Abstract
AIM: To clarify the association of vascular endothelial growth factor (VEGF) and microvascular density (MVD) expression with the angiogenesis and prognosis of colorectal cancer.
METHODS: A total of 97 cases of colorectal carcinomas were examined by immunohistochemical staining (SP method), using anti-VEGF and anti-factor CD34+ monoclonal antibodies.
RESULTS: VEGF positive staining was obtained in 68 out of 97 cases (70.1%), and observed mainly in the cytoplasm of tumor cells, and also frequently in stromal cells. VEGF expression was more intense in poorly differentiated adenocarcinoma in comparison with others, but there was no significant correlation between VEGF expression and age, sex and stage. A significant correlation was found between the MVD and grades, and there was no significant relationship between the MVD and age, sex, and stage. The MVD in the VEGF positive group (68 cases) was higher than that in the negative group. Upon multivariate analysis, the significant variables were stage, tumor grade and MVD; VEGF expression was not an independent prognostic factor.
CONCLUSION: The expression of VEGF has a significant correlation with MVD; MVD expression has prognostic value but VEGF has not in colon cancer.
INTRODUCTION
Angiogenesis is an essential process required for the growth and metastatic ability of solid tumors[1]. Some studies demonstrated that an increase in microvascular density (MVD) was found to be closely associated with the expression of vascular endothelial growth factor (VEGF), and that MVD and VEGF expression had a prognostic value in predicting metastasis of various malignant solid tumors[2,3]. Several studies have noted that the level of VEGF expression, a strong angiogenic factor, correlates with neovascularity and tumor progression in human breast and brain cancers and experimental tumor models[4,5]. In this study, we investigated the correlation of the VEGF and MVD in the tumor tissue of patients with colon cancer.
MATERIALS AND METHODS
Patients and tumor specimens
Tumor specimens from 97 patients resected for colorectal cancers, from the Second Affiliated Hospital of Zhejiang University (Hangzhou, China) from March 1993 to September 1995 were assessed. The age of the patients ranged from 36 to 74 years; 58 were male and 39 were female; average age, male 57.5 years old, female 61.5 years old. The patients were staged according to operation and pathological findings with UICC TNM classification: 9 (9.3%) in stage I, 38 (39.2%) in stage II, 32 (32.9%) in stage III, and 18 (18.6) in stage IV.
Immunohistochemistry
Specimens were fixed in a 10% formaldehyde solution and embedded in paraffin. Sections, 5 μm thick, were cut and mounted on glass slides. Immunohistochemical staining was performed using the avidin-biotin method. Staining for VEGF was performed using an anti-VEGF monoclonal antibody (MAb) (Calbiochem, Cambridge, UK). Staining for vascular endothelial cells was performed using an anti-CD34 MAb (DAKO, Copenhagen, Denmark). Briefly, formalin-fixed, paraffin-embedded 5 μm tissue sections were deparaffinised with xylene, dehydrated in ethanol and incubated with 3% hydrogen peroxidase for 5 min. After washed with phosphate-buffered saline (PBS), tissue sections were incubated in 10% normal bovine serum for 20 min, followed by an overnight incubation with anti-VEGF (1:50) antibody or anti-CD34 antibody (1:50). Biotinylated goat antimouse and antirabbit immunoglobulins were used as secondary antibodies. Peroxidase-conjugated avidin was used as a dilution of 1:500. Finally, 0.02% diaminobenzidine and 1% hydrogen peroxide in PBS were used as the substrate. Normal mouse IgG diluted to an equivalent protein concentration was used as a control in place of the primary antibody. Counterstaining was performed with haematoxylin.
Any single brown-stained cell that indicates an endothelial cell stained with CD34 was counted as a single vessel. Branching structures were counted as a single vessel, unless there was a break in the continuity of the structure. The stained sections were screened at 5 times magnification, to identify the areas of highest vascular density. After the area of highest neovascularization was identified, individual vessel counts were performed at × 200 magnification.
Evaluation of VEGF expression
For the evaluation of VEGF expression, immunostaining was classified in two groups, corresponding to the percentage of immunoreactive cells; the cut-off point to distinguish low from high VEGF expression was 25% of positive carcinoma cells.
Statistical analysis
Statistical comparisons for significance were made with the Student's t test and χ2 test. Multivariate analysis was performed using the Cox's regression multiple hazard model. P < 0.05 was considered statistically significant.
