Abstract
AIM: Irritable bowel syndrome (IBS) is characterized by abdominal pain and changes in stool habits. Visceral hypersensitivity is a key factor in the pathophysiology of IBS. The aim of this study was to examine the effect of rectal balloon-distention stimulus by blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI) in visceral pain center and to compare the distribution, extent, and intensity of activated areas between IBS patients and normal controls.
METHODS: Twenty-six patients with IBS and eleven normal controls were tested for rectal sensation, and the subjective pain intensity at 90 mL and 120 mL rectal balloon-distention was reported by using Visual Analogue Scale. Then, BOLD-fMRI was performed at 30 mL, 60 mL, 90 mL, and 120 mL rectal balloon-distention in all subjects.
RESULTS: Rectal distention stimulation increased the activity of anterior cingulate cortex (35/37), insular cortex (37/37), prefrontal cortex (37/37), and thalamus (35/37) in most cases. At 120 mL of rectal balloon-distention, the activation area and percentage change in MR signal intensity of the regions of interest (ROI) at IC, PFC, and THAL were significantly greater in patients with IBS than that in controls. Score of pain sensation at 90 mL and 120 mL rectal balloon-distention was significantly higher in patients with IBS than that in controls.
CONCLUSION: Using fMRI, some patients with IBS can be detected having visceral hypersensitivity in response to painful rectal balloon-distention. fMRI is an objective brain imaging technique to measure the change in regional cerebral activation more precisely. In this study, IC and PFC of the IBS patients were the major loci of the CNS processing of visceral perception.
INTRODUCTION
Irritable bowel syndrome (IBS) is the most common disorder seen in gastroenterological practice[1,2]. The disorder affects approximately 15% to 20% of the world's population and is predominately found in women[2]. It comprises a group of functional bowel disorders in which abdominal discomfort or pain is associated with defecation or a change in bowel habit, and with features of disordered defecation[3,4]. The pathophysiology of the symptom remains unclear, and visceral hypersensitivity or decreased pain thresholds to distension of the gut is considered to be a biologic marker for IBS and is present in most patients with this gastrointestinal disorder[5]. Possibly, there are dysfunctions in the processing of sensory stimuli in the "brain-gut" axis that may cause visceral hypersensitivity and secondary motility changes[6]. The central nervous system is believed to play a strong modulatory or etiological role in the pathophysiology of the disease[7].
In animals, the perception of somatovisceral pain is derived from the expression of the immediate early gene c-fos[8-11]. Numerous positron emission tomography (PET)[12-15] or functional magnetic resonance imaging (fMRI)[16-18] studies have dealt with the central processing of somatic pain in humans. In contrast, the neural networks involved in the perception of visceral pain in humans, especially rectal pain, have been the subjects of a limited number of functional brain imaging studies[19-23].
Previous studies of somatic pain using PET scanning to measure the regional cerebral blood flow have suggested that the anterior cingulate cortex (ACC), prefrontal cortex (PFC), insular cortex (IC), and thalamus (THAL) are important loci in pain perception[12,24]. Studies of visceral pain have generally suggested that these brain centers are important in sensation. fMRI is an alternative technique to measure changes in regional cerebral activity during stimulation. Using fMRI, the cerebral loci activated by rectal distention were also characterized in healthy volunteers[22].
In this study, we examined the effect of rectal balloon-distention stimulus by blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI) in the visceral pain center and to compare the distribution, extent, and intensity of activated areas between irritable bowel Syndrome (IBS) patients and normal controls.
MATERIALS AND METHODS
Subjects
Eleven normal right-handed control subjects (6 men and 5 women; age, 24-49 years; average age, 39 years) and twenty-six right-handed patients with IBS (12 men and 14 women; age, 18-61 years; average age, 47 years) participated in the study. All volunteers were free of any gastrointestinal complaint. The IBS patients were all diagnosed in Ruijin Hospital, and met the Rome II criteria for IBS[4], which include at least 12 wk, not necessarily to be consecutive in the preceding 12 mo of abdominal discomfort or pain that has two of the following three features: (1) Relieved with defecation; (2) Onset associated with a change in frequency of stool; (3) Onset associated with a change in form (appearance) of stool. Each patient underwent a basic evaluation to exclude organic disease including a history, physical examination, and colonoscopy.
