Abstract
AIM: To investigate the correlation between ezrin expression and invasive phenotype formation in malignantly transformed esophageal epithelial cells.
METHODS: The experimental cell line employed in the present study was originated form the progressive induction of a human embryonic esophageal epithelial cell line (SHEE) by the E6E7 genes of human papillomavirus (HPV) type 18. The cells at the 35th passage after induction called SHEEIMM were in a state of immortalized phase and used as the control, while that of the 85th passage denominated as SHEEMT represented the status of cells that were malignantly transformed. The expression changes of ezrin and its mRNA in both cell passages were respectively analyzed by RT-PCR and Western blot. Invasive phenotype was assessed in vivo by inoculating these cells into the severe combined immunodeficient (SCID) mice via subcutaneous and intraperitoneal injection, and in vitro by inoculating them on the surface of the amnion membranes, which then was determined by light microscopy and scanning electron microscopy.
RESULTS: Upregulated expression of ezrin protein and its mRNA was observed in SHEEMT compared with that in SHEEIMM cells. The SHEEMT cells inoculated in SCID mice were observed forming tumor masses in both visceral organs and soft tissues in a period of 40 d with a special propensity to invading mesentery and pancreas, but did not exhibit hepatic metastases. Pathologically, these tumor cells harboring larger nucleus, nucleolus and less cytoplasm could infiltrate and destroy adjacent tissues. In the in vitro study, the inoculated SHEEMT cells could grow in cluster on the amniotic epithelial surface and intrude into the amniotic stroma. In contrast, unrestricted growth and invasiveness were not found in SHEEIMM cells in both in vivo and in vitro experiment.
CONCLUSION: The upregulated ezrin expression is one of the important factors that are possibly associated with the invasive phenotype formation in malignantly transformed esophageal epithelial cells.
INTRODUCTION
It has been well known that cancer cells can invade and destroy surrounding tissues by their disseminative potency. This acquired malignant property is believed recently to be determined by the abnormal changes of expression patterns of certain genes such as c-met, urokinase type plasminogen activator receptor or ezrin and so on[1-9]. Therefore, the correlation between the invasive phenotype of tumor cells and the aberrant expression of these genes has become a focus of attention by the worldwide oncologists.
Induced by E6E7 genes of human papilloma virus (HPV) type 18, we have established both kinds of immortalized and malignantly transformed cells from a human embryonic esophageal epithelial cell line (SHEE)[10]. At early passages after induction, the cell did not display any abnormal growth ability in soft agar and nude mice[11-13]. At the 20th passage, the cell was noted to be immortalized with the appearance of telomerase[14,15]. At the 30th passage, the cell manifested as a phenotype of biphasic differentiation[16]. While cultivated over 60 passages, the cell exhibited atypical morphological changes and chromosomal alterations that were suspected to be a kind of premalignant transformation[17]. Over 85 passages, the cell was observed having harbored a property of malignant transformation shown by its unrestricted growth in the nude mice and invasion to the surrounding tissues[18,19]. Obviously, this process of malignant transformation of SHEE cells induced by E6E7 genes of HPV type 18 possesses a progressive and stepwise characteristic, which is no doubt a good cell model suitable for the study of correlations between abnormal gene expression and corresponding malignant phenotype formation in tumorigenesis.
Differential display analysis of RNA samples isolated from the SHEEIMM and SHEEMT cells has been performed in our previous study. There were 15 up-regulated and 6 down-regulated genes being identified by the cDNA microarray method (Data to be published), in which ezrin was one of the up-regulated genes that have been found existed in certain diseases and tumors. Being a membrane-cytoskeleton linker, ezrin protein is located in cytoplasm and is rich in microvilli and cell surface structures with the function involved in the formation of microvilli and intercellular junctions, as well as the cell motility and invasive behavior of malignant tumors[20-28]. Up to the present, however, there have been few reports concerned about how the ezrin expressed in the malignant transformation of esophageal epithelial cells and what was its relation to the invasiveness formation of tumor cells, which are of both theoretical and practical importance in the investigation of cancerogenesis. Thus, our present study was conducted to identify the correlation between the ezrin gene expression and invasive phenotype formation in malignantly transformed esophageal epithelial cells.
