Abstract
AIM: To develop an oral DNA vaccine against gastric cancer and evaluate its efficacy in mice.
METHODS: The genes of the MG7-Ag mimotope and a universal Th epitope (Pan-DR epitope, PADRE) were included in the PCR primers. By PCR, the fusion gene of the two epitopes was amplified. The fusion gene was confirmed by sequencing and was then cloned into pcDNA3.1 (+) plasmid. The pcDNA3.1 (+)-MG7/PADRE was used to transfect an attenuated Salmonella typhimurium. C57BL/6 mice were orally immunized with 1 × 108 cfu Salmonella transfectants. Salmonella harboring the empty pcDNA3.1 (+) plasmid and phosphate buffer saline (PBS) were used as negative controls. At the 6th week, serum titer of MG7-Ag specific antibody was detected by ELISA. At the 8th week cellular immunity was detected by an unprimed proliferation test of the spleenocytes by using a [3H]-thymidine incorporation assay. Ehrlich ascites carcinoma cells expressing MG7-Ag were used as a model in tumor challenge assay to evaluate the protective effect of the vaccine.
RESULTS: Serum titer of antibody against MG7-Ag was significantly higher in mice immunized with the vaccine than that in control groups (0.841 vs 0.347, P < 0.01; 0.841 vs 0.298, P < 0.01), while in vitro unprimed proliferation assay of the spleenocytes showed no statistical difference between those three groups. Two weeks after tumor challenge, 2 in 7 immunized mice were tumor free, while all the mice in the control groups showed tumor formation.
CONCLUSION: Oral DNA vaccine against the MG7-Ag momitope of gastric cancer is immunogenic. It can induce significant humoral immunity against tumor in mice, and the vaccine has partially protective effects.
INTRODUCTION
Gastric cancer is the most common malignant tumor in China and the second most common malignancy around the world. Conventional intervention measures such as operation and chemotherapy work poorly in treating gastric cancer. And with few tumor-specific antigens identified, there are few effective vaccines developed to combat gastric cancer. MG7-Ag, discovered by our institute, is a kind of gastric cancer-specific tumor-associated antigen. Detecting the serum anti-MG-Ag antibody serves as a preliminary test in the diagnosis for gastric cancer and could be used for the surveillance of relapse and the appraisal of treatment efficacy[1]. MG7-Ag can be used as an indicator for high risk of malignant change in stomach mucosa dysplasia[2]. Primary study showed that MG7-Ag could elicit significant specific immune response against gastric cancer, suggesting that it could be an excellent target for cancer vaccine development. However, due to its unknown identity, it is much difficult to isolate and purify MG7-Ag from tumor tissues. Recently, we have identified the mimotopes of MG7-Ag by screening the phage display library, and the mimotopes could mimic the primary antigen efficiently, as shown by in vitro and in vivo assays[3,4]. We reported here for the first time the development of an oral DNA vaccine by using the MG7-Ag mimotope of gastric cancer.
MATERIALS AND METHODS
Plasmids and bacteria
The plasmid pcDNA3.1 (+) was purchased from Invitrogen Corporation. And the pEGFP plasmid containing the enhanced green fluorescence protein (EGFP) gene was purchased from Clontech Corporation. Attenuated Salmonella typhimurium SL3261 strain was used as the oral vector to develop the vaccine.
