Abstract
AIM: To investigate the expression of inducible nitric oxide synthase (iNOS) in aberrant crypt foci (ACF) -adenoma-carcinoma sequence and its relation with tumor cell apoptosis, proliferation and angiogenesis.
METHODS: The expression of iNOS, proliferating cell nuclear antigen (PCNA) and microvessel density (MVD) in different stages of colorectal cancer were studied by immunohistochemical method from 30 normal tissues, 30 nonhyperplastic ACF, 30 hyperplastic ACF, 30 dysplastic ACF, 30 adenomas and 60 carcinomas. The apoptotic cells were detected by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) method using an Apop Tag in situ detection kit.
RESULTS: The immunoreactivity of iNOS significantly increased in the transition from hyperplastic ACF to dysplastic ACF. This transition was associated with a significant decrease in the apoptotic index (AI) (0.73 ± 0.37 vs 0.61 ± 0.35, P < 0.05) and significant increases in the PCNA labeling index (LI) (27.3 ± 2.80 vs 40.3 ± 3.11, P < 0.01) and microvessel density (MVD) (55 ± 11.5 vs 70 ± 13.2, P < 0.01). The expression of iNOS was in low levels and positively correlated with PCNA-LI (r = 0.812, P < 0.01) and MVD (r = 0.863, P < 0.01) during transition from normal mucosa to nonhyperplastic ACF and hyperplastic ACF. The expression of iNOS was in high levels and positively correlated with AI (r = 0.901, P < 0.01) after transition from hyperplastic ACF to dysplastic ACF, adenoma and carcinoma.
CONCLUSION: The results suggest that the transition from hyperplastic ACF to dysplastic ACF may be a crucial step in the ACF-adenoma-carcinoma sequence, in which iNOS plays an important role by regulating tumor cell apoptosis, proliferation and angiogenesis.
INTRODUCTION
Aberrant crypt foci (ACF) are recently described colorectal lesions that might be related to the earliest steps in multistage colorectal carcinogenesis[1]. Nitric oxide (NO) is an important bioactive agent and signaling molecule that mediates a variety of physical actions and may contribute to the pathogenesis of a variety of disorders including cancer[2-6]. Several studies implicate iNOS in colorectal tumorigenesis[7-9], but none has evaluated the expression of iNOS in ACF-adenoma-carcinoma sequence. In this study, TUNEL technique and immunohistochemical staining were used to detect biologic parameters of tumor and compare the state of apoptosis, proliferation, MVD and iNOS expression in ACF-adenoma-carcinoma sequence. The purpose was to find out the relationship between aberrant expression of iNOS and colorectal carcinogenesis and the possible mechanism.
MATERIALS AND METHODS
Tissue specimens
Human colorectal tissues were obtained from Zhongnan Hospital and the People's Hospital of Wuhan University and Hubei Cancer Hospital. We collected 90 ACF and their adjacent normal mucosa from 32 colorectal carcinomas (CRC) (19 males and 13 females; mean age, 54 ± 7.5 years). 30 adenomas (18 males and 12 females; mean age, 51 ± 7.3 years) and 60 carcinomas were included in this study. The cancer patients included 32 males and 28 females that had 7 Dukes stage A, 24 stage B, 12 stage C, and 17 stage D. There were 22 well-differentiated CRCs, 26 moderately differentiated and 12 poorly differentiated CRCs. All specimens were routinely fixed in 10% buffered formalin, embedded in paraffin, and cut into 5 mm sections. Each of the 5 sections was stained with hematoxylin and eosin for classification.
Sampling of ACF
Immediately after bowel resection, freshly resected colorectal segments were opened longitudinally, and the mucosa from macroscopically normal segments were dissected from the underlying layers, spread over on a piece of filter paper, and fixed in 10% buffered formalin solution.24 h later, the fixed mucosal strips were immersed in 0.2% methylene blue solution for 5 to 10 min and screened under 40 magnification for ACF. Methylene blue-stained ACF were easily distinguished from normal crypts by their deeper blue color, larger diameter, and the shape of their crypt orifices (oval, serrated, or slit-like)[10]. Randomly selected ACF were microdissected with a rim of normal surrounding mucosa, paraffin embedded, and serially cut perpendicular to the surface.
