Abstract
Dental calculus (calcified dental plaque) is a source of multiple types of data on life history. Recent research has targeted the plant microremains preserved in this mineralised deposit as a source of dietary and health information for recent and past populations. However, it is unclear to what extent we can interpret behaviour from microremains. Few studies to date have directly compared the microremain record from dental calculus to dietary records, and none with long-term observation dietary records, thus limiting how we can interpret diet, food acquisition and behaviour. Here we present a high-resolution analysis of calculus microremains from wild chimpanzees (Pan troglodytes verus) of Taï National Park, Côte d’Ivoire. We test microremain assemblages against more than two decades of field behavioural observations to establish the ability of calculus to capture the composition of diet. Our results show that some microremain classes accumulate as long-lived dietary markers. Phytolith abundance in calculus can reflect the proportions of plants in the diet, yet this pattern is not true for starches. We also report microremains can record information about other dietary behaviours, such as the age of weaning and learned food processing techniques like nut-cracking.
Understanding feeding ecology is crucial for recognising the evolutionary pressures that shaped the great apes and humans. It is long recognized that factors such as dietary specialization, tool-assisted food acquisition and the weaning age of infants are important in great apes and humans, and differ significantly among species1,2,3,4.
However, many approaches to dietary reconstruction leave unanswered specific questions on diet and related life history events, especially for fossil specimens. There is a need for new methods to reconstruct food acquisition from populations that can avoid some of the shortfalls of other techniques like direct observation, stable isotope analysis and microwear studies5,6. In some contexts direct observation is simply not possible, for example with extinct great apes and human groups. Stable isotope analysis and dental microwear studies fail to provide total dietary data, and instead only give a picture of broad dietary patterns such as consumption of particular plant categories or mechanical properties of diet7,8. Furthermore, even where direct observational data on food acquisition is available, data collection is frequently constrained because observation is only feasible over a short window of the lifetime of an individual that may live up to several decades.
Dental calculus sampled from living or dead individuals is rapidly gaining recognition as an invaluable material for the reconstruction of life history. Since Armitage9 first recognized plant remains from the teeth of ungulates, studies have reported starch grains, phytoliths, pollen grains, diatoms, mineral particles, proteins and DNA from diverse human and animal populations10,11,12,13,14,15. Using dental calculus from present day forager-horticulturalists, Leonard and colleagues16 showed for the first time that recovered microremains also occur in consumed foods, verifying the link between microremains in calculus and diet. As demand grows for dietary history data, analysis of phytoliths and starches in dental calculus is been increasingly used to reconstruct dietary ecology and ecological niches13,17,18,19,20,21,22,23.
Despite the promise of calculus dietary studies, they are hindered by the lack of research that cross-validates the dietary material recovered in calculus with the organism’s actual feeding ecology and life history. Until recently, our understanding of what the plant matter preserved in calculus precisely represents has been speculative. The initial effort to characterise the microremain record by Leonard and colleagues16 reported that calculus captured only a limited proportion of dietary breadth. In this study many vegetable foods lacked phytoliths and starches, and cooking may have significantly reduced starch abundance even if present. Dietary patterns were established through interviewing and short-term camp stays by Leonard, and though the recovered microremains corresponded to the average diet of the population, the dietary records lacked insight into the long term life history of individuals. Without dietary records that cross intra- and inter-annual cycles, our knowledge of the nature of the calculus record and its potential for archaeological studies is incomplete. Furthermore, it is unclear if the calculus dietary record has input from non-dietary sources (e.g. preparation of plant-based tools, oral hygiene and consumption of stomach contents24,25,26), with bias from diagenetic and taphonomic factors rendering it ultimately purely stochastic.
In our study, we compare the plant microremains from the calculus of the chimpanzees of the Taï Forest to 22 years of group averaged (comprising of 128 chimpanzees) dietary observation data in order to validate the calculus record and explore its potential as a source of information on life histories. For this purpose, the study of chimpanzees provides several strengths as a model. First, the chimpanzee mouth is analogous in that chimpanzees often accumulate large deposits of calculus unlike some mammals. Secondly, chimpanzees produce salivary amylase unlike some primates27, although it is less abundant than in humans28. Thirdly, Taï chimpanzees have a broad diet that includes nearly all food classes (e.g. fruits, piths, leaves, mammals, birds, invertebrates and honey) and is thus relevant to understanding hominin evolution in the African tropics and dietary ecology of hunter-gatherers living in other tropical regions.
