of the first contraction during the distension procedure was noted. The time to induce the first contraction at the distension site is generally thought to be dependent on a local short enteric reflex arc. The time to induce the first contraction 6 cm proximal to the bag depends on a longer reflex arc that is more likely affected by extrinsic pathways (Figure 2). Hence, the ratio between these contractile responses serves as a proxy of the function of the neural pathway between the bag and proximal pressure measurement site. The number of contractions during the first minute and the frequency of all contractions were also noted. Furthermore, the pressure amplitude and duration of the strongest contraction were calculated. A proxy of the contractile work was computed by multiplying the amplitude and the duration of the strongest contraction. In the duodenum the frequency of contractions at the proximal pressure measurement site was also calculated before and after the distension procedure.
Figure 2.
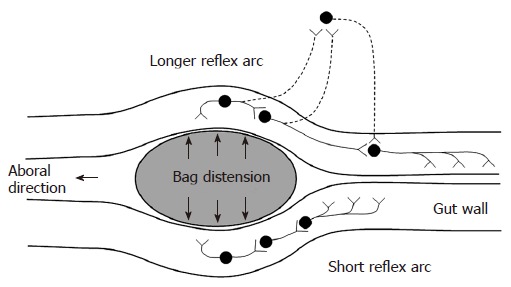
Schematic illustration of the neural pathways involved in distension-induced contraction. The primary afferent neurons, the excitatory interneurons and efferent neurons are shown. The dotted neurons illustrate extrinsic pathways. The induction of contraction on the bag depends on the local reflex arc (bottom). The measurement 6 cm proximal to the bag depends on a longer reflex arc including extrinsic pathways (top).
Biomechanical analysis of esophageal and duodenal distensions
The biomechanical properties including stress-strain and tension-strain relations, esophageal wall thickness and multidirectional deformation were measured and calculated from the intraluminal ultrasound images (Figure 3) and pressure recordings (see Appendix).
Figure 3.
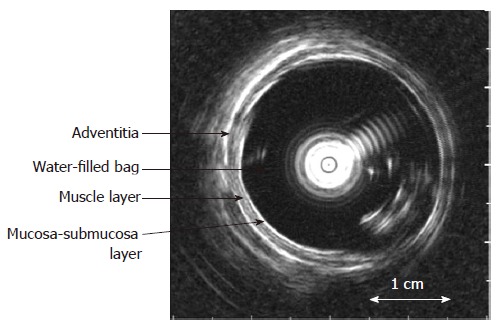
The cross-sectional ultrasound image of the distended distal esophagus allows identification of the esophageal layers, i.e. mucosa-submucosa, muscle and adventitia layers. The white round shadow in the centre is caused by the intraluminal ultrasound probe.
Statistical analysis
Data are given as mean ± SE unless otherwise stated. The contractile activity of the patients and controls was analyzed using one-way analysis of variance (ANOVA). The contraction frequencies in the duodenum in relation to the distension and the duodenal wall stiffness were analyzed using two-way ANOVA, which was also used for the esophageal stress, strain, stress-strain ratio, multi-directional deformation and wall thickness, and duodenal tension, strain and tension-strain ratio at maximum perception. The esophageal wall stiffness was analyzed using three-way ANOVA with the factors: (1) controls vs patients, (2) before vs after butylscopolamine, and (3) mucosal surface vs submucosa-muscle interface vs outer surface. The association between the clinical data and the contractile activity and biomechanical properties was analyzed using Spearman correlation test. P-values less than 0.05 were considered significant. If the data were not normal-distributed they were logarithmically transformed before the parametric tests were performed. SPSS version 11.0 was used for the statistical analysis.
RESULTS
The study was completed in 12 (10 males, 2 females, mean age 40 years, range 25-62 years) of the 15 diabetic patients. Three patients interrupted the study in its initial phase due to severe throat irritation, nausea and vomiting. All healthy controls completed the study.
Blood glucose levels
At the beginning of the 12 h fasting period the diabetic patients had a mean blood glucose level of 10.6 (range 7.0-16.5) mmol/L with fluctuations during the first hours. The glucose level was adjusted and stabilized to a mean level of 8.1 (range 5.2-10.5) mmol/L during the study period. The controls had a mean blood glucose level (after 6 h fasting) of 4.6 (range 3.5-5.2) mmol/L during the study period.
Distension-induced contractile activity
Data on the contractile activity induced by the bag distensions are provided in Table 2.
Table 2.