Histochemical detection of apoptosis and determination of apoptotic index
Apoptotic cell tissue sections were detected by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) method using an Apop Tag in situ detection kit according to the manufacturer's instructions. Morphologic characteristics of apoptosis were chromatin condensation, nuclear disintegration, and formation of crescent caps of condensed chromatin at the nuclear periphery. The apoptotic index (AI) was expressed as the ratio of positively stained cells to total cells evaluated for each tissue section after counting 1000 cells at 5 areas randomly selected for counting less than 400-fold magnification.
Immunohistochemical staining and evaluation of the sections
SP kit was used. The primary antibodies were iNOS polyclonal antibody (ready to use, Boster, Wuhan), PCNA monoclonal antibody (ready to use, Maxin, Fujian) and CD34 monoclonal antibody (ready to use, Maxin, Fujian), respectively. Before staining, the sections were heated in microwave heated in 0.01 mol/L citric acid solution for antigen retrieval. PBS was substituted for primary antibodies as negative control. The stained sections were reviewed and scored independently by two investigators using an Olympus microscope. The extent and intensity of immunoreactivity for iNOS of all specimens were recorded. The following scale was used to express the extent of positivity: 0, ≤ 5%; 1, > 5%-25%; 2, > 25%-50%; 3, > 50%-75%; 4, > 75%. The intensity of iNOS expression was scored as follows: 0, negative; 1, weak; 2, moderate; 3, strong. The final score, obtained by multiplying the intensity and extent of positivity scores, ranged from 0-12. Scores of 0-4 were defined as "markedly reduced" or "no expression"; Scores 5-8 were defined as "intermediate expression"; and scores of 9-12 were defined as"strong expression"[11]. The PCNA labeling index (LI) was expressed as the ratio of cells positively stained for PCNA to all epithelial cells in at least 5 areas randomly selected for counting less than 200-fold magnification. For microvessel density (MVD) determination, 5 areas were randomly selected and counted less than 200-fold magnification. The average count was recorded and expressed as the absolute number of vessels per 0.74 mm2 (per × 200 field) for each case.
Statistical analysis
T test was used for comparison of the means. The positivity of iNOS protein was analyzed by Fisher exact probability method. For the tendency of AI, LI and MVD in the ACF-adenoma-carcinoma sequence analysis of variance was performed based on the trend test. P value less than 0.05 was regarded as statistically significant.
RESULTS
Expression of iNOS in ACF-adenoma-carcinoma sequence
Although most cases of normal mucosa, nonhyperplastic ACF and hyperplastic ACF showed intermediate, weak or absent iNOS expression, the expression of iNOS increased markedly during transition from hyperplastic ACF to dysplastic ACF (P < 0.01) (Table 1, Figure 1). Greater than 70% of cases of dysplastic ACF, adenoma or carcinoma showed strong expression of iNOS. We found no relationship between expression of iNOS and the Ducks’ classification and the differentiation of cancer.
Table 1.
iNOS expression in normal mucosa-ACF-adenoma-carcinoma sequence
|
iNOS expression |
Total | |||
| Strong | Intermediate | Weak or absent | ||
| Normal mucosa | 3 | 6 | 21 | 30 |
| Nonhyperplastic ACF | 6 | 8 | 16 | 30 |
| Hyperplastic ACF | 8 | 10 | 12 | 30 |
| Dysplastic ACF | 26 | 2 | 2 | 30 |
| Adenoma | 24 | 4 | 2 | 30 |
| Carcinoma | 41 | 10 | 9 | 60 |
Figure 1.