Construction of eukayotic expression vector of MG7-Ag momitope fused with a helper T cell epitope PADRE
Two pairs of PCR primers (P1.1, P1.2 and P2.1, P2.2) were designed by using Primer Premiere 5.0 software. The sense primers (P1.1 and P2.1) were both 5'-CGATGTACGGGCCAGATATACGCG-3', corresponding to the 209-232 bp sequence of pcDNA3.1 (+). Reverse primer P1.2 was 5'-ACTTCCTCCTCCTTTTGTATGCACA TGAGGTTTCATGGTGGCAAGCTTCCTACCGCCCATTTGCGT, corresponding to the reverse complementary sequence of 768-785 bp sequence of pcDNA3.1 (+), Hind III digestion site, Kozak sequence, and the sequence of the MG7-Ag mimotope. Reverse primer P2.2 was 5'-TTAAGCAGCAGCTTTAAGTGTCCAAGCAGCCAC AAATTTAGCACTTCCTCCTCCTTTTGTATGCA-3', corresponding to the reverse complementary sequence of the MG7-Ag mimotope and the universal Th epitope PADRE. Two PCR reactions were performed to incorporate the mimotope and the PADRE into a fragment of pcDNA3.1 (+) plasmid. In this case, the pcDNA3.1 fragment was used as a carrier to facilitate further manipulations. For the first PCR reaction, template was plasmid pcDNA3.1 (+), and primers were P1.1 and P1.2. By using the product of the first PCR as template, a second PCR was performed with primers P2.1 and P2.2 to incorporate the PADRE epitope into the yielding fragment. The final PCR product was visualized by agarose electrophoresis and was then cloned into pUCm-T vector and sequenced on ABI PRISMTM 377 sequencer. Then, the PCR product was subcloned into pcDNA3.1 (+) vector from the pUCm-T vector. By restrictive enzyme digestion with Hind III, the non-relevant plasmid fragment was removed from the final recombinant vector pcDNA3.1 (+)-MG7/PADRE. The vector was sequenced to confirm the proper encoding sequence.
Construction of oral DNA vaccine and in vitro experiment using attenuated Salmonella typhimurium as the oral DNA vector
To get the oral DNA vaccine, the pcDNA3.1 (+)-MG7/PADRE vector was transduced into the attenuated Salmonella typhimurium SL3261 by electroporation (2.5 kV, 25 μF, 200 Ω, pulse time 0.0326S). Plasmid in the Salmonella transfectant was extracted and used as template, and PCR was performed by using primer P1.1 and primer P2.2 to verify the successful transfection. The PCR product underwent agarose electrophoresis for visualization. An in vitro experiment was performed by using the SL3261 strain as the oral vector of DNA vaccine according to reference[5]. Briefly, eukaryotic expression vector of the enhanced green fluorescence protein was transduced into the attenuated Salmonella typhimurium SL3261. The transfectants were coincubated with murine peritoneal macrophage at 37 °C for 30 min. SL3261 harboring the empty pcDNA3.1 (+) plasmid was used as negative control. Gentamycin was added into the culture media to kill the extracellular beacteria. Four hours later, tetracycline was added to kill the intracellular bacteria. The infected macrophages were cultured for 48 h, and were then examined by flow cytometry (FCM) for the expression of green fluorescence protein.
Immunization of the mice and immune response examination
Thirty-five female 4-week-aged C57BL/6J mice weighing 15-20 g were used in the immunization assay. They were randomly divided into 3 groups, which were orally given the PBS solution (10 mice, PBS control), the attenuated Salmonella SL3261 harboring the empty pcDNA3.1 (+) plasmid (10 mice, empty control) or the oral DNA vaccine SL3261 strain harboring the pcDNA3.1 (+)-MG7/PADRE (15 mice, immunization group). Before immunization, all the mice were starved overnight and pre-administered with 100 μL 10 g/L NaHCO3 solution. Each time, 100 μL PBS (pH7.6) was given to the mice in PBS control group, and 1 × 108 Salmonella typhimurium were given to the mice in the empty control and immunization group. PBS and Salmonella typhimurium were given to the mice by orogastric inoculation. Immunization was repeated every two weeks. At the 6th week after the first immunization, sera from the mice (5 mice from each group) were prepared and 1:80 diluted. By coating KATO III cells expressing the MG7-Ag on the plates, a cellular ELISA was performed to detect the antibody against MG7-Ag. At the 8th week, the splenocyte suspension was prepared, and an unprimed proliferation test of the splenocytes was performed by a [3H]-thymidine incorporation assay[6] with a few modifications. Briefly, splenocyte suspension of the mice from three groups was prepared. 1 × 106 cells were plated into each well of 96-well plate. Into each well, 1 × 105 mytocin C-pretreated autologous antigen-presenting cells (APC) were added. The cells were incubated with 50 μg/mL synthetic MG7-Ag mimotope peptide for 4 h. The cells were washed and cultured for 3 d. Then cells were incubated for 6 h with 74 MBq/L of [3H]thymidine. The plates were harvested, and the proliferative response of the splenocytes was examined by measuring the β counts of the cells. To further investigate the efficacy of the oral DNA vaccine, the tumor challenge assay was performed. Ehrlich ascites carcinoma cells (EAC) were immunostained with MG7 antibody to verify the presence of the MG7-Ag. Then 1 × 107 EAC cells were injected into the abdominal cavity of the mice in each group. The number of tumor-bearing mice and the survival rate of each group were observed.