The contractile activity on the bag and at the proximal pressure recording site are given for both the esophagus and duodenum (mean ± SEM)
|
Esophagus |
Duodenum |
|||||||
|
Bag |
Proximal |
Bag |
Proximal |
|||||
| Controls | Diabetes | Controls | Diabetes | Controls | Diabetes | Controls | Diabetes | |
| Time to first | 17.5 ± 6.0 | 9.9 ± 2.7 | 35 ± 9.0 | 6.6 ± 2.3 | 16.5 ± 5.9 | 15.9 ± 5.2 | 28 ± 8 | 6.5 ± 2.5 |
| contraction (s) | F = 3.8 | P = 0.07 | F = 16 | P < 0.001 | F = 0.4 | P = 0.6 | F = 2.2 | P = 0.16 |
| Ratio bag/proximal, time | 0.5 ± 0.1 | 1.5 ± 0.6 | 0.6 ± 0.2 | 1.2 ± 0.2 | ||||
| to first contraction | F = 5.8 | P = 0.03 | F = 3.6 | P = 0.08 | ||||
| Number of contractions | 2.6 ± 0.5 | 5.9 ± 0.7 | 2.2 ± 0.6 | 4.5 ± 0.8 | 1.6 ± 0.4 | 1.9 ± 0.5 | 1.2 ± 0.2 | 2.1 ± 0.7 |
| in first minute | F = 14 | P = 0.001 | F = 5.8 | P = 0.03 | F = 0.2 | P = 0.7 | F = 2.9 | P = 0.09 |
| Frequency | 3.3 ± 0.5 | 6.0 ± 0.6 | 3.0 ± 0.7 | 4.6 ± 0.8 | 1.7 ± 0.4 | 1.7 ± 0.5 | 1.2 ± 0.3 | 1.7 ± 0.4 |
| (min-1) | F = 13 | P = 0.001 | F = 2.6 | P = 0.11 | F = 0.03 | P = 0.9 | F = 0.5 | P = 0.5 |
| Maximum amplitude | 95 ± 11 | 63 ± 10 | 37 ± 7 | 34 ± 5 | 19.1 ± 4.8 | 19.5 ± 5.1 | 15.9 ± 2.6 | 14.6 ± 3.8 |
| (mm H2O) | F = 4.9 | P = 0.04 | F = 0.09 | P = 0.8 | F = 0.01 | P = 0.9 | F = 0.08 | P = 0.8 |
| Contractile work | 1470 ± 257 | 592 ± 165 | 386 ± 57 | 181 ± 43 | 106 ± 50 | 88 ± 43 | 60 ± 15 | 42 ± 16 |
| (mm H2O x s) | F = 7.8 | P = 0.01 | F = 7.5 | P = 0.01 | F = 0.9 | P = 0.8 | F = 0.6 | P = 0.4 |
P- and F-values are given, and bold P-values indicate significant difference between the diabetes patients and healthy controls.
Esophagus: The diabetic patients had shorter time to the first contraction on the bag (borderline), increased number of bag contractions during the first minute, increased frequency of contractions, reduced pressure amplitudes and reduced contractile work of the bag contractions. Similar findings were found 6 cm proximal to the bag where the diabetic patients had shorter time to the first contraction, increased number of contractions during the first minute, and reduced contractile work of the contractions. The ratio between the time until the first contraction on the bag and the time until the first contraction 6 cm proximal to the bag was increased in the diabetic patients. This likely reflects dysfunction of local intestinal neural pathways.
Duodenum: The contractile activity between the diabetic patients and controls did not differ in the duodenum. The ratio between the time until the first contraction on the bag and the time until the first proximal contraction tended to increase in the diabetic patients. Furthermore, the frequency of contractions in the diabetic duodenum was 2.3 ± 1.0 min-1 before, 2.1 ± 0.8 min-1 during and 2.8 ± 0.6 min-1 after the distension and in the controls the numbers were 1.5 ± 0.4 min-1, 1.6 ± 0.6 min-1 and 1.9 ± 0.8 min-1, respectively. Hence, no difference between the patients and controls (F = 0.8, P = 0.4) and in relation to the distension (F = 0.5, P = 0.6) were found.
Esophageal wall thickness
The thickness of the entire esophageal wall, the muscle layer and mucosa-submucosa layer obtained during the distensions (after administration of butylscopolamine) are illustrated in Figure 4. The wall and the mucosa-submucosa layer were thicker (0.2-0.3 mm) in the diabetic patients compared to the control subjects (F = 13, P < 0.001, and F = 13, P < 0.001). The muscle layer thickness showed a borderline increase (F = 3.7, P = 0.055).
Figure 4.
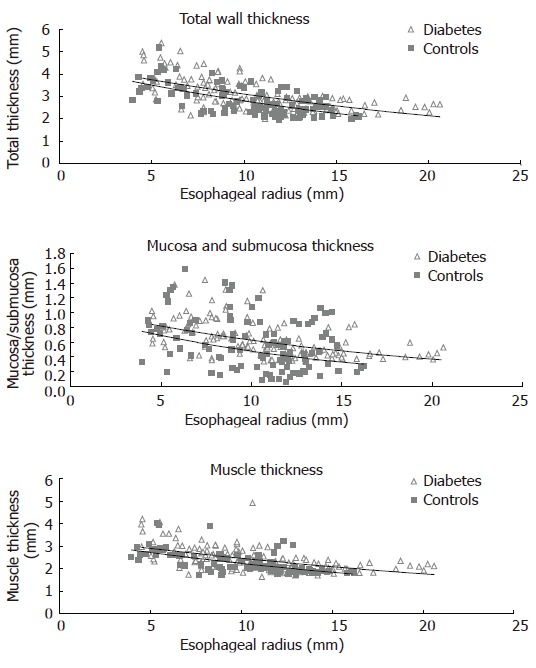
The distension-induced change in thicknesses of the total esophageal wall structure, the muscle layer, and mucosa-submucosa layer during smooth muscle relaxation with butylscopolamine are illustrated as function of the esophageal radius. The data points represent the multiple measuring points during each distension of the patients and controls. Exponential trend lines (solid lines) of the patients and controls are shown. The total wall thickness and the mucosa-submucosa layer were increased in the diabetic patients.