RESULTS
VEGF expression
Positive staining was obtained in 68 out of 97 cases (70.1%) and a typical immunohistochemical staining is shown in Figure 1. VEGF immunoreactivity was observed mainly in the cytoplasm of tumor cells, and also frequently in stromal cells. The distribution of VEGF-staining was not continuous in the whole slide of the specimen. VEGF expression was more intense in poorly differentiated adenocarcinoma in comparison with other tumors (P = 0.014), but there was no significant correlation between VEGF expression and age, sex and stage (Table 1).
Figure 1.
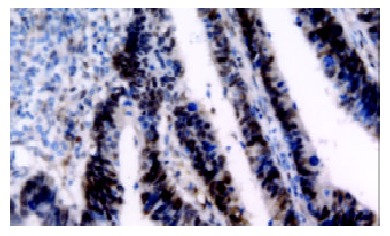
VEGF expression in colorectal cancer specimen. VEGF immunoreactivity was observed mainly in the cytoplasm of tumor cells, and also frequently in stromal cells.
Table 1.
Relationship between clinicopathologic factors and VEGF expression (n = 97)
| Variables | n (%) |
VEGF expression |
||
| + n(%) | - n(%) | P value | ||
| Age | ||||
| < 45 years | 36 | 26(72.2) | 10(27.8) | |
| > 45 years | 61 | 42(68.8) | 19(31.2) | |
| Sex | ||||
| Male | 58 | 41(74.1) | 17(25.9) | |
| Female | 39 | 27(68.9) | 12(31.1) | |
| Different differentiation | ||||
| Well | 28 | 15(51.1) | 13(48.9) | |
| Moderate | 36 | 26(72.2) | 10(27.8) | |
| Poor | 33 | 27(81.8) | 6(18.2) | 0.014 |
| Stage | ||||
| I | 9 | 7(77.8) | 2(22.2) | |
| II | 38 | 27(71.1) | 11(28.9) | |
| III | 32 | 23(72.2) | 9(27.8) | |
| IV | 18 | 11(61.5) | 7(38.5) | |
P < 0.05 vs Well differentiated group.
Microvascular density (MVD)
Any single brown-stained cell that indicates an endothelial cell stained with CD34 was counted as a single vessel (Figure 2). The median MVD was 187.6 ± 17.3. A significant correlation was found between the MVD and different grade (0.028), and there was no significant relationship between the MVD and age, sex, and stage (Table 2).
Figure 2.
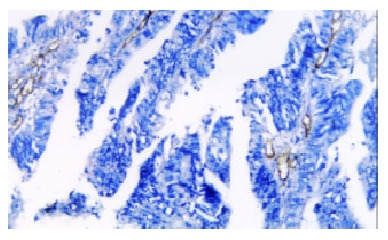
Microvascular density in colorectal cancer specimen. The single brown-stained cell indicates an endothelial cell that was stained for the presence of CD34.
Table 2.
Relationship between clinicopathologic factors and MVD (n = 97)
| Variables | n |
MVD |
||
| high MVD | low MVD | P value | ||
| Age | ||||
| < 45 years | 36 | 20(55.6) | 16(44.4) | |
| > 45 years | 61 | 33(54.3) | 28(45.7) | |
| Sex | ||||
| Male | 58 | 32(55.2) | 26(44.8) | |
| Female | 39 | 21(53.8) | 18(46.2) | |
| Different differentiation | ||||
| Well | 28 | 12(42.9) | 16(57.1) | |
| Moderate | 36 | 20(55.6) | 16(44.4) | |
| Poor | 33 | 21(63.6) | 12(36.4) | 0.028 |
| Stage | ||||
| I | 9 | 5(55.6) | 4(44.4) | |
| II | 38 | 20(52.6) | 18(47.4) | |
| III | 32 | 18(56.3) | 14(43.7) | |
| IV | 18 | 10(55.6) | 8(44.4) | |
P < 0.05 vs Well differentiated group.
Association between VEGF expression and MVD
The MVD in the VEGF positive group was 213.4 ± 12.8, and that in the negative group was 138.7 ± 19.4. MVD in the VEGF positive group was higher than that in the negative group (P = 0.027) (Table 3).
Table 3.
Relationship between MVD and VEGF expression
| Variable | MVD | P value |
| VEGF (+) | 213.4 ± 12.8 | |
| Expression (-) | 138.7 ± 19.4 | 0.027 |
P < 0.05 vs VEGF (-) group.
Multivariate analysis
Upon multivariate analysis of all patients, the significant variables were stage, tumor grade and MVD. Age, sex, VEGF expression were not independent prognostic factors (Table 4).
Table 4.