Distention protocol
Subjects reclined on the magnetic resonance imaging (MRI) table with head resting on a beanbag saddle that reduced head motion. A plastic balloon (Medtronic Synectics, USA) was placed in rectum at 10-15 cm from the anal margin. First, the subjects were tested for the rectal sensation, including the thresholds for sensation of gas, defecation and pain. Second, subjects reported the subjective pain intensity at 90 mL and 120 mL rectal balloon-distention by using visual analogue scale (0 = no pain, 10 = unbearable pain). Then, fMRI scanning was begun. Subjects were instructed to expect 4 series of rectal stimuli. Each set of distention included 3 stimuli of the same volume lasting 30 s each, with a 30-second rest period in between. The balloon inflation and deflation for each stimulus required an average of 6 s. The baseline volume during the rest periods was 0 mL. The first series of stimulus volume was 30 mL, the second 60 mL, the third 90 mL, and the last 120 mL.
MRI scanning
BOLD imaging was performed on a 1.5-T GE Signa MRI system. Each scanning consisted of a T1-weighted (the parameters included TR/TE = 400 ms/14 ms, matrix = 256 × 256, NEX = 1). Next, four 10-mm-thickslices aligned at an approximately 20o angle above the anterior commissure-posterior commissure line to include the ACC, IC, PFC, and THAL. A functional scan was performed using echo planar imaging, and a matrix of 64 × 64, NEX = 1. The pulse sequence parameters included a 90o flip-angle with a TR (image repetition rate)/TE (effective echo time) of 3000 ms/60 ms. Each run consisted of 3 repetitions of 30 s of rest, followed by 30 s of stimulus. In each 30-second period, 10 parameters were collected, 60 data during each series.
Data processing and analysis
Data analysis was performed using correlation-coefficient tool in Functional software (AW3.2, SUN workstation). The confidence level was 0.05. Brain areas thought to mediate painful sensation included the ACC, the IC, the PFC, and THAL. These regions of interest (ROI) were identified and circled on the high-resolution anatomic images by a radiologist, who was blind to the identity of the patient and to the active pixels. The regional cerebral activation was evaluated by the percentage area of ROI and the percentage change in MR signal intensity of ROI. The percentage area of ROI was calculated by the formula: the percentage area of ROI = the pixels of ROI/the total pixels of selected pain center × 100%. The percentage change in MR signal intensity of ROI = (the MR signal intensity during stimulation - the Mean baseline signal)/the mean baseline signal × 100%. The average percentage change in MR signal intensity was calculated for each subject at each stimulus volume in each ROI. To exclude the influence of balloon inflation and deflation, the first and the tenth MR signal intensity of the rest and stimulus phase were eliminated.
Statistical analysis
The data were expressed as means ± SEM. For comparison of means, an unpaired Student's t test was used. The primary comparisons were the thresholds for sensation and VAS score between IBS patients and controls. Secondary analysis included the percentage of ROI and the average percentage change in MR signal intensity of ROI comparing IBS patients with controls. Statistically significant differences by 2-tailed t tests were defined by P < 0.05.
RESULTS
Rectal sensation test
In the control group, the average thresholds for sensation of gas, defecation and pain were 28 mL, 127 mL, and 208 mL. They were 24 mL, 90 mL, and 150 mL respectively in IBS group. The thresholds for sensation of defecation and pain were significantly lower in IBS group than in control group (P < 0.05, Figure 1).
Figure 1.
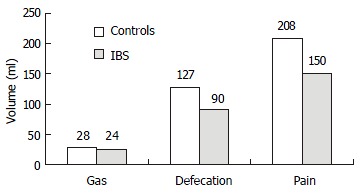
Rectal sensation test (the thresholds for sensation).