Multidirectional deformation of the esophagus
The circumferential, longitudinal and radial stretch ratios during distension in both the diabetic patients and control subjects are shown in Figure 5. The curves were obtained from the muscle layer and after administration of butylscopolamine. The same pattern was seen in all sub-layers both before and after administration of butylscopolamine. During distensions the patients tended to stretch less in the circumferential direction (F = 0.007, P = 0.1). The compressive deformation (shortening) in the longitudinal and radial directions during distension was clearly reduced in the diabetic patients (F = 150, P < 0.001 for longitudinal direction and F = 180, P < 0.001 for the radial direction). Thus, the esophageal wall appeared to be deformed less in all normal directions in diabetics compared to healthy volunteers.
Figure 5.
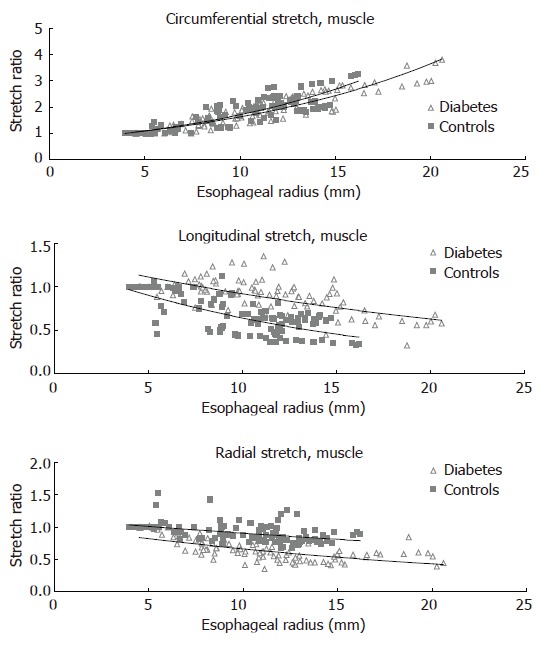
The distension-induced change in circumferential, longitudinal and radial stretch ratios are illustrated as function of the esophageal radius. The curves were obtained during smooth muscle relaxation with butylscopolamine. The data points represent the multiple measuring points during each distension of the patients and controls. Exponential trend lines (solid lines) of the patients and controls are shown. The shortening during distension was clearly reduced in the diabetic patients while the radial stretch was decreased.
Stress-strain and tension-strain relations
The tissue stiffness (approximated by the mechanical alpha-constant, see Appendix) in circumferential direction of the different esophageal layers and the duodenum are provided in Figure 6.
Figure 6.
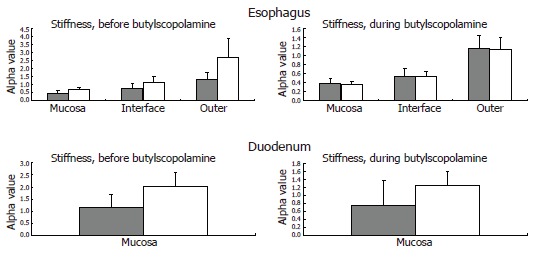
The computed esophageal stiffness (circumferential [alpha] constant) of the mucosa, submucosa-muscle interface and outer surface are illustrated. The solid grey bars represent the controls, while the grey-white bars represent the diabetic patients. The stiffness tends to be higher in the diabetic patients and is normalized during smooth muscle relaxation with butylscopolamine. This indicates an increased esophageal resting tone in diabetes. The computed duodenal stiffness is also illustrated. Mean and SEM values are shown.
Esophagus: The circumferential stiffness increased throughout the esophageal wall (F = 14, P < 0.001). The difference in tissue stiffness between the patients and the control subjects was non-significant (F = 0.3, P = 0.6) and unaffected by butylscopolamine (F = 2.2, P = 0.15). At moderate induced pain, the circumferential stress, the circumferential strain and the ratio between them (which also indicates the wall stiffness) obtained in the patients were not significantly different from those obtained in the control subjects (F = 0.7, P = 0.4; and F = 1.3, P = 0.3; and F = 0.7 P = 0.4), and unaffected by butylscopolamine (all P > 0.4).
Duodenum: The difference in tissue stiffness (Figure 6) between the patients and the controls was non-significant (F = 2.2, P = 0.14). The stiffness was not changed by butylscopolamine (F = 1.1, P = 0.3). At moderate pain the circumferential tension, the circumferential strain and the ratio between them (indicating the wall stiffness) did not differ between patients and control subjects (F = 1.0, P = 0.3; and F = 0.02, P = 0.9; and F = 2.3, P = 0.14), and was unaffected by butylscopolamine (all P > 0.5).
Correlation to clinical data
The distension induced contractile activity in the diabetic patients was not affected by the mean glucose level during the study (P > 0.2 for all comparisons). The disease duration was clearly associated with the distension-induced contractile activity. Thus, the disease duration correlated with increased frequency of the contractions and with the number of contractions during the first minute proximal to the esophageal bag (correlation coefficient rs = 0.71, P = 0.009 and rs = 0.69, P = 0.01), and to increased duration of the strongest contraction on the esophageal bag (rs = 0.71, P = 0.009). In the duodenum the disease duration correlated with increased frequency (bag: rs = 0.58, P = 0.04; proximal: rs = 0.68, P = 0.01), increased number of contractions during the first minute (bag: rs = 0.57, P = 0.04; proximal: rs = 0.68, P = 0.04), increased pressure amplitude (bag: rs = 0.59, P = 0.04; proximal: rs = 0.70, P = 0.01), duration (bag: rs = 0.62, P = 0.03; proximal: rs = 0.69, P = 0.01) and contractile work (bag: rs = 0.63, P = 0.03; proximal: rs = 0.72, P = 0.01) of the strongest contraction both at and proximal to the bag.