MATERIALS AND METHODS
Cell lines and cell culture
The experimental cells came from our laboratory, and were established by the progressive induction of SHEE cell line with the E6E7 genes of HPV type 18. The 35th, 85th passages of induced cells were employed in the present study. The cells at the 35th passage called SHEEIMM were in a state of immortalized phase, while that of the 85th passage denominated as SHEEMT represented the status of cells that were malignantly transformed. Both passages of cells were continuously cultivated in flasks with medium 199 (GIBCO) supplemented by 10% fetal bovine serum (FBS), 100 U•mL-1 penicillin and 100 U•mL-1 streptomycin at 37 °C in a humidified atmosphere containing 5% of CO2.
RT-PCR
Total RNA was extracted from SHEEIMM and SHEEMT cells using the Trizol reagent (Invitrogen). The first-strand cDNA synthesis was performed with 1 μg total RNA and carried out at 42 °C for 1 h followed by at 95 °C for 5 min and at 0-5 °C for 5 min according to protocol of reverse transcription system (Promega). The synthesized cDNA was diluted to 100 μL with TE and stored at -20 °C until use. In the following experiment, 5 μL of cDNA was amplified in a 25 μL PCR reaction volume with Advantage 2 PCR Kit (CLONTECH). Both of the ezrin primer 5'-GGGCGCTCTAAGGGTTCT-3' (sense), 5'-GCCTTTGCAAAGCTTTTATTTCA-3' (antisense) and GAPDH primer 5'-AAGGTGAAGGTCGGAGTC-3' (sense), 5'-AAGATGGTGATGGGATTTC-3 '(antisense) were synthesized by Genecore Company (Shanghai). After prepared by routine procedures, PCR products were visualized by electrophoresis on 1% agarose gel stained with ethidium bromide and quantitated with Gelworks 1D Intermediate software (version 3.51, Kodak).
Western blot
Western blot was used to detect ezrin protein expressed by experimental cells. Confluent cells of 3 flasks were washed three times with ice-cold PBS and then lysed in buffer containing 50 mM Tris-HCl (pH8.0), 150 mM NaCl, 100 μg•mL-1 phenyl-methyl-sulfonyl fluoride (PMSF) and 1% Triton X-100 for 30 min on ice. After removal of cell debris by centrifugation at 12000 g for 5 min, 50 mL of supernatant was boiled for 5 min in the sample buffer and separated by 10% SDS-PAGE, which was then transferred onto the nitrocellulose membrane (Pall Corporation). After non-specific reactivity was blocked by 5% fat-free milk in TBST (10 mM Tris-HCl, pH7.5, 150 mM NaCl, 0.05% Tween 20) for 1 h at room temperature, the membrane was incubated in turn with monoclonal antibody of mouse against human ezrin p81 (Maixin-Bio) and anti-mouse IgG-HRP antibody. Reactive protein was finally detected by ECL chemiluminescence system (Santa Cruz).
Oncogenesis and invasive potency of SHEE in vivo
SCID mice (C.B-17/IcrJ-scid nu/nu) were from Animal Laboratory Center, Chinese Academy of Medical Sciences. For the determination of their oncogenesis and invasive potency, SHEEIMM and SHEEMT cells were cultured in flasks with fresh medium to reconstitute their surface protein. After digested and washed twice with PBS, they were counted and resuspended in PBS solution (10 × 106•mL-1) until use. Then SCID mice were anesthetized with 7% chloralhydrate followed by intraperitoneal and subcutaneous inoculation with the both passages of cells. That is, 2106 SHEEMT or SHEEIMM cells in 0.2 mL PBS were injected into the peritoneal cavity in 5 mice and into the right axilla of the same number of animals. Instead, the control mice were injected only with 0.2 mL of PBS. The experimental animals were checked daily and all were killed on after day 40 inoculation by an overdose of anesthetic. Tissues from tumor mass, mesentery, pancreas and gastrointestinal tract were sampled and prepared with the routine method to produce thin paraffin sections (5 μm) stained by hematoxylin and eosin for the assessment of tumor invasion.