RESULTS
Construction of the oral DNA vaccine
By PCR, MG7-Ag mimotope and PADRE were fused together and incorporated into a fragment of the pcDNA3.1 (+) (Figure 1). The proper coding of the epitopes was confirmed by sequencing (Figure 2). The PCR product was subcloned into pcDNA3.1 (+). And by Hind III digestion, the fragment of the pcDNA3.1 in the PCR product was removed (Figure 3).
Figure 1.
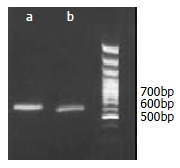
Incorporation of the epitope gene into the pcDNA3.1 fragment by PCR: A product of 620bp was amplified by the first PCR (a), and a fragment of 660bp was amplified by the second PCR (b).
Figure 2.

Hind III digestion of the recombinant plasmid after subcloning the PCR product into pcDNA3.1 (+) from the pUCm-T vector: A fragment of 660bp was released (c), which corresponded to the size of the carrier fragment.
Figure 3.

Sequencing of PCR product (partial sequence): By PCR, the two epitopes were fused together and incorporated into a pcDNA3.1 fragment. The amino acid sequence of KPHVHTKGGGS correspondeds to the sequence of MG7-Ag mimotope. AKFVAAWTLKAAZ corresponds to the sequence of universal Th epitope PADRE.
The pcDNA3.1 (+)-MG7/PADRE was transduced into the attenuated Salmonella typhimurium SL3261 by eletroporation. Plasmid from the transfectant was extracted and used as template. By PCR, it was verified that the plasmid harbored by the SL3261 transfectant was pcDNA3.1 (+)-MG7/PADRE (Figure 4).
Figure 4.

PCR identification of the pcDNA3.1 (+)-MG7/PADRE plasmid harbored by the Salmonella typhimurium SL3261: By PCR, a fragment of 800bp was amplified (d), suggesting the existence of epitope genes and removal of carrier fragment.
In vitro assay using Salmonella typhimurium as oral DNA vaccine vector
Forty-eight hours after infection with Salmonella typhimurium, fluorescence intensity of macrophages infected by the Salmonella harbouring the pcDNA3.1 (+)-EGFP was 0.927 and the percentage of fluorescent cells was 40.6%, which was significantly higher than that of than the control (0.345 P < 0.01 and 3.8% P < 0.01 respectively). Our result showed that pcDNA3.1 (+)-EGFP plasmid was transferred into the macrophages from the bacteria and was expressed by the macrophages, suggesting that the SL3261 strain could be used as the oral DNA vaccine vector.