DISCUSSION
An ultrasound based testing approach was applied to assess the contractile activity, geometry and biomechanical properties of the esophagus and duodenum in patients affected by gastrointestinal symptoms and diabetic autonomic neuropathy. Overall, this study illustrates that the patients had increased reactivity to standardized esophageal distension including dyscoordination and reduced contractile work of the contractions. The disease duration was associated with increased contractile reactivity. Finally, the esophageal wall thickness and the pattern of deformation in longitudinal and radial directions were affected in diabetic patients indicating remodeling.
Both acute and chronic hyperglycemia is known to impair the GI motor responses to stimulation[12,36] and to reduce the perceived sensation[37]. This study was designed to minimize the influence of the blood glucose level on the contractile activity. However, the patients in the present study typically suffered from severe fluctuating glucose levels and fluctuations in GI symptoms with intermittent nausea and vomiting which makes glycemic control very challenging. Even though the mean blood glucose level during the study was higher in the diabetic patients, the glucose level itself did not seem to affect the contractile activity. Hence, the differences found in this study are more likely due to neuromuscular changes. However, comparison of data obtained in different studies of diabetic patients must be done with caution.
The present study utilizes a cross-sectional imaging technique. Conventional methods based on pressure-volume measurements obtained during bag distension do not directly measure variables used in analysis of tissue deformation and stiffness[38]. Since the esophagus is thick-walled, only cross-sectional ultrasound imaging with good temporal and spatial resolution provides data for computation of the circumferential stress, wall thickness and multi-directional deformation[30].
All patients included in the study had, as per inclusion criteria, severe and long-lasting upper GI symptoms (nausea, vomiting, bloating and pain). The observed esophageal contractile hyperreactivity may be explained by both primary neuropathic changes of the gut nerves and secondary changes due to reflex mechanisms caused by increased sensitivity to the distensions (central sensitization)[39]. However, esophageal motor abnormalities in terms of decreased contraction amplitude, decreased number of esophageal peristaltic contractions[13,23,24,40] and impaired coordination[41,42] have also been recorded in diabetic patients without GI symptoms. Disordered esophageal motility and acid reflux may be related to diabetic neuropathy[43]. Such changes correspond to the changes observed during distension in the present study. The observed reduction in time until the first contraction on the bag in the diabetic patients suggests that a local neuronal dysfunction is responsible for the hyperreactivity. This is consistent with the finding that the hyperreactivity is associated with the disease duration. A neuronal dysfunction can theoretically be addressed to the mechanoreceptor, afferent fibers, interneurons, or efferent fibers located in the gut wall. Impaired balance of the inhibitory and excitatory pathways from the central nervous system can potentially also affect the motor response. The time until the first contraction 6 cm proximal to the bag was also reduced in the diabetic patients, which may indicate hyperactivity and dysfunction of local intestinal neural pathways (a long reflex arc which may also include extra-intestinal pathways, Figure 2). Since this long reflex arc is more affected than the short arc (evidenced in Table 2 and by the computed ratio), the neuronal pathways rather than the mechanoreceptors seem to be affected. Even though neuropathic damage to the nerve fibers is expected, central (and peripheral) neuronal hyperexcitability in response to the distensions may counteract the response. Such hyperreactivity and hyperexcitability have for example been shown in patients with non-cardiac chest pain[44]. In contrast, in structural GI disorders such as systemic sclerosis, the local mechanoreceptors in the gut wall seem to be affected (resetting) more than the neuronal pathways[45]. However, the present study cannot definitively distinguish between neuronal changes restricted to the enteric nervous system and the effect of changes in the central inhibitory and excitatory neuronal pathways. Advanced methods using evoked brain potentials and functional magnetic resonance imaging may shed more light on this important aspect. Also one should take into consideration that the neuronal changes observed could be due to both diabetes-induced autonomic neuropathy and changes in the afferent visceral nervous system (sensitization and hypersensitivity) evoked by the long-standing GI symptoms[39]. Finally, the vagal innervation of different organs and central vs peripheral levels can easily be affected to different degrees[46]. As an indicator of dysfunction of the esophageal neuromuscular apparatus, the diabetic patients also seem to have an increased resting tone of the muscle component in the esophagus. This is indicated by the mechanical constants in Figure 6 where smooth muscle relaxation is shown to abolish/normalize the increased stiffness observed in the diabetic patients.
Studies of the small intestinal contractile activity in diabetes have revealed a wide spectrum of motor patterns ranging from normal to grossly abnormal[5]. The distension-induced duodenal contractile activity recorded in this study was not clearly affected in diabetes compared to the control subjects. However, the duodenal reactivity increased with the disease duration.