Multivariate analysis of overall survival by Cox proportional hazards model
| Variable | Categories | Hazard ratio | SEM | P value |
| Age | > 45 years versus < 45 years | 1.877 | 0.3796 | |
| Sex | Male versus female | 1.216 | 0.4071 | |
| Differentiation | Poor versus others | 2.361 | 0.2438 | 0.0217 |
| Stage | I, II versus III, IV | 2.973 | 0.1976 | 0.0012 |
| VEGF | (+) versus (-) | 1.164 | 0.3874 | |
| MVD | > 187.6 versus < 187.6 | 2.526 | 0.2126 | 0.0314 |
P < 0.05 vs Stage I, II group.
DISCUSSION
Microvascularity is important in cancer growth and metastasis because it is involved in the transport of various nutrients to the tumor cells[1,2]. In this process of tumor growth and angiogenesis numerous angiogenic factors are involved. In recent years several of these factors have been identified[3-6]. One of the most important regulators of angiogenesis is VEGF. It induces the vascular stroma not only as a direct endothelial cell mitogen, but also as a potent mediator of microvessel permeability. This ability of VEGF to induce fenestrations on microvessels has been demonstrated in experimental tumors[7,8].
The VEGF is overexpressed in a variety of benign tissues and malignant human tumors. The expression of VEGF suggests that it plays a role in luminal secretion by increasing local vascular permeability[9]. In neuroendocrine tumors the high levels of VEGF expression could indicate a role for VEGF in the release of gastrointestinal hormones through the regulation of baseline permeability of the normal microcirculation[10]. The high level of VEGF expression in some malignant tumors, such as breast cancer, non-small cell lung cancer, bladder cancer and gastric cancer, is a characteristic feature of these tumors. Several studies have demonstrated that high microvessel density is a useful indicator for poor prognosis in these cancers[11-15].
CD34 antigen is expressed on immature human haematopoietic precursor cells and is progressively lost during maturation[16,17]. In normal resting tissues, anti-CD34 antibodies are predominantly neated with the luminal endothelial membrane, whereas the abluminal membrane is negative or only weakly positive. In contrast, significant staining of the endothelial abluminal microprocesses (EAM) has been found in tumor stroma[18]. It has been shown that CD34 is a marker for EAM during angiogenesis and the antigenicity of CD34 is preserved by freezing, or ethanol, and formalin fixation[19].
Microvessel density (MVD) and expression of VEGF act as a highly specific inducer of angiogenesis. Strong VEGF expression and high MVD are considered important parameters of tumor angiogenesis and related to poor survival probability in vulvar cancer patients[20,21]. In primary breast cancer, MVD and VEGF serve as a parameter for determining tumor biological, metastatic potential and prognosis[22]. VEGF is highly related to angiogenesis of gastric carcinoma and promotes growth, invasion and metastasis of gastric carcinoma, VEGF expression and MVD are predictors for the biological behavior of gastric carcinoma[21,23]. In primary liver cancer, besides tumor stage, satellite nodules and portal vein embolus, the MVD and VEGF expressions are also of prognostic significance[24]. Intense VEGF staining was found in the majority of advanced primary SCCs, lymph node metastases, and human SCCs in severe combined immunodeficent mice, where no dysplasia, CIS, or early SCCs showed intense immunostaining. Suggesting a role for VEGF in both clinical and experimental HNSCC[25,26]. By univariate analysis, VEGF expression and MVD in the biopsy specimens were significant predictors of bladder cancer recurrence. By multivariate analysis, only VEGF expression was an independent prognostic factor[27]. The metastatic potency of NPC tissue and the prognosis of the patients with NPC could be estimated by measuring MVD and the expression of VEGF in NPC tissue[28]. Previously it was demonstrated that in prostate tumors, angiogenesis measured as microvessel density (MVD) was associated with tumor stage as well as WHO grade and was an independent predictor of clinical outcome. Vascular endothelial growth factor (VEGF) is a major inducer of angiogenesis[29]. The relationship between VEGF expression and MVD in ovarian carcinoma suggests that in conjunction with the established clinicopathologic prognostic parameters of ovarian carcinoma, VEGF expression may enhance the predictability of patients at high risk for tumor progression who are potential candidates for further aggressive therapy[30]. But MVD in synovial sarcomas did not correlate with prognosis or VEGF expression, angiogensis in synovial sarcoma might be controlled by angiogenesis activators other than VEGF[31]. In patients with invasive cervical cancer VEGF expression has no prognostic value in contrast with the MVD[32].