VAS score
At 30 mL rectal distention, subjects generally sensed a very-low-intensity stimulation, using the terms "gas", "mildly felt". At 60 mL rectal distention, some IBS patients expressed sensation of defecation. At 90 mL rectal distention, a large number of IBS patients and some normal controls expressed sensation of stool, associated with mild-moderate pain, and VAS score was 4.42 ± 2.00 vs 2.71 ± 1.78. At 120 mL rectal distention, most IBS patients reported moderate-severe painful sensation, and VAS score was 5.90 ± 1.84 vs 3.95 ± 2.04. In this study, three IBS patients could not tolerate 120 mL rectal distention. The VAS score of 90 mL and 120 mL rectal distention (painful rectal distention) was significantly higher in IBS patients than in control (P < 0.05, Figure 2).
Figure 2.
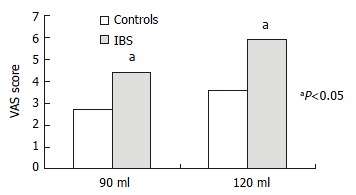
Subjective pain intensity.
Functional brain imaging
The time course of rectal stimulation sensation center response indicated immediate increase and rapid decline in BOLD signal in parallel with the mechanical distending stimulus (Figure 3). For both IBS patients and control subjects, rectal distention stimulation increased the activity of anterior cingulate cortex (35/37), insular cortex (37/37), prefrontal cortex (37/37), and thalamus (35/37) in most cases.
Figure 3.
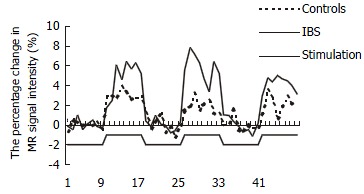
The percentage change in MR signal intensity time course of PFC in response to 3 rectal distentions at 90 mL.
In patients with IBS, the average percentage area of ROI increased in parallel with rectal distention volumes in the IC, PFC, and THAL, only that in PFC had statistical significance (P < 0.05). In controls, this increasing tendency only occurred in the ACC (Figure 4). At 120 mL rectal distention, the average percentage area of ROI and the average percentage change in MR signal intensity of ROI in the IC, PFC, and THAL were significantly greater in patients with IBS than in control subjects (P < 0.05, Figure 5 and Figure 6).
Figure 4.
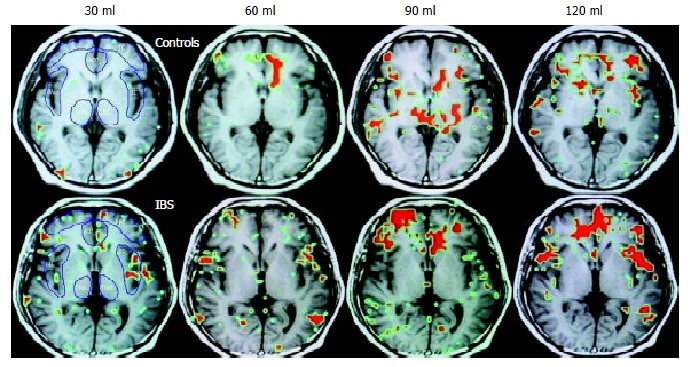
Functional brain map. The red area is the ROI.
Figure 5.
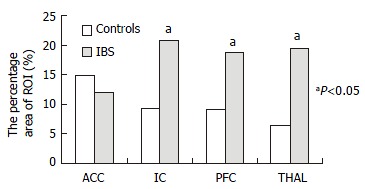
The average area of ROI at 120 mL rectal distention.
Figure 6.
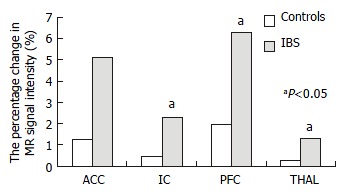
The percentage change in MR signal intensity of ROI at 120 mL rectal distention.
DISCUSSION
FMRI is a useful technology to measure changes in regional CNS blood oxygenation, which is in parallel with regional metabolic activity[25-27]. The BOLD technology detects changes in the ratio of deoxyhemoglobin to oxyhemoglobin. When brain-center neurons are metabolically active, there is an increase in local blood flow and a relative increase in the amount of oxyhemoglobin, and an increase in magnetic resonance signal[25-29]. Accordingly, the magnetic resonance signal in a given pixel will increase above baseline if the region is activated in response to stimulation. FMRI offers advantages over PET such as direct anatomic correlation, avoidance of radioisotopes, and acceptable signal-to-noise ratio that do not require large numbers of stimuli. fMRI images give similar results as PET[30,31].