Immunohistochemical analysis for iNOS protein in ACF. (A) weak staining was observed in the cytoplasm of nonhyperplastic ACF. (SP method, × 200). (B) weak staining was observed in the cytoplasm of hyperplastic ACF. (SP method, × 200). (C) strong staining was observed in the cytoplasm of dysplastic ACF. (SP method, × 200).
Changes in AI, PCNA-LI, and MVD in ACF-adenoma-carcinoma sequence
Apoptotic index was highest in hyperplastic ACF, whereas it significantly decreased after transition to dysplastic ACF (Figure 2), adenoma and carcinoma (P < 0.05). Conversely, the proliferative activity as determined by PCNA-LI gradually increased during ACF (Figure 3)-adenoma-carcinoma sequence (P < 0.01). Microvessel density significantly increased after the transition to dysplastic ACF (Figure 4), and further elevated in adenoma and carcinoma (P < 0.01) (Table 2).
Figure 2.
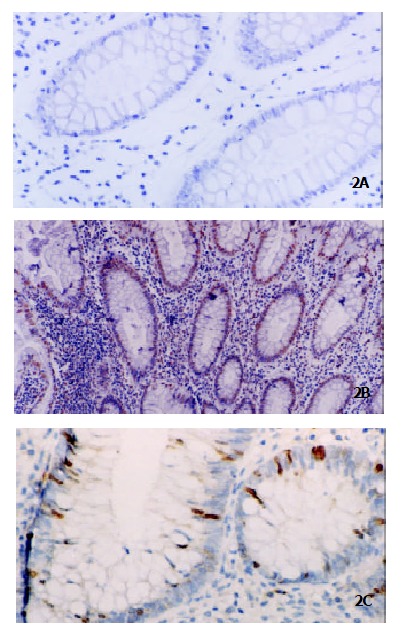
Histochemical detection of apoptosis by TUNEL in ACF. Few apoptotic cells were detected in nonhyperplastic ACF (Panel A, original magnification × 200), and more apoptotic cells were detected in hyperplastic ACF (Panel B, original magnification × 100) whereas they decreased significantly after transition to dysplastic ACF (Panel C, original magnification × 200).
Figure 3.
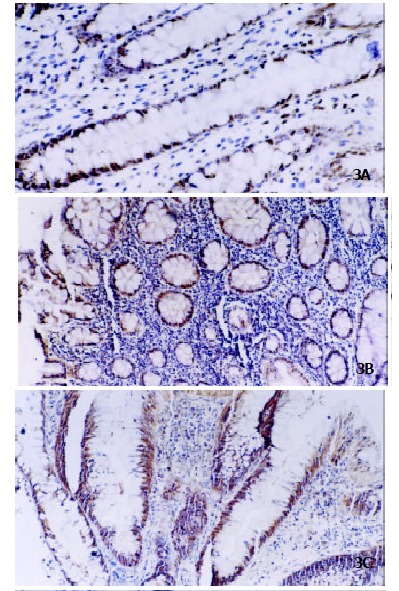
Immunohistochemistry of PCNA protein in ACF. PCNA expression was gradually increased from nonhyperplastic ACF (Panel A, original magnification × 200), hyperplastic ACF (Panel B, original magnification × 100) to dysplastic ACF (Panel C, original magnification × 100).
Figure 4.
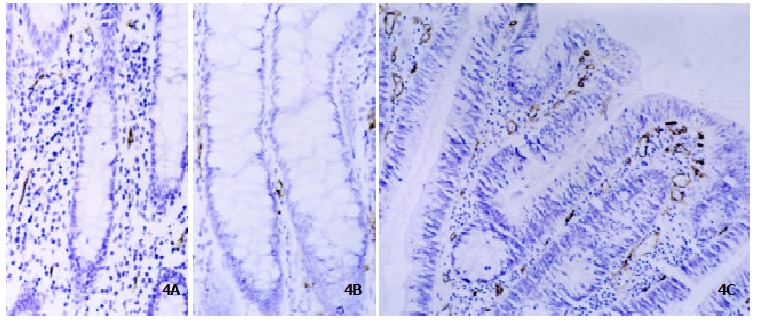
Immunohistochemistry of CD34 protein in ACF. MVD determined by anti-CD34 antibody was low in nonhyperplastic ACF (Panel A, original magnification × 200) and hyperplastic ACF (Panel B, original magnification × 200) whereas significantly increased in dysplastic ACF (Panel C, original magnification × 200).