In this study, we found positive VEGF staining was obtained in 68 out of 97 cases (70.1%), VEGF immunoactivity was observed mainly in the cytoplasm of tumor cells, and also frequently in stromal cells. VEGF expression was more intense in poorly differentiated adenocarcinoma in comparison with other tumors, but there was no significant correlation between VEGF expression and age, sex and stage. A significant correlation was found between the MVD and different grade, and there was no significant relationship between the MVD and age, sex, and stage. MVD in the VEGF positive group was higher than that in the negative group. Upon multivariate analysis, the significant variables were stage, tumor grade and MVD; VEGF expression was not an independent prognostic factor. We conclude that MVD but not VEGF expression has prognostic value in colon cancer.
Footnotes
Edited by Zhang JZ
References
- 1.Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- 2.Decaussin M, Sartelet H, Robert C, Moro D, Claraz C, Brambilla C, Brambilla E. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999;188:369–377. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 4.Burian M, Quint C, Neuchrist C. Angiogenic factors in laryngeal carcinomas: do they have prognostic relevance. Acta Otolaryngol. 1999;119:289–292. doi: 10.1080/00016489950181846. [DOI] [PubMed] [Google Scholar]
- 5.Giatromanolaki A, Koukourakis MI, Stathopoulos GP, Kapsoritakis A, Paspatis G, Kakolyris S, Sivridis E, Georgoulias V, Harris AL, Gatter KC. Angiogenic interactions of vascular endothelial growth factor, of thymidine phosphorylase, and of p53 protein expression in locally advanced gastric cancer. Oncol Res. 2000;12:33–41. doi: 10.3727/000000001108747426. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Shimada Y, Uchida S, Maeda M, Kawabe A, Mori A, Itami A, Kano M, Watanabe G, Imamura M. TGF-alpha as well as VEGF, PD-ECGF and bFGF contribute to angiogenesis of esophageal squamous cell carcinoma. Int J Oncol. 2000;17:453–460. doi: 10.3892/ijo.17.3.453. [DOI] [PubMed] [Google Scholar]
- 7.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108(Pt 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Doi R, Imamura M. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34:1439–1447. doi: 10.1016/s0959-8049(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Scott PA, Turley H, Leek R, Lewis CE, Gatter KC, Harris AL, Mackenzie IZ, Rees MC, Bicknell R. Validation of anti-vascular endothelial growth factor (anti-VEGF) antibodies for immunohistochemical localization of VEGF in tissue sections: expression of VEGF in the human endometrium. J Pathol. 1998;185:402–408. doi: 10.1002/(SICI)1096-9896(199808)185:4<402::AID-PATH112>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Terris B, Scoazec JY, Rubbia L, Bregeaud L, Pepper MS, Ruszniewski P, Belghiti J, Fléjou J, Degott C. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology. 1998;32:133–138. doi: 10.1046/j.1365-2559.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 11.Uchida S, Shimada Y, Watanabe G, Tanaka H, Shibagaki I, Miyahara T, Ishigami S, Imamura M. In oesophageal squamous cell carcinoma vascular endothelial growth factor is associated with p53 mutation, advanced stage and poor prognosis. Br J Cancer. 1998;77:1704–1709. doi: 10.1038/bjc.1998.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jong JS, van Diest PJ, van der Valk P, Baak JP. Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: Correlations with proliferation and angiogenesis. J Pathol. 1998;184:53–57. doi: 10.1002/(SICI)1096-9896(199801)184:1<53::AID-PATH6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Koomägi R, Volm M. Tissue-factor expression in human non-small-cell lung carcinoma measured by immunohistochemistry: correlation between tissue factor and angiogenesis. Int J Cancer. 1998;79:19–22. doi: 10.1002/(sici)1097-0215(19980220)79:1<19::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Aikawa H, Takahashi H, Fujimura S, Sato M, Endo C, Sakurada A, Kondo T, Tanita T, Matsumura Y, Ono S, et al. Immunohistochemical study on tumor angiogenic factors in non-small cell lung cancer. Anticancer Res. 1999;19:4305–4309. [PubMed] [Google Scholar]
- 15.Neuchrist C, Quint C, Pammer A, Burian M. Vascular endothelial growth factor (VEGF) and microvessel density in squamous cell carcinomas of the larynx: an immunohistochemical study. Acta Otolaryngol. 1999;119:732–738. doi: 10.1080/00016489950180711. [DOI] [PubMed] [Google Scholar]