Invasive potency of SHEE in vitro
Tumor cell invasion in vitro was assessed by using a fresh fetal amnion, which was cultured in 199 medium supplemented with 10% FBS. In the experiment, a piece of amnion and 50000 SHEEIMM or SHEEMT cells were in turn added to one well of a 24-well plate and incubated together for 24 h and 72 h, and then were washed for several times with PBS. Then the samples, including the piece of amnion and cells adhering to it, were fixed with 2.5% glutaraldehyde and post-fixed with 2% osmium tetraoxide. After full dehydration with gradient concentrations of ethanol, the samples were adhered to an aluminium stub and sprayed plating with gold for 3 min (EIKD, IB-3, Hitachi), which were further examined by Hitachi H300 electron microscope with the attachment of scanning apparatus.
RESULTS
Expressive alterations of ezrin and its mRNA
The ezrin mRNA detected by electrophoresis on 1% agarose gel was shown as a band of 3032 bp segment, whose expression was observed to be significantly up-regulated in SHEEMT by RT-PCR assay compared with that expressed in SHEEIMM cells as displayed in Figure 1. The same difference was also noted in the expression of ezrin protein between both of the cell passages (Figure 2).
Figure 1.
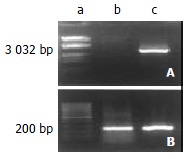
RT-PCR assay of ezrin mRNA. The expression of ezrin mRNA was significantly increased in SHEEMT compared with that in SHEEIMM cells. A. Ezrin; B. GAPDH: a. Marker; b. SHEEIMM; c. SHEEMT.
Figure 2.

Western blot analysis of ezrin protein expression. The ezrin protein was exhibited as a band of 81-kDa segment, whose expression was up-regulated in SHEEMT compared with that in SHEEIMM cells. Lane a: SHEEIMM; lane b: SHEEMT.
Oncogenesis and invasiveness in SCID mice
After inoculated into the SCID mice, SHEEMT cells were noted growing rapidly and forming tumor masses in 40 d. In peritoneal cavity, tumors were observed occurring on the mesentery, pancreas, urinary cyst, sub-diaphragm etc. with a special propensity for invading mesentery and pancreas, but did not exhibit any signs of hepatic metastases. Histological examination revealed that these tumor cells did not only grow on the organ surface, but also invade into adjacent tissues (Figure 3). In the subcutaneous tissue of the right axilla, tumor mass was observed macroscopically forming on the thoracic wall and penetrating into the thoracic cavity. Pathologically, these tumor cells harboring larger nucleus, nucleolus and less cytoplasm could infiltrate and destroy adjacent muscular fibers (Figure 4). Once transplanted, tumors could keep be passed to other SCID mice. In contrast, SHEEIMM cells were not found to form tumors in inoculated tissue by gross examination.
Figure 3.
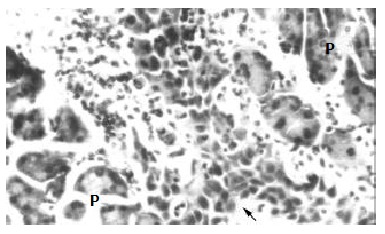
The inoculated SHEEMT cells (arrow) invaded into the parenchyma of pancreas (P) (HE, × 200).
Figure 4.
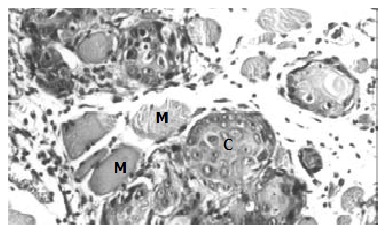
Tumor formation in subcutaneous tissue inoculated with SHEEMT cells (C), the latter was also shown to invade and destroy nearby muscle fibers (M). (HE, × 200).
Invasive potency in vitro
After inoculated on the amnion, SHEEMT cells were shown growing in cluster on the epithelial surface with the formation of pseudopod that intruded into the gap between intercellular conjunctions (Figure 5A). On the cutting surface, SHEEMT cells were observed invading into the amnion stroma (Figure 5B). It was not found that the inoculated SHEEIMM cells could adhere to or colonize on the amniotic epithelium.
Figure 5.
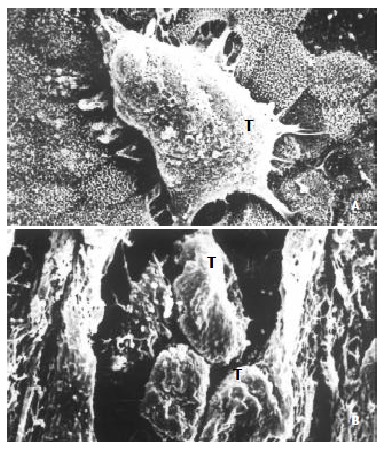
The invasiveness of inoculated SHEEMT cells on amniotic epithelium was demonstrated by scanning electron microscopy. A. A cluster of SHEEMT cells (T) grew on the amniotic epithelial surface (bar, 5 μ); B. On the cutting surface, SHEEMT cells (T) were observed invading into the amnion stroma (bar, 50 μ).