The question is if the disordered contractile activities observed in these studies are only due to the neuronal changes and dysfunction (diabetes-induced autonomic neuropathy) or if primary diabetes-induced remodeling in the GI tract may also play a role. Animal studies have shown that diabetes may induce crypt hyperplasia, change in the villous microvasculature and increase in the mucosal and muscle mass[14,17,19,47]. A histopathological study of the human stomach in diabetic patients with severe gastroparesis showed prominent collagenization and smooth muscle atrophy of the muscle layer[48]. Studies on diabetes and aging show that advanced glycation end-products are causing cross linking of collagen molecules responsible for basement membrane thickening and loss of matrix elasticity[15,16,49]. Animal studies support the presence of structural and biomechanical changes with increased stiffness, weight per unit length and wall thickness (i.e. increased stiffness and thickness of the GI wall)[19-22]. Even though the wall stiffness and circumferential deformation induced by the circumferential distensions in the present study were not significantly affected in diabetes, the esophageal deformations in longitudinal and radial directions and the wall thickness were abnormal with reduced deformation. The fact that the deformation is reduced but the stiffness appears unchanged can be attributed to the stress-dependent growth law for soft tissues stating that tissue remodeling is determined by the stress and remodels towards a tissue-specific stress level[35]. The esophageal wall and the mucosa-submucosa layer were thickened in diabetes, which indicates growth processes of the intestinal wall. The observed increase in esophageal wall thickness can theoretically be caused by increased muscle tone, even though an attempt to abolish smooth muscle contractions was done and repeated if contractions were still present. Butylscopolamine diminishes cholinergic mediated tone and from the tracings it appeared that relaxation was obtained. Also the decreased ability of esophageal shortening during distension and the change in radial deformation supports that structural changes occur in diabetic esophagus. The contractile work of the esophageal contractions was decreased in spite of increased neuronal activity, increased muscle thickness and the increased esophageal resting tone. This indicates that the ability of the muscle to contract could be restricted by structural remodeling (accumulation of connective tissue). The fact that the esophageal wall deformed less in diabetic patients supports this hypothesis. Alternatively, myopathic abnormalities with diabetes-induced reduced action of the muscle fibers may also be important[48].
The present study shows that both the neuronal function of the contractile system and structural apparatus of the GI tract are affected in patients with long-standing diabetes and autonomic neuropathy. This may contribute to the understanding of the pathophysiology of diabetic gut dysfunction and may have impact on future management of these patients.
ACKNOWLEDGMENTS
The Danish Health Research Council (SSVF), The Danish Diabetes Association, the Research Council of North Jutland County, the Toyota Foundation and the SparNord Foundation are acknowledged for funding this project.
APPENDIX
Analysis of esophageal distensions
Ultrasound images were captured corresponding to the start of the distension (the zero-pressure state) and between contractions during the distensions (tonic state) by a video converter (jpg format, 787 × 576 pixels, A4 Video Converter v. 2.3, www.a4video.com)[35]. The mucosal surface (bag-mucosa interface), the submucosa-muscle interface, and the outer surface (muscle-adventitia interface) contours of the esophageal wall were identified by visual inspection by an experienced radiologist using image measurement software (SigmaScan Pro v. 5.0.0, SPSS Inc., Chicago, IL, USA) (Figure 3). This allowed computation of the cross-sections encircled by the mucosal surface (CSAmuc), submucosa-muscle interface (CSAin) and outer surface (CSAout), and the corresponding circumferences (cmuc, cin and cout).
For each of the three sur- and interfaces the circumferential stretch ratio (a deformation measure) was computed as the relative elongation of each circumference: λθ = c/c zero-pressure, where c zero-pressure denotes the circumference of the unloaded segment. The c zero-pressure was measured directly from ultrasound images at the start of the butylscopolamine distension. The circumferential strain at each circumference (mucosa, submucosa-muscle and outer, respectively) was represented by the Green strain:
Math 1
Math 1.

Math(A1).
The radial deformation of the mucosal and muscle layers were computed as radial stretch ratio: λr = h/h zero-pressure, where h and hzero-pressure represents the thickness of the distended and butylscopolamine relaxed layers.
Assuming a circular shape, the wall thicknesses were calculated as:
Math 2
Math 2.
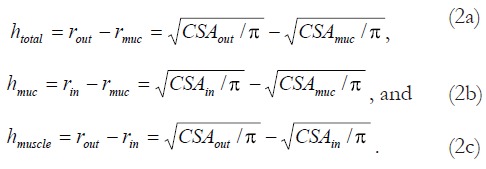
Math(A1).
The longitudinal stretch ratio of each layer which indicate the degree of longitudinal deformation was calculated as assuming incompressibility (λθλrλz = 1) of the tissue[35].
Since the esophagus is thick-walled the pressure and stress decay through the wall is expected to be non-linear[50]. Hence, the distribution of the circumferential stress through the wall was computed as:
Math 3
Math 3.
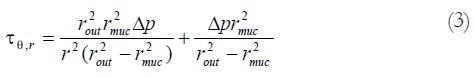
Math(A1).
where r denotes the radial location inside the wall[30,50]. The radii rout and rmuc was calculated as: and . represents the bag pressure corrected for the baseline pressure (representing the mediastinal resting pressure) before the butylscopolamine distensions.