It showed that fMRI has adequate sensitivity to measure regional cerebral blood flow changes in response to visceral - in this case rectal - stimulation. Activity in the 4 selected CNS pain centers, ACC, IC, PFC, and THAL, promptly increase with rectal distention stimulation. The 4 selected pain centers are components of the brain's pain-processing system[12,24,32,33]. Current studies pointed to the THAL as a relay center, connecting afferent signals from the spinothalamic tract and spinoreticular tracts to higher centers such as the cingulate, prefrontal, and insular cortices[34,35]. The IC is believed to mediate primarily visceral sensations (taste, smell, gastric, colonic, and other visceral inputs) including rectal stimuli, whereas the ACC is thought to mediate the affective or "emotional" content of sensory information. The insular cortex neurons distributed between the taste area and the visceral area receive convergent inputs from baroreceptor, chemoreceptor, gustatory and nociceptive organs and may have roles in taste aversion or in regulation of visceral responses[36]. Using positron emission tomography (PET), Craig's[37] group found contralateral activity correlated with graded cooling stimuli only in the dorsal margin of the middle/posterior insula in humans. Furthermore, Krushel[38] referred to the region of convergence in the agranular insular cortex as the visceral cortex, and suggested its involvement in the efficient integration of specific visceral sensory stimuli with correlated limbic or motivational consequences. The visceral cortex may help regulate the organism's visceral response to stress.
The PFC is thought to exert higher executive functions in pain perception[39]. There are several different functional divisions of the PFC, including the dorsolateral, ventromedial, and orbital sectors. Each of these regions plays some role in affective processing that shares the feature of representing affect in the absence of immediate rewards and punishments as well as in different aspects of emotional regulation[40].
In this study, the thresholds for sensation of defecation and pain were significantly lower in IBS group than in control group, and VAS score was significantly higher in IBS patients than in controls. The results were similar to the previous studies[41]. Normal volunteers and IBS patients had significant cerebral activation in the 4 selected brain centers (ACC, IC, PFC, and THAL) during distention stimulation at 30 mL, 60 mL, 90 mL, and 120 mL. Significant differences in cerebral activation (both the percentage area and the percentage change in MR signal intensity of ROI) were found between IBS patients and controls. In IBS patients, there was significantly greater area of ROI of the PFC with 120 mL distention than with other volume distention. Conversely, in control subjects there was no significant increase in activation of these areas with 120 mL distention compared with others. Furthermore, at 120 mL rectal distention, there was significantly greater activation of the IC, PFC, and THAL in patients with IBS than in control subjects. In summary, by a variety of measures, it is possible that IC and PFC responses to visceral pain in IBS are greater than that in controls.
There were several studies about the CNS activity in response to visceral stimulation by using PET and fMRI, but the results differed. Silverman[20] found a lack of activation within the ACC or PFC with nonpainful stimuli, and reported activation of PFC in response to rectal pain only in IBS and the ACC only in normal subjects. In contrast, more recently Mertz[23], using fMRI, observed that pain led to a greater activation of the ACC than nonpainful stimuli. In Bernstein's[42] study, they also found that normal controls and subjects with IBD and IBS shared similar loci of activations to visceral sensations of stool and pain. A significantly higher percentage of pixels activated in the anterior cingulate gyrus over both pain and stool conditions for the control group than for the IBS group and for the IBS group than for the IBD group (P < 0.035). In another study, Bonaz et al[7] revealed significant deactivations within the right insula, the right amygdala, and the right striatum. There were gender differences in cortical representation of rectal distension in healthy humans. Male subjects showed localized clusters of fMRI activity primarily in the sensory and parietooccipital regions, whereas female subjects also showed activity in the anterior cingulate and insular regions[43]. Thus, somatic and visceral sensation including pain perception can be studied noninvasively in humans with functional brain imaging techniques. Positron emission tomography and functional magnetic resonance imaging have identified a series of cerebral regions involved in the processing of somatic pain, including the anterior cingulate, insular, prefrontal, inferior parietal, primary and secondary somatosensory, and primary motor and premotor cortices, the thalamus, hypothalamus, brain stem, and cerebellum[44]. Experimental evidence supports possible specific roles for individual structures in processing the various dimensions of pain[44].