DISCUSSION
Invasive potency is one of the most important features of malignant tumors and is involved in a critical cascade of events such as extracellular matrix degradation, cell migration and colonization in the assaulted tissue. The invasive phenotype formation of malignant cells requires up-regulated expression of certain adhesive molecules, enzymes and related genes responsible for the interaction between cancer cells and extracellular matrix[29]. Several co-factors have been reported to be involved in the process of invasion and metastasis[30-32] besides ezrin that has been considered as an important molecule contributing to the malignantly transformation of cells. In addition to combining with adhesion molecules such as E-cadherin and catenin implicated in cell-cell and cell-matrix adhesion[33], ezrin plays a critical role in the determination of invasiveness of cancer cells[34].
In the present study, we investigated the expression of ezrin and its mRNA in a malignantly transformed esophageal epithelial cell line SHEEMT and further demonstrated its unrestricted growth and invasive potency in both in vivo and in vitro circumstances. The experimental results revealed that the expression of ezrin protein and its mRNA was significantly upregulated in SHEEMT compared with that in SHEEIMM cells. The SHEEMT cells inoculated to SCID mice could form tumor masses in both visceral organs and soft tissues in a period of 40 d. Pathologically, these tumor cells harboring larger nucleus, nucleolus and less cytoplasm could infiltrate and destroy adjacent tissues. In the in vitro study, the inoculated SHEEMT cells could grow in cluster on the amniotic epithelial surface and intrude into the amniotic stroma. In contrast, unrestricted growth and invasive property were not found in the SHEEIMM cells that were used as the control in both in vivo and in vitro experiments. As to our knowledge, this is one of the few reports concerned about how the ezrin gene expresses and what is its relation to the invasiveness formation in malignantly transformed esophageal epithelial cells.
New technology in molecular medicine allows global descriptions of complex expression patterns of genes responsible for the malignant properties of tumors[35-38]. With the two representative passages of cells described above, we have defined the profile of genes involved in the invasiveness of tumor cells using a sensitive cDNA microarray in a previous study. We found that increased expression of ezrin protein in SHEEMT cells was in accordance with their acquisition of the invasive potency[39]. Based on these observations, we therefore speculated that ezrin might be an important candidate of genes in charge of the invasive behaviors of malignantly transformed esophageal epithelial cells, as reported in lymphoma and astrocytic tumors[40,41]. However, recent work in this field has also focused on the identification of ezrin-binding molecules, including CD44, CD43, intercellular adhesion molecule[42-44] and syndecan-2[45]. Moreover, modulation of the ERM protein ezrin by Merlin and NF-2 has been reported in other literatures[46-48]. In the meanwhile, we have demonstrated that following expression of MMP2 and MMP9, highly invasive potency was developed in SHEEMT cells (Data to be published). All these suggest the invasiveness of tumor cells is determined by multiple genes and co-factors with complicated cellular signal passways. Therefore, future works are necessitated to demonstrate more exactly the roles of ezrin and related molecules in the formation of invasive potency of cancer cells by the gene knockout technique and other powerful tools.