Immune response induced by the oral DNA vaccine
No diarrhea was seen in the mice given the Salmonella typhimurium harbouring either the vaccine DNA or the empty pcDNA3.1 (+) vector. And no Salmonella infection-associated death occurred in the end of the experiments. Six weeks after the first immunization, serum titre of the antibody against MG7-Ag was significantly increased in the vaccine-immunized mice, while no significant titre of MG7 antibody was detected in the control groups. There was a significant difference between the vaccine-immunized group and the control groups (P < 0.05), while no difference was detected between the two control groups (P < 0.05, Table 1). In vitro unprimed proliferation assay of the splenocytes showed no statistical difference between those three groups (Table 2), suggesting no significant cellular immunity was elicited.
Table 1.
Cellular ELISA detection of antibody in the immune serum (OD450, ¯x± s)
| Group | OD450 |
| PBS control | 0.298 ± 0.017 |
| Empty vector control | 0.347 ± 0.062 |
| Vaccination group | 0.841 ± 0.136 |
Paired Student t-test was used to investigate the difference between two groups.
Table 2.
In vitro unprimed assay of spleenocytes (Radiation count/min cpm ¯x± s)
| Group | Radiation count (cpm) |
| PBS control | 2981 ± 389.8 |
| Empty vector control | 3158 ± 416.7 |
| Vaccination group | 2896 ± 335.4 |
Paired student t-test was used to investigate the difference between two groups.
By immunohistochemical staining, it was found that MG7-Ag was expressed in the EAC cells, both on the membrane and in the cytoplasm (Figure 5), indicating that the EAC cells can be used to challenge the mice to investigate the protective ability of the oral DNA vaccine. Two weeks after the challenge, all the mice in the control groups developed neoplastic ascites, which was confirmed by microscopic observation of the cells in ascites. However, 2 in the 7 vaccinated mice were tumor free. All the mice in the control group died 4 wk after the primary challenge, while the tumor-free mice in the immunization group survived 8 wk after the challenge with no signs of tumor formation. Our results suggested that the oral DNA vaccine was partially protective.
Figure 5.
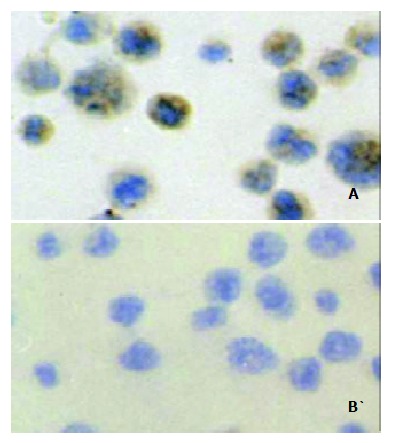
Immunohistochemical staining of the Ehrlich ascites carcinoma cells (EAC): Positive signal was seen in the cytoplasm and membrane of the EAC cells (A). When stained with a negative control monoclonal antibody (anti-E-tag antibody), the EAC cells showed no positive staining (B).
DISCUSSION
Attenuated strains of Salmonella typhimurium have been widely used as vehicles for delivery and expression of vaccine antigens. Attenuated Salmonella typhimurium strains expressing antigens from bacteria, viruses and parasites have been proved efficient as well as safe in combating respective pathogens[7]. Due to mutations in their genome, attenuated Salmonella typhimurium lost their pathogenicity but remained to be invasive. When used as the oral vehicle, they can invade the M cells and the intestinal epithelial cells and penetrate the mucosal barrier of the intestine. Subsequently, they can be uptaken by the macrophages and dendritic cells (DC) in the lamina propria and the Peyer's patch. The intracellular bacteria would not undergo lysis in the lysosomes immediately but survive for a period of time due to unknown reasons. The intracellular bacteria could provide a reservoir of antigen, so these features of the Salmonella typhimurium strains make them an excellent vehicle of oral vaccines. The use of attenuated Salmonella typhimurium as oral DNA vaccine carrier was first reported by Darji and his colleagues[8]. They found that when used as the carrier of DNA vaccines, the Salmonella could deliver the eukaryotic vector to the host cells through the unknown mechanism and the eukaryotic vector could be expressed by the host cells. Further study by Paglia et al[9] suggested that attenuated Salmonella typhimurium could deliver the eukaryotic vector to the dendritic cells in the spleen. Other studies suggested the expression of eukaryotic vectors in dendritic cells in the mesenteric nodes and Peyer’s patch was detected early after inoculation[10-12]. Oral DNA vaccines developed by using Salmonella typhimurium as carrier can provide a context similar to intracellular bacteria infection and render the body danger signals. Besides, the DC cells are excellent antigen presenting cells and have high expression of costimulatory molecules. So it is not surprising that the oral DNA vaccines can evoke significant immune response, especially CTL response[5,11,13,14]. In this study, we used an attenuated Salmonella typhimurium strain SL3261 (S. typhimurium WARY hisG46 aroA del407 Fusaricres etc, R+M+), which genotype is much the same as SL7207. We found that it could also act as the carrier of DNA vaccine, which was safe to mice.