Table 2.
Changes in AI, PCNA-LI, and MVD in ACF-adenoma-carcinoma sequence (¯x± s)
| n | AI(%) | PCNA-LI (%) | MVD (MVD/0.74mm2) | |
| Normal mucosa | 30 | 0.21 ± 0.13 | 12.1 ± 2.48 | 50 ± 10.3 |
| Nonhyperplastic ACF | 30 | 0.28 ± 0.16 | 14.7 ± 2.47 | 52 ± 10.6 |
| Hyperplastic ACF | 30 | 0.73 ± 0.37ab | 27.3 ± 2.80ab | 55 ± 11.5 |
| Dysplastic ACF | 30 | 0.61 ± 0.35abc | 40.3 ± 3.11abd | 70 ± 13.2abd |
| Adenoma | 30 | 0.58 ± 0.25abc | 45.4 ± 3.24abd | 80 ± 14.7abde |
| Carcinoma | 60 | 0.49 ± 0.43abde | 52.2 ± 3.17abdf | 95 ± 13.3abdf |
P < 0.01, vs normal mucosa;
P < 0.01, vs nonhyperplastic ACF;
P < 0.05, vs hyperplastic ACF;
P < 0.01, vs Hyperplastic ACF;eP < 0.05, vs dysplastic ACF;
P < 0.01, vs dysplastic ACF.
Correlations between iNOS expression and biologic parameters
The expression of iNOS was in low level and positively correlated with PCNA-LI (r = 0.812, P < 0.01) and MVD (r = 0.863, P < 0.01) during transition from normal mucosa to nonhyperplastic ACF and hyperplastic ACF. The expression of iNOS was in high level and positively correlated with AI (r = 0.901, P < 0.01) after transition to dysplastic ACF, adenoma and carcinoma.
DISCUSSION
The development of colorectal carcinoma usually occurs via the adenoma-carcinoma sequence[12,13]. The search for the earliest morphological precursors led to the description of ACF. Currently, there is a tendency to consider ACF as putative preneoplastic lesions that could represent one of the earliest stages of the multistep colorectal carcinogenesis[1,14]. Alteration of enzymes, specifically hexosaminidase, and carcinoembryonic antigen expression have been identified in ACF. Genetic mutations that include APC suppressor gene and k-ras gene as well as beta-catenin gene have been found in ACF[15,16]. Genomic instability, manifested as altered lengths of microsatellites and oligo A sequences similar to alterations that occur in neoplasm, has been identified in some ACF[17]. Morphological alterations from nonhyperplasia to hyperplasia to varying degrees of dysplasia have been found in ACF[18]. One study suggested that some hyperplastic ACF can develop into the adenomatous type[19]. In a carcinogen-induced colonic tumorigenesis model, these lesions may show dysplastic morphology and precede formation of adenomas and adenocarcinomas. Phenotypic and genetic abnormalities in ACF have been postulated as evidence that ACF are preneoplastic and the smallest lesions preceding adenoma and carcinoma. Recently, there was a direct evidence of the existence of an ACF-adenoma-carcinoma sequence[20].
A disturbance in the balance between cell proliferation and cell loss, or apoptosis may underlie neoplastic development[21-27]. In this study, we noticed that in normal mucosa the apoptotic cells were identified in colorectal surface epithelium and formed "an apoptotic zone". Proliferative cells were seen in the basal region of the mucosa glands and formed "a proliferating zone". However in colorectal carcinoma, apoptotic cells and proliferative cells clustered all over the tumor tissue. This phenomenon elucidated that the regulation of apoptosis and proliferation had already been beyond control. Angiogenesis also played a crucial role in tumorigenesis[28-32]. In this study, we demonstrated that MVD increased gradually in ACF-adenoma-carcinoma sequence. This change indicated that the regulation of growth of vessel was also disordered.