Footnotes
Supported by the National Natural Science Foundation of China (No. 39830380, 39900069), Research and Development Foundation of Shantou University (L00012) and the Chinese National Human Genome Center, Beijing
Edited by Zhu L and Wang XL
References
- 1.Ueda M, Terai Y, Yamashita Y, Kumagai K, Ueki K, Yamaguchi H, Akise D, Hung YC, Ueki M. Correlation between vascular endothelial growth factor-C expression and invasion phenotype in cervical carcinomas. Int J Cancer. 2002;98:335–343. doi: 10.1002/ijc.10193. [DOI] [PubMed] [Google Scholar]
- 2.Nestl A, Von Stein OD, Zatloukal K, Thies WG, Herrlich P, Hofmann M, Sleeman JP. Gene expression patterns associated with the metastatic phenotype in rodent and human tumors. Cancer Res. 2001;61:1569–1577. [PubMed] [Google Scholar]
- 3.Comoglio PM, Tamagnone L, Boccaccio C. Plasminogen-related growth factor and semaphorin receptors: a gene superfamily controlling invasive growth. Exp Cell Res. 1999;253:88–99. doi: 10.1006/excr.1999.4684. [DOI] [PubMed] [Google Scholar]
- 4.Janneau JL, Maldonado-Estrada J, Tachdjian G, Miran I, Motté N, Saulnier P, Sabourin JC, Coté JF, Simon B, Frydman R, et al. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab. 2002;87:5336–5339. doi: 10.1210/jc.2002-021093. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Pakneshan P, Gladu J, Slack A, Szyf M, Rabbani SA. Regulation of DNA methylation in human breast cancer. Effect on the urokinase-type plasminogen activator gene production and tumor invasion. J Biol Chem. 2002;277:41571–41579. doi: 10.1074/jbc.M201864200. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Xu W, Lu J, He F, Yang X. Invasiveness of hepatocellular carcinoma cell lines: contribution of hepatocyte growth factor, c-met, and transcription factor Ets-1. Biochem Biophys Res Commun. 2001;286:1123–1130. doi: 10.1006/bbrc.2001.5521. [DOI] [PubMed] [Google Scholar]
- 7.Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loktionov A, Watson MA, Stebbings WS, Speakman CT, Bingham SA. Plasminogen activator inhibitor-1 gene polymorphism and colorectal cancer risk and prognosis. Cancer Lett. 2003;189:189–196. doi: 10.1016/s0304-3835(02)00556-6. [DOI] [PubMed] [Google Scholar]
- 9.Tokunou M, Niki T, Eguchi K, Iba S, Tsuda H, Yamada T, Matsuno Y, Kondo H, Saitoh Y, Imamura H, et al. c-MET expression in myofibroblasts: role in autocrine activation and prognostic significance in lung adenocarcinoma. Am J Pathol. 2001;158:1451–1463. doi: 10.1016/S0002-9440(10)64096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen ZY, Xu LY, Chen MH, Shen J, Cai WJ, Zeng Y. Progressive transformation of immortalized esophageal epithelial cells. World J Gastroenterol. 2002;8:976–981. doi: 10.3748/wjg.v8.i6.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shgn Z, Cen S, Zeng Y. [Immortalization of human fetal esophageal epithelial cells induced by E6 and E7 genes of human papilloma virus 18] Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 1999;13:121–123. [PubMed] [Google Scholar]
- 12.Shen ZY, Xu LY, Chen XH, Cai WJ, Shen J, Chen JY, Huang TH, Zeng Y. The genetic events of HPV-immortalized esophageal epithelium cells. Int J Mol Med. 2001;8:537–542. doi: 10.3892/ijmm.8.5.537. [DOI] [PubMed] [Google Scholar]
- 13.Shen Z, Shen J, Zeng Y. [Biological characteristics of human fetal esophageal epithelial cell line immortalized by the E6 and E7 gene of HPV type 18] Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 1999;13:209–212. [PubMed] [Google Scholar]
- 14.Shen ZY, Xu LY, Li C, Cai WJ, Shen J, Chen JY, Zeng Y. A comparative study of telomerase activity and malignant phenotype in multistage carcinogenesis of esophageal epithelial cells induced by human papillomavirus. Int J Mol Med. 2001;8:633–639. doi: 10.3892/ijmm.8.6.633. [DOI] [PubMed] [Google Scholar]