CD4+ T cells (T helper cells, Th) play important roles in modulating immune response: They can facilitate the activation of CTL cells by cross-priming, produce various cytokines for the activation of T and B cells, upregulate the costimulatory molecules on APC cells and enhance their ability of antigen processing and presenting. In order to enhance the efficacy of the DNA vaccine, we fused the MG7-Ag mimotope with a universal T helper cell epitope PADRE, which was developed by Alexander et al[15]. They introduced the anchoring motif of MHC molecules into the polyalanine backbone and found that the PADRE could bind to most of the human HLA-DR alleles and certain mouse class II alleles and elicit strong CD4+ T cell response; the epitopes were approximately 1000 times more powerful than natural T cell epitopes. PADRE has been used as the adjuvant for various epitopes including B cell epitope, CTL epitope and carbohydrate epitope and was proved to be efficient in enhancing the immunogenicity of these epitopes. Ishioka et al[16] developed a minigene vaccine by including PADRE in tandem with multiple CTL epitopes with no spacer between them, and found that though the affinity of the CTL epitopes varied much, PADRE could still provide adjuvant effects on the induction of strong CTL response. Their study suggested that spacers were not necessarily needed in designing the multi-epitope minigene DNA vaccines. Therefore we fused the PADRE directly with the mimotope gene. To guarantee the efficient expression of the minigene, we included a Kozak sequence to right upstream the ATG initiation codon of the fusion gene. Kozak sequence is a specific sequence for the recognition and initiation of the transcription for the eukaryotic ribosome. The study suggested that Kozak sequence was necessary for correct and efficient expression of minigene vaccine[17].
MG7-Ag of gastric cancer was discovered by our institute, and we found that the immonogenicity of the MG7-Ag was located in the carbohydrate chain of the glycoprotein. Due to its unknown identity, MG7-Ag is hard to be isolated from tumor tissue. Our institute has successfully identified the anti-MG-Ag antibodies using phage display method[18,19]. Thus we identified the mimic peptides of MG7-Ag by screening the phage display peptide library with the MG7 antibody and then used it as the target for vaccine development. DNA vaccine of mimotope was first reported by Kieber-Emmons et al[20], and the feasibility to develop DNA vaccine of mimotopes was further confirmed by Lesinski et al[21]. In both studies, the DNA vaccine of mimotope induced strong humoral immune response. And in the former study, no significant CTL response was elicited. As shown by Monzavi-Karbassi et al[22], the mimic peptides could induce carbohydrate antigen reactive T cell response. Therefore, absence of significant specific T cell response in Kiever-Emmons's study and our study might be due to the immunogen type of the primary antigen. However, the oral DNA vaccine developed by us was shown to be partially protective as shown by the tumor challenge assay though no significant difference was seen in the 3H-Tdr incorporation assay. The inconsistency between the results of tumor challenge assay and the 3H-Tdr incorporation assay might be due to the following reasons: (1) The inaccuracy of the 3H-Tdr incorporation assay in determining the T cell response. More accurate methods include 51Cr-releasing assay and ELISPOT. Moreover in our study, we did not purify CD8+ T cells for 3H-Tdr incorporation assay. The existence of other types of cells might affect the results. (2) The sample included in the tumor challenge assay was too small. Much larger samples would be needed to further confirm the protective effect of the oral DNA vaccine.