We investigated iNOS expression in human colorectal cancer with respect to tumor staging. Only low levels were found in the surrounding normal tissue, nonhyperplastic ACF and hyperplastic ACF. However, we found markedly increased iNOS in dysplastic ACF, adenoma and carcinoma. After transition to dysplastic ACF, adenoma and carcinoma, iNOS activity decreased with increasing staging. The markedly increased iNOS expression during hyperplastic ACF-dysplastic ACF transition suggested that the increase of iNOS expression might be an early event in the development of colorectal tumor. The different expression of iNOS in the epithelial cells in ACF-adenoma-carcinoma sequence may provide important clues that host factor regulates iNOS differently in different tumor stages.
During transition from normal mucosa to nonhyperplastic ACF and hyperplastic ACF, the expression of iNOS was at low levels. This transition was associated with a gradual increase in the AI, PCNA-LI and MVD. Several reports suggest that low concentrations of NO and iNOS induce angiogenesis and enhance the growth rate of tumor[33]. Increased proliferation might result in a state of lacking of nutrients, competition for growth factors or oxygen starvation and then in turn induce apoptosis[34]. Our results demonstrated that the initial neoplastic transformation was associated with a remarkable increase in rates of both proliferation and apoptosis, which suggested the increased instability of colorectal mucosa in this process. The immunoreactivity of iNOS significantly increased during the transition from hyperplastic ACF to dysplastic ACF. The perturbation of tissue homeostasis derived from the increased proliferation and decreased apoptosis detected at this transition. Microvessel density (MVD) was found markedly increased at this transition. These results suggested that the transition from hyperplastic ACF to dysplastic ACF might be a crucial step in the ACF-adenoma-carcinoma sequence, in which iNOS might play an important role. The expression of iNOS was in high level and associated with a significant increase in the PCNA-LI, MVD and marked decrease in the AI after transition to dysplastic ACF, adenoma and carcinoma. With the decreased expression of iNOS, AI decreased gradually and was lowest in carcinoma in this process. Several reports suggest that high concentrations of NO and iNOS are cytotoxic and can induce apoptosis[33]. Because the increased instability of colorectal mucosa in former stages led to the formation of "supper clone" and the micro-environmental changes such as expression of EGF and TGF-a in the tumor tissues[35,36], epithelial cells maintained at hyperproliferation and increased angiogenesis was present after transition to dysplastic ACF, adenoma and carcinoma. These results elucidate that iNOS might be an important factor of colorectal carcinogenesis by regulating tumor cell apoptosis, proliferation and angiogenesis. What may cause the overexpression of iNOS after transition to dysplastic ACF, adenoma and carcinoma remains unclarified and awaits further study.