In conclusion, our data conform that fMRI is an objective brain imaging technique to measure the change in regional cerebral activation exactly. Using fMRI, some patients with IBS could be detected having visceral hypersensitivity in response to painful rectal balloon-distention. In this study, IC and PFC of IBS patients are the major loci in CNS processing of visceral perception.
Footnotes
Edited by Wu XN
References
- 1.Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology. 2002;122:1701–1714. doi: 10.1053/gast.2002.33741. [DOI] [PubMed] [Google Scholar]
- 2.Foxx-Orenstein AE, Clarida JC. Irritable bowel syndrome in women: the physician-patient relationship evolving. J Am Osteopath Assoc. 2001;101:S12–S16. [PubMed] [Google Scholar]
- 3.Camilleri M, Prather CM. The irritable bowel syndrome: mechanisms and a practical approach to management. Ann Intern Med. 1992;116:1001–1008. doi: 10.7326/0003-4819-116-12-1001. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002;4:322–328. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- 6.Blomhoff S, Diseth TH, Jacobsen MB, Vatn M. [Irritable bowel syndrome--a multifactorial disease in children and adults] Tidsskr Nor Laegeforen. 2002;122:1213–1217. [PubMed] [Google Scholar]
- 7.Bonaz B, Baciu M, Papillon E, Bost R, Gueddah N, Le Bas JF, Fournet J, Segebarth C. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol. 2002;97:654–661. doi: 10.1111/j.1572-0241.2002.05545.x. [DOI] [PubMed] [Google Scholar]
- 8.Menétrey D, Gannon A, Levine JD, Basbaum AI. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol. 1989;285:177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- 9.Bonaz B, Plourde V, Taché Y. Abdominal surgery induces Fos immunoreactivity in the rat brain. J Comp Neurol. 1994;349:212–222. doi: 10.1002/cne.903490205. [DOI] [PubMed] [Google Scholar]
- 10.Traub RJ, Silva E, Gebhart GF, Solodkin A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci Lett. 1996;215:165–168. doi: 10.1016/0304-3940(96)12978-5. [DOI] [PubMed] [Google Scholar]
- 11.Bonaz B, Rivière PJ, Sinniger V, Pascaud X, Junien JL, Fournet J, Feuerstein C. Fedotozine, a kappa-opioid agonist, prevents spinal and supra-spinal Fos expression induced by a noxious visceral stimulus in the rat. Neurogastroenterol Motil. 2000;12:135–147. doi: 10.1046/j.1365-2982.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- 12.Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 13.Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 14.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 15.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 16.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 17.Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 18.Apkarian AV, Gelnar PA, Krauss BR, Szeverenyi NM. Cortical responses to thermal pain depend on stimulus size: a functional MRI study. J Neurophysiol. 2000;83:3113–3122. doi: 10.1152/jn.2000.83.5.3113. [DOI] [PubMed] [Google Scholar]
- 19.Rothstein RD, Stecker M, Reivich M, Alavi A, Ding XS, Jaggi J, Greenberg J, Ouyang A. Use of positron emission tomography and evoked potentials in the detection of cortical afferents from the gastrointestinal tract. Am J Gastroenterol. 1996;91:2372–2376. [PubMed] [Google Scholar]
- 20.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 21.Bouras EP, O'Brien TJ, Camilleri M, O'Connor MK, Mullan BP. Cerebral topography of rectal stimulation using single photon emission computed tomography. Am J Physiol. 1999;277:G687–G694. doi: 10.1152/ajpgi.1999.277.3.G687. [DOI] [PubMed] [Google Scholar]
- 22.Baciu MV, Bonaz BL, Papillon E, Bost RA, Le Bas JF, Fournet J, Segebarth CM. Central processing of rectal pain: a functional MR imaging study. AJNR Am J Neuroradiol. 1999;20:1920–1924. [PMC free article] [PubMed] [Google Scholar]