- 15.Shen ZY, Xu LY, Li EM, Cai WJ, Chen MH, Shen J, Zeng Y. Telomere and telomerase in the initial stage of immortalization of esophageal epithelial cell. World J Gastroenterol. 2002;8:357–362. doi: 10.3748/wjg.v8.i2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen ZY, Xu LY, Chen MH, Cai WJ, Shen J, Chen JY, Hon CQ, Zeng Y. Biphasic differentiation of immortalized esophageal epitheliums induced by HPV18E6E7. Bingdu Xuebao. 2001;17:210–214. [Google Scholar]
- 17.Shen Z, Cen S, Shen J, Cai W, Xu J, Teng Z, Hu Z, Zeng Y. Study of immortalization and malignant transformation of human embryonic esophageal epithelial cells induced by HPV18 E6E7. J Cancer Res Clin Oncol. 2000;126:589–594. doi: 10.1007/pl00008469. [DOI] [PubMed] [Google Scholar]
- 18.Shen ZY, Cai WJ, Shen J, Xu JJ, Cen S, Teng ZP, Hu Z, Zeng Y. Human papilloma virus 18E6E7 in synergy with TPA induced malignant transformation of human embryonic esophageal epithelial cells. Bingdu Xuebao. 1999;15:1–6. [Google Scholar]
- 19.Shen Z, Shen J, Cai W, Chen J, Zeng Y. [Malignant transformation of the immortalized esophageal epithelial cells] Zhonghua Zhongliu Zazhi. 2002;24:107–109. [PubMed] [Google Scholar]
- 20.Mangeat P, Roy C, Martin M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999;9:187–192. doi: 10.1016/s0962-8924(99)01544-5. [DOI] [PubMed] [Google Scholar]
- 21.Scherer SS, Xu T, Crino P, Arroyo EJ, Gutmann DH. Ezrin, radixin, and moesin are components of Schwann cell microvilli. J Neurosci Res. 2001;65:150–164. doi: 10.1002/jnr.1138. [DOI] [PubMed] [Google Scholar]
- 22.Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F. Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett. 1999;147:31–38. doi: 10.1016/s0304-3835(99)00272-4. [DOI] [PubMed] [Google Scholar]
- 23.Mäkitie T, Carpén O, Vaheri A, Kivelä T. Ezrin as a prognostic indicator and its relationship to tumor characteristics in uveal malignant melanoma. Invest Ophthalmol Vis Sci. 2001;42:2442–2449. [PubMed] [Google Scholar]
- 24.Ohtani K, Sakamoto H, Rutherford T, Chen Z, Kikuchi A, Yamamoto T, Satoh K, Naftolin F. Ezrin, a membrane-cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett. 2002;179:79–86. doi: 10.1016/s0304-3835(01)00857-6. [DOI] [PubMed] [Google Scholar]
- 25.Tokunou M, Niki T, Saitoh Y, Imamura H, Sakamoto M, Hirohashi S. Altered expression of the ERM proteins in lung adenocarcinoma. Lab Invest. 2000;80:1643–1650. doi: 10.1038/labinvest.3780174. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MW, Miyata H, Vinters HV. Ezrin and moesin expression within the developing human cerebrum and tuberous sclerosis-associated cortical tubers. Acta Neuropathol. 2002;104:188–196. doi: 10.1007/s00401-002-0540-x. [DOI] [PubMed] [Google Scholar]
- 27.Mykkänen OM, Grönholm M, Rönty M, Lalowski M, Salmikangas P, Suila H, Carpén O. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–3073. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:7300–7305. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez RR, Devoto L, Campana A, Bischof P. Effects of leptin, interleukin-1alpha, interleukin-6, and transforming growth factor-beta on markers of trophoblast invasive phenotype: integrins and metalloproteinases. Endocrine. 2001;15:157–164. doi: 10.1385/ENDO:15:2:157. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Fadiel A, Feng Y, Ohtani K, Rutherford T, Naftolin F. Ovarian epithelial carcinoma tyrosine phosphorylation, cell proliferation, and ezrin translocation are stimulated by interleukin 1alpha and epidermal growth factor. Cancer. 2001;92:3068–3075. doi: 10.1002/1097-0142(20011215)92:12<3068::aid-cncr10149>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Tran Quang C, Gautreau A, Arpin M, Treisman R. Ezrin function is required for ROCK-mediated fibroblast transformation by the Net and Dbl oncogenes. EMBO J. 2000;19:4565–4576. doi: 10.1093/emboj/19.17.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stapleton G, Malliri A, Ozanne BW. Downregulated AP-1 activity is associated with inhibition of Protein-Kinase-C-dependent CD44 and ezrin localisation and upregulation of PKC theta in A431 cells. J Cell Sci. 2002;115:2713–2724. doi: 10.1242/jcs.115.13.2713. [DOI] [PubMed] [Google Scholar]