Footnotes
Edited by Wu XN
References
- 1.Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995;93:55–71. doi: 10.1016/0304-3835(95)03788-X. [DOI] [PubMed] [Google Scholar]
- 2.Holian O, Wahid S, Atten MJ, Attar BM. Inhibition of gastric cancer cell proliferation by resveratrol: role of nitric oxide. Am J Physiol Gastrointest. Liver Physiol. 2002;282:G809–G816. doi: 10.1152/ajpgi.00193.2001. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K, Deng CS, Zhu YQ, Yang YP, Zhang YM. Significance of nuclear factor-κB, cyclooxgenase 2 and inducible nitric oxide synthase expression in human ulcerative colitis tissues. Shijie Huaren XiaoHua Zazhi. 2002;10:575–578. [Google Scholar]
- 4.Diao TJ, Yuan TY, Li YL. Immunologic role of nitric oxide in acute rejection of golden hamster to rat liver xenotransplantation. World J Gastroenterol. 2002;8:746–751. doi: 10.3748/wjg.v8.i4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bing RJ, Miyataka M, Rich KA, Hanson N, Wang X, Slosser HD, Shi SR. Nitric oxide, prostanoids, cyclooxygenase, and angiogenesis in colon and breast cancer. Clin Cancer Res. 2001;7:3385–3392. [PubMed] [Google Scholar]
- 6.Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS, VEGF, tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591–595. doi: 10.3748/wjg.v8.i4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- 8.Rao CV, Kawamori T, Hamid R, Reddy BS. Chemoprevention of colonic aberrant crypt foci by an inducible nitric oxide synthase-selective inhibitor. Carcinogenesis. 1999;20:641–644. doi: 10.1093/carcin/20.4.641. [DOI] [PubMed] [Google Scholar]
- 9.Nozoe T, Yasuda M, Honda M, Inutsuka S, Korenaga D. Immunohistochemical expression of cytokine induced nitric oxide synthase in colorectal carcinoma. Oncol Rep. 2002;9:521–524. [PubMed] [Google Scholar]
- 10.Nucci MR, Robinson CR, Longo P, Campbell P, Hamilton SR. Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum Pathol. 1997;28:1396–1407. doi: 10.1016/s0046-8177(97)90230-6. [DOI] [PubMed] [Google Scholar]
- 11.Hao XP, Willis JE, Pretlow TG, Rao JS, MacLennan GT, Talbot IC, Pretlow TP. Loss of fragile histidine triad expression in colorectal carcinomas and premalignant lesions. Cancer Res. 2000;60:18–21. [PubMed] [Google Scholar]
- 12.Li A, Yonezawa S, Matsukita S, Hasui K, Goto M, Tanaka S, Imai K, Sato E. Comparative study for histology, proliferative activity, glycoproteins, and p53 protein between old and recent colorectal adenomas in Japan. Cancer Lett. 2001;170:45–52. doi: 10.1016/s0304-3835(01)00610-3. [DOI] [PubMed] [Google Scholar]
- 13.Luo MJ, Lai MD. Identification of differentially expressed genes in normal mucosa, adenoma and adenocarcinoma of colon by SSH. World J Gastroenterol. 2001;7:726–731. doi: 10.3748/wjg.v7.i5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird RP, Good CK. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol Lett. 2000;112-113:395–402. doi: 10.1016/s0378-4274(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 15.Yuan P, Sun MH, Zhang JS, Zhu XZ, Shi DR. APC and K-ras gene mutation in aberrant crypt foci of human colon. World J Gastroenterol. 2001;7:352–356. doi: 10.3748/wjg.v7.i3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319–1327. [PubMed] [Google Scholar]
- 17.Pedroni M, Sala E, Scarselli A, Borghi F, Menigatti M, Benatti P, Percesepe A, Rossi G, Foroni M, Losi L, et al. Microsatellite instability and mismatch-repair protein expression in hereditary and sporadic colorectal carcinogenesis. Cancer Res. 2001;61:896–899. [PubMed] [Google Scholar]
- 18.Bouzourene H, Chaubert P, Seelentag W, Bosman FT, Saraga E. Aberrant crypt foci in patients with neoplastic and nonneoplastic colonic disease. Hum Pathol. 1999;30:66–71. doi: 10.1016/s0046-8177(99)90302-7. [DOI] [PubMed] [Google Scholar]