To obtain mechanical constants of each layer the circumferential stress-strain relationship of the mucosal surface, the interface between submucosa and muscle layer, and the outer surface layers for each subject were plotted. α and β constants were obtained using non-linear curve fitting (Microcal Origon 6.0, Microcal Software Inc., Northampton, MA, USA) approximating the equation[51]:
Math 4
Math 4.

Math(A1).
The computed stiffness parameter is dependent on passive stretch and the contribution from active muscle contraction or tone. Administration of butylscopolamine abolishes smooth muscle contractions whereby the passive properties can be assessed.
Analysis of duodenal distensions
The duodenal ultrasound images were captured as described at the start of each distension and between the distension-induced contractions (see above). This allowed computation of the luminal duodenal CSA and mucosal circumference c. The circumferential stretch ratio was computed as the relative elongation of the mucosal surface (circumference c) during distension:
Math 5
Math 5.

Math(A1).
Since the ultrasound imaging not always allowed measurements at the start of the distension (artifacts due to air), c zero-pressure was in some cases approximated by a double logarithmic fitting of the circumference-pressure data. The validity of the fitting procedure was verified from the experiments with good imaging quality. A good agreement between fitted and measured values was found[31]. The circumferential strain was computed as the Green strain, see Eq. 1.
As an approximation the wall thickness was not taken into account[35] and consequently the circumferential tension was computed using Laplace’s law:
Math 6
Math 6.

Math(A1).
where r denotes the inner duodenal radius assuming circular shape: . The wall tension is the integration of the stresses through the wall (the stress moment).
The circumferential tension-strain relationships for each subject were plotted to obtain the curve fitting constants. The α and β constants were obtained using non-linear curve fitting (Microcal Origon 6.0, Microcal Software Inc., Northampton, MA, USA) using a modification of Fung’s approach[51]:
Limitation of the ultrasound technique
The ultrasound technique suffers from limitations too. At low degrees of distension convolutions of the plastic bag resulted in artifacts (air and plastic folds) and in many cases only the bag-mucosa, submucosa-muscle and muscle-adventitia interfaces could be clearly identified. At low degrees of distension it was difficult to clearly identify the inner circumference of the duodenum. This was due to small amounts of air outside the bag and folds in the bag resulting in artifacts and the fact that the bag might not always be in contact with the wall in the entire circumference at low degrees of distension. To compensate for this problem, the mucosal circumference at low degrees of distension was approximated using double-logarithmic curve fitting. Other limitations were that the ultrasound system used in this study did not provide sufficient image quality for accurate measurement of the wall thickness in the entire duodenal circumference, especially at high degrees of distension.
Footnotes
S- Editor Liu Y L- Editor Negro F E- Editor Ma WH
References
- 1.Rundles RW. Diabetic Neuropathy. Medicine. 1945;24:111–160. [Google Scholar]
- 2.Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:670–674. doi: 10.1046/j.1464-5491.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 3.Folwaczny C, Riepl R, Tschöp M, Landgraf R. Gastrointestinal involvement in patients with diabetes mellitus: Part I (first of two parts). Epidemiology, pathophysiology, clinical findings. Z Gastroenterol. 1999;37:803–815. [PubMed] [Google Scholar]
- 4.Spångéus A, El-Salhy M, Suhr O, Eriksson J, Lithner F. Prevalence of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroenterol. 1999;34:1196–1202. doi: 10.1080/003655299750024706. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz M, Samsom M. Gastrointestinal Function in Diabetes Mellius. Chichester: John Wiley & Sons Ltd; 2004. pp. 1–27. [Google Scholar]
- 6.Mearin F, Malagelada JR. Gastroparesis and dyspepsia in patients with diabetes mellitus. Eur J Gastroenterol Hepatol. 1995;7:717–723. [PubMed] [Google Scholar]
- 7.Horowitz M, Edelbroek M, Fraser R, Maddox A, Wishart J. Disordered gastric motor function in diabetes mellitus. Recent insights into prevalence, pathophysiology, clinical relevance, and treatment. Scand J Gastroenterol. 1991;26:673–684. doi: 10.3109/00365529108998583. [DOI] [PubMed] [Google Scholar]
- 8.Britland ST, Young RJ, Sharma AK, Lee D, Ah-See AK, Clarke BF. Vagus nerve morphology in diabetic gastropathy. Diabet Med. 1990;7:780–787. doi: 10.1111/j.1464-5491.1990.tb01492.x. [DOI] [PubMed] [Google Scholar]
- 9.Holtmann G, Goebell H, Talley NJ. Gastrointestinal sensory function in functional dyspepsia. Gastroenterology. 1995;109:331–332. doi: 10.1016/0016-5085(95)90308-9. [DOI] [PubMed] [Google Scholar]