Due to its various mechanisms of multi-drug resistance, gastric cancer often responds poorly to chemotherapy[23,24]. Cancer vaccines have been proved a powerful adjuvant intervention in gastric cancer management. Study showed that gastric cancer antigens could induce specific immune response[25]. We reported here the first attempt to develop an oral DNA vaccine against gastric cancer antigen mimotope. The oral DNA vaccine against MG7-Ag mimotope is immunogenic. It can induce significant immune response against gastric cancer and may be partially protective in mice. Our results verified the feasibility to develop oral DNA vaccine of mimotopes and efficacy as well as safety of Salmonella typhimurium as oral DNA vaccine carriers.
Footnotes
Supported by the National Natural Science Foundation of China, No. 39870742
Edited by Zhang JZ
References
- 1.Zhang XY. Some recent works on diagnosis and treatment of gastric cancer. World J Gastroenterol. 1999;5:1–3. doi: 10.3748/wjg.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Hu JL, Zhang XY, Qiao TD, Chen XT, Wu KC, Ding J, Fan DM. The value of MG7 antigen in predicting cancerous change in dysplastic gastric mucosa. Int J Clin Pract. 2002;56:169–172. [PubMed] [Google Scholar]
- 3.Xu L, Qiao T, Chen B. [Mimic epitope recognized by monoclonal antibody MG7 against gastric cancer through screening phage displayed random peptide library] Zhonghua Yixue Zazhi. 2000;80:304–307. [PubMed] [Google Scholar]
- 4.Xu L, Xu H, Ma F. [Immunogenicity of phage-displayed tumor antigen-mimic peptide] Zhonghua Zhongliu Zazhi. 2001;23:187–189. [PubMed] [Google Scholar]
- 5.Xiang R, Lode HN, Chao TH, Ruehlmann JM, Dolman CS, Rodriguez F, Whitton JL, Overwijk WW, Restifo NP, Reisfeld RA. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci U S A. 2000;97:5492–5497. doi: 10.1073/pnas.090097697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinilla-Ibarz J, Cathcart K, Korontsvit T, Soignet S, Bocchia M, Caggiano J, Lai L, Jimenez J, Kolitz J, Scheinberg DA. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood. 2000;95:1781–1787. [PubMed] [Google Scholar]
- 7.Sirard JC, Niedergang F, Kraehenbuhl JP. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol Rev. 1999;171:5–26. doi: 10.1111/j.1600-065x.1999.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 8.Darji A, Guzmán CA, Gerstel B, Wachholz P, Timmis KN, Wehland J, Chakraborty T, Weiss S. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91:765–775. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 9.Paglia P, Medina E, Arioli I, Guzman CA, Colombo MP. Gene transfer in dendritic cells, induced by oral DNA vaccination with Salmonella typhimurium, results in protective immunity against a murine fibrosarcoma. Blood. 1998;92:3172–3176. [PubMed] [Google Scholar]
- 10.Darji A, zur Lage S, Garbe AI, Chakraborty T, Weiss S. Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS Immunol Med Microbiol. 2000;27:341–349. doi: 10.1111/j.1574-695X.2000.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 11.Cochlovius B, Stassar MJ, Schreurs MW, Benner A, Adema GJ. Oral DNA vaccination: antigen uptake and presentation by dendritic cells elicits protective immunity. Immunol Lett. 2002;80:89–96. doi: 10.1016/s0165-2478(01)00313-3. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins SA, Niedergang F, Corthesy-Theulaz IE, Kraehenbuhl JP. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell Microbiol. 2000;2:59–68. doi: 10.1046/j.1462-5822.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 13.Niethammer AG, Primus FJ, Xiang R, Dolman CS, Ruehlmann JM, Ba Y, Gillies SD, Reisfeld RA. An oral DNA vaccine against human carcinoembryonic antigen (CEA) prevents growth and dissemination of Lewis lung carcinoma in CEA transgenic mice. Vaccine. 2001;20:421–429. doi: 10.1016/s0264-410x(01)00362-0. [DOI] [PubMed] [Google Scholar]