- 33.Si HX, Tsao SW, Lam KY, Srivastava G, Liu Y, Wong YC, Shen ZY, Cheung AL. E-cadherin expression is commonly downregulated by CpG island hypermethylation in esophageal carcinoma cells. Cancer Lett. 2001;173:71–78. doi: 10.1016/s0304-3835(01)00646-2. [DOI] [PubMed] [Google Scholar]
- 34.Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999;112 Pt 18:3081–3090. doi: 10.1242/jcs.112.18.3081. [DOI] [PubMed] [Google Scholar]
- 35.Schindelmann S, Windisch J, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. Expression profiling of mammary carcinoma cell lines: correlation of in vitro invasiveness with expression of CD24. Tumour Biol. 2002;23:139–145. doi: 10.1159/000064030. [DOI] [PubMed] [Google Scholar]
- 36.Zhan F, Cao L, Hu C, Li G. [Differentially expressed cDNA sequences homologous with known genes in human nasopharyngeal carcinoma] Hunan Yike Daxue Xuebao. 1999;24:103–106. [PubMed] [Google Scholar]
- 37.Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C, Trepel J, Meltzer P, Helman L. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res. 2001;61:3750–3759. [PubMed] [Google Scholar]
- 38.Wang KC, Cheng AL, Chuang SE, Hsu HC, Su IJ. Retinoic acid-induced apoptotic pathway in T-cell lymphoma: Identification of four groups of genes with differential biological functions. Exp Hematol. 2000;28:1441–1450. doi: 10.1016/s0301-472x(00)00546-4. [DOI] [PubMed] [Google Scholar]
- 39.Shen J, Chen MH, Zheng RM, Chen JJ, Shen ZY. Detection of tumor cell invasion in vitro by scanning electron microscopy. Dianzi Xianwei Xuebao. 2000;19:313–314. [Google Scholar]
- 40.Geiger KD, Stoldt P, Schlote W, Derouiche A. Ezrin immunoreactivity is associated with increasing malignancy of astrocytic tumors but is absent in oligodendrogliomas. Am J Pathol. 2000;157:1785–1793. doi: 10.1016/S0002-9440(10)64816-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akisawa N, Nishimori I, Iwamura T, Onishi S, Hollingsworth MA. High levels of ezrin expressed by human pancreatic adenocarcinoma cell lines with high metastatic potential. Biochem Biophys Res Commun. 1999;258:395–400. doi: 10.1006/bbrc.1999.0653. [DOI] [PubMed] [Google Scholar]
- 42.Harrison GM, Davies G, Martin TA, Jiang WG, Mason MD. Distribution and expression of CD44 isoforms and Ezrin during prostate cancer-endothelium interaction. Int J Oncol. 2002;21:935–940. [PubMed] [Google Scholar]
- 43.Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM. A novel PKC-regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol. 2002;4:399–407. doi: 10.1038/ncb797. [DOI] [PubMed] [Google Scholar]
- 44.Guan XQ, Wang CJ, Li YY. [Effects of Ezrin on differentiation and adhesion of hepatocellular carcinoma] Ai Zheng. 2002;21:281–284. [PubMed] [Google Scholar]
- 45.Granés F, Urena JM, Rocamora N, Vilaró S. Ezrin links syndecan-2 to the cytoskeleton. J Cell Sci. 2000;113(Pt 7):1267–1276. doi: 10.1242/jcs.113.7.1267. [DOI] [PubMed] [Google Scholar]
- 46.Grönholm M, Sainio M, Zhao F, Heiska L, Vaheri A, Carpén O. Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin. J Cell Sci. 1999;112(Pt 6):895–904. doi: 10.1242/jcs.112.6.895. [DOI] [PubMed] [Google Scholar]
- 47.Meng JJ, Lowrie DJ, Sun H, Dorsey E, Pelton PD, Bashour AM, Groden J, Ratner N, Ip W. Interaction between two isoforms of the NF2 tumor suppressor protein, merlin, and between merlin and ezrin, suggests modulation of ERM proteins by merlin. J Neurosci Res. 2000;62:491–502. doi: 10.1002/1097-4547(20001115)62:4<491::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 48.Gutmann DH, Sherman L, Seftor L, Haipek C, Hoang Lu K, Hendrix M. Increased expression of the NF2 tumor suppressor gene product, merlin, impairs cell motility, adhesionand spreading. Hum Mol Genet. 1999;8:267–275. doi: 10.1093/hmg/8.2.267. [DOI] [PubMed] [Google Scholar]