- 19.Otori K, Sugiyama K, Hasebe T, Fukushima S, Esumi H. Emergence of adenomatous aberrant crypt foci (ACF) from hyperplastic ACF with concomitant increase in cell proliferation. Cancer Res. 1995;55:4743–4746. [PubMed] [Google Scholar]
- 20.Shpitz B, Hay K, Medline A, Bruce WR, Bull SB, Gallinger S, Stern H. Natural history of aberrant crypt foci. A surgical approach. Dis Colon Rectum. 1996;39:763–767. doi: 10.1007/BF02054441. [DOI] [PubMed] [Google Scholar]
- 21.Wang LD, Zhou Q, Wei JP, Yang WC, Zhao X, Wang LX, Zou JX, Gao SS, Li YX, Yang C. Apoptosis and its relationship with cell proliferation, p53, Waf1p21, bcl-2 and c-myc in esophageal carcinogenesis studied with a high-risk population in northern China. World J Gastroenterol. 1998;4:287–293. doi: 10.3748/wjg.v4.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15–17. doi: 10.3748/wjg.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223–227. doi: 10.3748/wjg.v6.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia XD, Han C. Chemoprevention of tea on colorectal cancer induced by dimethylhydrazine in Wistar rats. World J Gastroenterol. 2000;6:699–703. doi: 10.3748/wjg.v6.i5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779–782. doi: 10.3748/wjg.v7.i6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oda T, Takahashi A, Miyao N, Yanase M, Masumori N, Itoh N, Sato MA, Kon S, Tsukamoto T. Cell proliferation, apoptosis, angiogenesis and growth rate of incidentally found renal cell carcinoma. Int J Urol. 2003;10:13–18. doi: 10.1046/j.1442-2042.2003.00558.x. [DOI] [PubMed] [Google Scholar]
- 27.Hao X, Du M, Bishop AE, Talbot IC. Imbalance between proliferation and apoptosis in the development of colorectal carcinoma. Virchows Arch. 1998;433:523–527. doi: 10.1007/s004280050284. [DOI] [PubMed] [Google Scholar]
- 28.Tanigawa N, Amaya H, Matsumura M, Lu C, Kitaoka A, Matsuyama K, Muraoka R. Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res. 1997;57:1043–1046. [PubMed] [Google Scholar]
- 29.Xiong B, Gong LL, Zhang F, Hu MB, Yuan HY. TGF beta1 expression and angiogenesis in colorectal cancer tissue. World J Gastroenterol. 2002;8:496–498. doi: 10.3748/wjg.v8.i3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minagawa N, Nakayama Y, Hirata K, Onitsuka K, Inoue Y, Nagata N, Itoh H. Correlation of plasma level and immunohistochemical expression of vascular endothelial growth factor in patients with advanced colorectal cancer. Anticancer Res. 2002;22:2957–2963. [PubMed] [Google Scholar]
- 31.Fan YF, Huang ZH. Angiogenesis inhibitor TNP-470 suppresses growth of peritoneal disseminating foci of human colon cancer line Lovo. World J Gastroenterol. 2002;8:853–856. doi: 10.3748/wjg.v8.i5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao HQ, Lin YZ, Wang RN. Significance of vascular endothelial growth factor messenger RNA expression in gastric cancer. World J Gastroenterol. 1998;4:10–13. doi: 10.3748/wjg.v4.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomsen LL, Miles DW. Role of nitric oxide in tumour progression: lessons from human tumours. Cancer Metastasis Rev. 1998;17:107–118. doi: 10.1023/a:1005912906436. [DOI] [PubMed] [Google Scholar]
- 34.Sinicrope FA, Roddey G, McDonnell TJ, Shen Y, Cleary KR, Stephens LC. Increased apoptosis accompanies neoplastic development in the human colorectum. Clin Cancer Res. 1996;2:1999–2006. [PubMed] [Google Scholar]
- 35.Wang Q, Wu JS, Gao DM, Lai DL, Ma QJ. Significance of EGF receptor and TGF- messenger RNA expression in colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:590–592. [Google Scholar]
- 36.Xia L, Yuan YZ, Xu CD, Zhang YP, Qiao MM, Xu JX. Effects of epidermal growth factor on the growth of human gastric cancer cell and the implanted tumor of nude mice. World J Gastroenterol. 2002;8:455–458. doi: 10.3748/wjg.v8.i3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]