- 14.Zheng B, Woo PC, Ng M, Tsoi H, Wong L, Yuen K. A crucial role of macrophages in the immune responses to oral DNA vaccination against hepatitis B virus in a murine model. Vaccine. 2001;20:140–147. doi: 10.1016/s0264-410x(01)00272-9. [DOI] [PubMed] [Google Scholar]
- 15.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra HM, Kubo RT, Sette A. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 16.Ishioka GY, Fikes J, Hermanson G, Livingston B, Crimi C, Qin M, del Guercio MF, Oseroff C, Dahlberg C, Alexander J, et al. Utilization of MHC class I transgenic mice for development of minigene DNA vaccines encoding multiple HLA-restricted CTL epitopes. J Immunol. 1999;162:3915–3925. [PubMed] [Google Scholar]
- 17.An LL, Rodriguez F, Harkins S, Zhang J, Whitton JL. Quantitative and qualitative analyses of the immune responses induced by a multivalent minigene DNA vaccine. Vaccine. 2000;18:2132–2141. doi: 10.1016/s0264-410x(99)00546-0. [DOI] [PubMed] [Google Scholar]
- 18.Yu ZC, Ding J, Nie YZ, Fan DM, Zhang XY. Preparation of single chain variable fragment of MG(7) mAb by phage display technology. World J Gastroenterol. 2001;7:510–514. doi: 10.3748/wjg.v7.i4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie YZ, He FT, Li ZK, Wu KC, Cao YX, Chen BJ, Fan DM. Identification of tumor associated single-chain Fv by panning and screening antibody phage library using tumor cells. World J Gastroenterol. 2002;8:619–623. doi: 10.3748/wjg.v8.i4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieber-Emmons T, Monzavi-Karbassi B, Wang B, Luo P, Weiner DB. Cutting edge: DNA immunization with minigenes of carbohydrate mimotopes induce functional anti-carbohydrate antibody response. J Immunol. 2000;165:623–627. doi: 10.4049/jimmunol.165.2.623. [DOI] [PubMed] [Google Scholar]
- 21.Lesinski GB, Smithson SL, Srivastava N, Chen D, Widera G, Westerink MA. A DNA vaccine encoding a peptide mimic of Streptococcus pneumoniae serotype 4 capsular polysaccharide induces specific anti-carbohydrate antibodies in Balb/c mice. Vaccine. 2001;19:1717–1726. doi: 10.1016/s0264-410x(00)00397-2. [DOI] [PubMed] [Google Scholar]
- 22.Monzavi-Karbassi B, Cunto-Amesty G, Luo P, Lees A, Kieber-Emmons T. Immunological characterization of peptide mimetics of carbohydrate antigens in vaccine design strategies. Biologicals. 2001;29:249–257. doi: 10.1006/biol.2001.0307. [DOI] [PubMed] [Google Scholar]
- 23.Han Y, Han ZY, Zhou XM, Shi R, Zheng Y, Shi YQ, Miao JY, Pan BR, Fan DM. Expression and function of classical protein kinase C isoenzymes in gastric cancer cell line and its drug-resistant sublines. World J Gastroenterol. 2002;8:441–445. doi: 10.3748/wjg.v8.i3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR, et al. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54–59. doi: 10.3748/wjg.v8.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Ye YB, Chen Z. Activation of killer cells with soluble gastric cancer antigen combined with anti-CD(3) McAb. World J Gastroenterol. 1999;5:179–180. doi: 10.3748/wjg.v5.i2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]