- 10.Samsom M, Salet GA, Roelofs JM, Akkermans LM, Vanberge-Henegouwen GP, Smout AJ. Compliance of the proximal stomach and dyspeptic symptoms in patients with type I diabetes mellitus. Dig Dis Sci. 1995;40:2037–2042. doi: 10.1007/BF02208676. [DOI] [PubMed] [Google Scholar]
- 11.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 12.Hebbard GS, Samsom M, Sun WM, Dent J, Horowitz M. Hyperglycemia affects proximal gastric motor and sensory function during small intestinal triglyceride infusion. Am J Physiol. 1996;271:G814–G819. doi: 10.1152/ajpgi.1996.271.5.G814. [DOI] [PubMed] [Google Scholar]
- 13.Clouse RE, Lustman PJ, Reidel WL. Correlation of esophageal motility abnormalities with neuropsychiatric status in diabetics. Gastroenterology. 1986;90:1146–1154. doi: 10.1016/0016-5085(86)90379-3. [DOI] [PubMed] [Google Scholar]
- 14.Nowak TV, Harrington B, Weisbruch JP, Kalbfleisch JH. Structural and functional characteristics of muscle from diabetic rodent small intestine. Am J Physiol. 1990;258:G690–G698. doi: 10.1152/ajpgi.1990.258.5.G690. [DOI] [PubMed] [Google Scholar]
- 15.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez SS, Genta SB, Aybar MJ, Honoré SM, Villecco EI, Sánchez Riera AN. Changes in the expression of small intestine extracellular matrix proteins in streptozotocin-induced diabetic rats. Cell Biol Int. 2000;24:881–888. doi: 10.1006/cbir.2000.0581. [DOI] [PubMed] [Google Scholar]
- 17.Zoubi SA, Mayhew TM, Sparrow RA. The small intestine in experimental diabetes: cellular adaptation in crypts and villi at different longitudinal sites. Virchows Arch. 1995;426:501–507. doi: 10.1007/BF00193174. [DOI] [PubMed] [Google Scholar]
- 18.Zoubi SA, Williams MD, Mayhew TM, Sparrow RA. Number and ultrastructure of epithelial cells in crypts and villi along the streptozotocin-diabetic small intestine: a quantitative study on the effects of insulin and aldose reductase inhibition. Virchows Arch. 1995;427:187–193. doi: 10.1007/BF00196525. [DOI] [PubMed] [Google Scholar]
- 19.Jørgensen CS, Ahrensberg JM, Gregersen H, Flyvberg A. Tension-strain relations and morphometry of rat small intestine in experimental diabetes. Dig Dis Sci. 2001;46:960–967. doi: 10.1023/a:1010737323153. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Yang J, Gregersen H. Biomechanical and morphometric intestinal remodelling during experimental diabetes in rats. Diabetologia. 2003;46:1688–1697. doi: 10.1007/s00125-003-1233-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Liao D, Yang J, Gregersen H. Viscoelastic behavior of small intestine in streptozotocin-induced diabetic rats. Dig Dis Sci. 2003;48:2271–2277. doi: 10.1023/b:ddas.0000007862.50690.85. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Zhao J, Zeng Y, Gregersen H. Biomechanical properties of the rat oesophagus in experimental type-1 diabetes. Neurogastroenterol Motil. 2004;16:195–203. doi: 10.1111/j.1365-2982.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 23.Hollis JB, Castell DO, Braddom RL. Esophageal function in diabetes mellitus and its relation to peripheral neuropathy. Gastroenterology. 1977;73:1098–1102. [PubMed] [Google Scholar]
- 24.Loo FD, Dodds WJ, Soergel KH, Arndorfer RC, Helm JF, Hogan WJ. Multipeaked esophageal peristaltic pressure waves in patients with diabetic neuropathy. Gastroenterology. 1985;88:485–491. doi: 10.1016/0016-5085(85)90511-6. [DOI] [PubMed] [Google Scholar]
- 25.Assentoft JE, Gregersen H, O'Brien WD. Determination of biomechanical properties in guinea pig esophagus by means of high frequency ultrasound and impedance planimetry. Dig Dis Sci. 2000;45:1260–1266. doi: 10.1023/a:1005579214416. [DOI] [PubMed] [Google Scholar]
- 26.Jørgensen CS, Dall FH, Jensen SL, Gregersen H. A new combined high-frequency ultrasound-impedance planimetry measuring system for the quantification of organ wall biomechanics in vivo. J Biomech. 1995;28:863–867. doi: 10.1016/0021-9290(95)95275-a. [DOI] [PubMed] [Google Scholar]
- 27.Takeda T, Kassab G, Liu J, Puckett JL, Mittal RR, Mittal RK. A novel ultrasound technique to study the biomechanics of the human esophagus in vivo. Am J Physiol Gastrointest Liver Physiol. 2002;282:G785–G793. doi: 10.1152/ajpgi.00394.2001. [DOI] [PubMed] [Google Scholar]
- 28.Takeda T, Kassab G, Liu J, Nabae T, Mittal RK. Effect of atropine on the biomechanical properties of the oesophageal wall in humans. J Physiol. 2003;547:621–628. doi: 10.1113/jphysiol.2002.028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda T, Nabae T, Kassab G, Liu J, Mittal RK. Oesophageal wall stretch: the stimulus for distension induced oesophageal sensation. Neurogastroenterol Motil. 2004;16:721–728. doi: 10.1111/j.1365-2982.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- 30.Frøkjaer JB, Andersen SD, Lundbye-Christensen S, Funch-Jensen P, Drewes AM, Gregersen H. Sensation and distribution of stress and deformation in the human oesophagus. Neurogastroenterol Motil. 2006;18:104–114. doi: 10.1111/j.1365-2982.2005.00734.x. [DOI] [PubMed] [Google Scholar]