- 23.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 24.Derbyshire SW, Jones AK, Devani P, Friston KJ, Feinmann C, Harris M, Pearce S, Watson JD, Frackowiak RS. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. J Neurol Neurosurg Psychiatry. 1994;57:1166–1172. doi: 10.1136/jnnp.57.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Bihan D, Jezzard P, Haxby J, Sadato N, Rueckert L, Mattay V. Functional magnetic resonance imaging of the brain. Ann Intern Med. 1995;122:296–303. doi: 10.7326/0003-4819-122-4-199502150-00010. [DOI] [PubMed] [Google Scholar]
- 27.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 29.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 30.Belliveau JW, Kennedy DN, McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, Vevea JM, Brady TJ, Rosen BR. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 31.Yousry TA, Schmid UD, Jassoy AG, Schmidt D, Eisner WE, Reulen HJ, Reiser MF, Lissner J. Topography of the cortical motor hand area: prospective study with functional MR imaging and direct motor mapping at surgery. Radiology. 1995;195:23–29. doi: 10.1148/radiology.195.1.7892475. [DOI] [PubMed] [Google Scholar]
- 32.Rosen SD, Paulesu E, Nihoyannopoulos P, Tousoulis D, Frackowiak RS, Frith CD, Jones T, Camici PG. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med. 1996;124:939–949. doi: 10.7326/0003-4819-124-11-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Aziz Q, Andersson JL, Valind S, Sundin A, Hamdy S, Jones AK, Foster ER, Långström B, Thompson DG. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology. 1997;113:50–59. doi: 10.1016/s0016-5085(97)70079-9. [DOI] [PubMed] [Google Scholar]
- 34.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 35.Cechetto DF, Saper CB. Role of the cerebral cortex in autonomic function. In: Loewy AD, Spyer KM, editors. Central regulation of autonomic function. New York: Oxford University; 1990. pp. 208–223. [Google Scholar]
- 36.Hanamori T, Kunitake T, Kato K, Kannan H. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol. 1998;79:2535–2545. doi: 10.1152/jn.1998.79.5.2535. [DOI] [PubMed] [Google Scholar]
- 37.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 38.Krushel LA, van der Kooy D. Visceral cortex: integration of the mucosal senses with limbic information in the rat agranular insular cortex. J Comp Neurol. 1988;270:39–54, 62-63. doi: 10.1002/cne.902700105. [DOI] [PubMed] [Google Scholar]
- 39.Kupfermann I. Localization of higher cognitive and affective functions: the association cortices. In Kandell E, Schwartz J, Jessell T, editors. Principles of neuroscience. 3rd editor. East Norwalk, CT: Appleton & Lange; 1991. pp. 823–838. [Google Scholar]
- 40.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 41.Rogers J. Testing for and the role of anal and rectal sensation. Baillieres Clin Gastroenterol. 1992;6:179–191. doi: 10.1016/0950-3528(92)90026-b. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein CN, Frankenstein UN, Rawsthorne P, Pitz M, Summers R, McIntyre MC. Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. Am J Gastroenterol. 2002;97:319–327. doi: 10.1111/j.1572-0241.2002.05464.x. [DOI] [PubMed] [Google Scholar]
- 43.Kern MK, Jaradeh S, Arndorfer RC, Jesmanowicz A, Hyde J, Shaker R. Gender differences in cortical representation of rectal distension in healthy humans. Am J Physiol Gastrointest. Liver Physiol. 2001;281:G1512–G1523. doi: 10.1152/ajpgi.2001.281.6.G1512. [DOI] [PubMed] [Google Scholar]
- 44.Ladabaum U, Minoshima S, Owyang C. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications V. Central nervous system processing of somatic and visceral sensory signals. Am J Physiol Gastrointest. Liver Physiol. 2000;279:G1–G6. doi: 10.1152/ajpgi.2000.279.1.G1. [DOI] [PubMed] [Google Scholar]