- 16.Watt SM, Karhi K, Gatter K, Furley AJ, Katz FE, Healy LE, Altass LJ, Bradley NJ, Sutherland DR, Levinsky R. Distribution and epitope analysis of the cell membrane glycoprotein (HPCA-1) associated with human hemopoietic progenitor cells. Leukemia. 1987;1:417–426. [PubMed] [Google Scholar]
- 17.El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–1562. doi: 10.1002/hep.510270613. [DOI] [PubMed] [Google Scholar]
- 18.Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ, Greaves MF, Denekamp J, Ruiter DJ. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990;62:690–696. [PubMed] [Google Scholar]
- 19.Traweek ST, Kandalaft PL, Mehta P, Battifora H. The human hematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol. 1991;96:25–31. doi: 10.1093/ajcp/96.1.25. [DOI] [PubMed] [Google Scholar]
- 20.Ogura Y, Sato K, Kato T, Saito K, Enomoto K. [Immunohistochemical analysis of expression of angiogenic factors and tumor angiogenesis in superficial bladder cancer] Nihon Hinyokika Gakkai Zasshi. 1998;89:529–537. doi: 10.5980/jpnjurol1989.89.529. [DOI] [PubMed] [Google Scholar]
- 21.Obermair A, Kohlberger P, Bancher-Todesca D, Tempfer C, Sliutz G, Leodolter S, Reinthaller A, Kainz C, Breitenecker G, Gitsch G. Influence of microvessel density and vascular permeability factor/vascular endothelial growth factor expression on prognosis in vulvar cancer. Gynecol Oncol. 1996;63:204–209. doi: 10.1006/gyno.1996.0307. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, Huang X, Li J. [The correlation between tumor angiogenesis and lymph node metastasis in primary breast carcinoma] Zhonghua Waike Zazhi. 1997;35:583–585. [PubMed] [Google Scholar]
- 23.Lu M, Jiang Y, Wang R. [The relationship of vascular endothelial growth factor and angiogenesis to the progression of gastric carcinoma] Zhonghua Binglixue Zazhi. 1998;27:278–281. [PubMed] [Google Scholar]
- 24.Xia J, Yang B, Ye S. [Clinico-pathological significance of microvessel density and VEGF expression in primary liver cancer] Zhonghua Zhongliu Zazhi. 1998;20:440–442. [PubMed] [Google Scholar]
- 25.Ikeguchi M, Oka S, Saito H, Kondo A, Tsujitani S, Maeta M, Kaibara N. The expression of vascular endothelial growth factor and proliferative activity of cancer cells in gastric cancer. Langenbecks Arch Surg. 1999;384:264–270. doi: 10.1007/s004230050202. [DOI] [PubMed] [Google Scholar]
- 26.Sauter ER, Nesbit M, Watson JC, Klein-Szanto A, Litwin S, Herlyn M. Vascular endothelial growth factor is a marker of tumor invasion and metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 1999;5:775–782. [PubMed] [Google Scholar]
- 27.Inoue K, Slaton JW, Karashima T, Yoshikawa C, Shuin T, Sweeney P, Millikan R, Dinney CP. The prognostic value of angiogenesis factor expression for predicting recurrence and metastasis of bladder cancer after neoadjuvant chemotherapy and radical cystectomy. Clin Cancer Res. 2000;6:4866–4873. [PubMed] [Google Scholar]
- 28.Guang-Wu H, Sunagawa M, Jie-En L, Shimada S, Gang Z, Tokeshi Y, Kosugi T. The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope. 2000;110:2066–2069. doi: 10.1097/00005537-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Strohmeyer D, Rössing C, Bauerfeind A, Kaufmann O, Schlechte H, Bartsch G, Loening S. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000;45:216–224. doi: 10.1002/1097-0045(20001101)45:3<216::aid-pros3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Shen GH, Ghazizadeh M, Kawanami O, Shimizu H, Jin E, Araki T, Sugisaki Y. Prognostic significance of vascular endothelial growth factor expression in human ovarian carcinoma. Br J Cancer. 2000;83:196–203. doi: 10.1054/bjoc.2000.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawauchi S, Fukuda T, Tsuneyoshi M. Angiogenesis does not correlate with prognosis or expression of vascular endothelial growth factor in synovial sarcomas. Oncol Rep. 1999;6:959–964. doi: 10.3892/or.6.5.959. [DOI] [PubMed] [Google Scholar]
- 32.Tjalma W, Weyler J, Weyn B, Van Marck E, Van Daele A, Van Dam P, Goovaerts G, Buytaert P. The association between vascular endothelial growth factor, microvessel density and clinicopathological features in invasive cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2000;92:251–257. doi: 10.1016/s0301-2115(99)00295-x. [DOI] [PubMed] [Google Scholar]