- 31.Frøkjaer JB, Andersen SD, Drewes AM, Gregersen H. Ultrasound-determined geometric and biomechanical properties of the human duodenum. Dig Dis Sci. 2006;51:1662–1669. doi: 10.1007/s10620-005-9015-y. [DOI] [PubMed] [Google Scholar]
- 32.Gao C, Arendt-Nielsen L, Liu W, Petersen P, Drewes AM, Gregersen H. Sensory and biomechanical responses to ramp-controlled distension of the human duodenum. Am J Physiol Gastrointest Liver Physiol. 2003;284:G461–G471. doi: 10.1152/ajpgi.00456.2001. [DOI] [PubMed] [Google Scholar]
- 33.Frøkjaer JB, Andersen SD, Gale J, Arendt-Nielsen L, Gregersen H, Drewes AM. An experimental study of viscero-visceral hyperalgesia using an ultrasound-based multimodal sensory testing approach. Pain. 2005;119:191–200. doi: 10.1016/j.pain.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Drewes AM, Pedersen J, Liu W, Arendt-Nielsen L, Gregersen H. Controlled mechanical distension of the human oesophagus: sensory and biomechanical findings. Scand J Gastroenterol. 2003;38:27–35. [PubMed] [Google Scholar]
- 35.Gregersen H. Biomechanics of the Gastrointestinal Tract. London: Springer-Verlag; 2003. pp. 1–262. [Google Scholar]
- 36.Björnsson ES, Urbanavicius V, Eliasson B, Attvall S, Smith U, Abrahamsson H. Effects of hyperglycemia on interdigestive gastrointestinal motility in humans. Scand J Gastroenterol. 1994;29:1096–1104. doi: 10.3109/00365529409094894. [DOI] [PubMed] [Google Scholar]
- 37.Rayner CK, Smout AJ, Sun WM, Russo A, Semmler J, Sattawatthamrong Y, Tellis N, Horowitz M. Effects of hyperglycemia on cortical response to esophageal distension in normal subjects. Dig Dis Sci. 1999;44:279–285. doi: 10.1023/a:1026642114971. [DOI] [PubMed] [Google Scholar]
- 38.Gregersen H, Christensen J. Gastrointestinal tone. Neurogastroenterol Motil. 2000;12:501–508. doi: 10.1046/j.1365-2982.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- 39.Frøkjaer JB, Andersen SD, Ejskaer N, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Drewes AM. Gut sensations in diabetic autonomic neuropathy. Pain. 2007;131:320–329. doi: 10.1016/j.pain.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Silber W. Diabetes and oesophageal dysfunction. Br Med J. 1969;3:688–690. doi: 10.1136/bmj.3.5672.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell CO, Gannan R, Coatsworth J, Neilsen R, Allen F, Hill LD, Pope CE. Relationship among esophageal dysfunction, diabetic gastroenteropathy, and peripheral neuropathy. Dig Dis Sci. 1983;28:289–293. doi: 10.1007/BF01324943. [DOI] [PubMed] [Google Scholar]
- 42.Keshavarzian A, Iber FL, Nasrallah S. Radionuclide esophageal emptying and manometric studies in diabetes mellitus. Am J Gastroenterol. 1987;82:625–631. [PubMed] [Google Scholar]
- 43.Kinekawa F, Kubo F, Matsuda K, Fujita Y, Tomita T, Uchida Y, Nishioka M. Relationship between esophageal dysfunction and neuropathy in diabetic patients. Am J Gastroenterol. 2001;96:2026–2032. doi: 10.1111/j.1572-0241.2001.03862.x. [DOI] [PubMed] [Google Scholar]
- 44.Drewes AM, Pedersen J, Reddy H, Rasmussen K, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Central sensitization in patients with non-cardiac chest pain: a clinical experimental study. Scand J Gastroenterol. 2006;41:640–649. doi: 10.1080/00365520500442559. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen J, Gao C, Egekvist H, Bjerring P, Arendt-Nielsen L, Gregersen H, Drewes AM. Pain and biomechanical responses to distention of the duodenum in patients with systemic sclerosis. Gastroenterology. 2003;124:1230–1239. doi: 10.1016/s0016-5085(03)00265-8. [DOI] [PubMed] [Google Scholar]
- 46.Jermendy G, Fornet B, Koltai MZ, Pogátsa G. Correlation between oesophageal dysmotility and cardiovascular autonomic dysfunction in diabetic patients without gastrointestinal symptoms of autonomic neuropathy. Diabetes Res. 1991;16:193–197. [PubMed] [Google Scholar]
- 47.Tahara T, Yamamoto T. Morphological changes of the villous microvascular architecture and intestinal growth in rats with streptozotocin-induced diabetes. Virchows Arch A Pathol Anat Histopathol. 1988;413:151–158. doi: 10.1007/BF00749677. [DOI] [PubMed] [Google Scholar]
- 48.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, Edmonds ME, Howard ER, Purewal T, Thomas PK, Watkins PJ. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–495. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 49.Reddy GK. AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res. 2004;68:132–142. doi: 10.1016/j.mvr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Fung YC. A First Course in Continuum Mechanics. Englewood Cliffs. NJ: Prentice Hall; 1994. pp. 1–412 PMCid: PMC3208349. [Google Scholar]
- 51.Fung YC. Biomechanics, Motion, Flow and Growth. New York: Springer Verlag; 1990. pp. 499–546. [Google Scholar]