We sampled calculus from 24 individual chimpanzees using established methods18,29, and built a random forest model in R30 to allow us to identify the microremains based on multivariate comparison to reference material31,32,33,34,35 (see detailed methods below). We predict that if microremains reflect diet, they are accumulative in calculus and should increase with age of the individual. Chimpanzee sex might also influence the abundance of microremains, since male and female chimpanzee are known to vary in their time allocation to different food resources2,36,37,38. We also anticipate that the proportions of microremains from each plant will be determined by the frequency with which that plant is consumed and how abundant the microremains are in the plant tissue. Although we knew the taxonomic identity of the reference plants at the level of species, an important amount of dietary observation data was present only at the genus level. Therefore, we performed our analyses at the genus level in order to have a higher chance of capturing long term dietary averages for the group, and refer only to genera in the text. Except where otherwise noted, our analyses were done at the group level observational data, since the records for individual chimpanzees were not complete enough to provide a detailed overview of life history. We found the phytoliths in dental calculus to be an approximate record of diet, and furthermore that microremains can reflect important behaviour like nut-cracking and episodes of Taï Chimpanzee life history such as the age of weaning. The implications of our findings from this chimpanzee group are significant not only for diet but also for studying hominin major life history events and culture.
Results
Identification of the microremain assemblages
We were able to examine 91 of the 157 genera (113 of 230 species) of plants that the 128 Taï chimpanzees consumed during the observation period. Of these plants, only a small subset produced sufficiently diagnostic microremains to use for identification (thirteen starch- and five phytolith-producing genera, Table 1; Fig. 1). For each microremain-producing plant genus, we collected data from 50 microremains, to provide a range of measurements within each genus. We collected 9 types of measurements for phytoliths and 11 for starches from 900 microremains (Supplementary Data 1 and 2). By using a subset of the reference collection to test the model, we assessed the success rate of identification of each genus with the model. Some genera were reliably identified, and others were more difficult to identify. For example, Sarcophrynium phytoliths were identified successfully 94% of the time while Panda starch was only identified 22% of the time. Generally, phytoliths were identified more reliably (Supplementary Table 1, 2). Using this random forest model we were able to proceed with identification of microremains recovered from the calculus.
Table 1. Plant genera selected from reference collection species for use in the predictive identification model, with the microremain content of the dried plant material provided as a percent of dried plant material, and the frequency of observed consumption provided as number of minutes eaten.
| Plant category (Genus) | Microremain type | Plant part | % Microremains /Dry Weight | Minutes eaten |
|---|---|---|---|---|
| Elaeis | Phytolith | Fruit and Leaf | 4.81 | 9379 |
| Eremospatha | Phytolith | Pith | 1.72 | 25,046 |
| Laccosperma | Phytolith | Pith and Seed | 2.15 | 5311 |
| Aframomum | Phytolith | Seed and Leaf | 2.13 | 1704 |
| Sarcophrynium | Phytolith | Fruit | 3.32 | 1847 |
| Cola | Starch | Seed | 40 | 35,778 |
| Aframomum | Starch | Seed | 54.58 | 1704 |
| Piper | Starch | Seed | 39.22 | 492 |
| Sacoglottis | Starch | Fruit | 2.46 | 258,225 |
| Panda | Starch | Seed | 0.85 | 17,299 |
| Coula | Starch | Seed | 31.15 | 118,095 |
| Napoleona | Starch | Seed | 20.79 | 51 |
| Gilbertiodendron | Starch | Seed | 23.87 | 11,808 |
| Eremospatha | Starch | Pith | 2.93 | 25,046 |
| Calpocalyx | Starch | Fruit | 9.1 | 49,074 |
| Sarcophrynium | Starch | Fruit | 23.83 | 1847 |
| Xylia | Starch | Seed | 19.58 | 46,587 |
| Treculia | Starch | Seed | 23.87 | 58,093 |
We chose to use genus as the taxonomic rank as some dietary records only identify genus.
Figure 1. Starch and phytolith morphotypes used in the identification model.
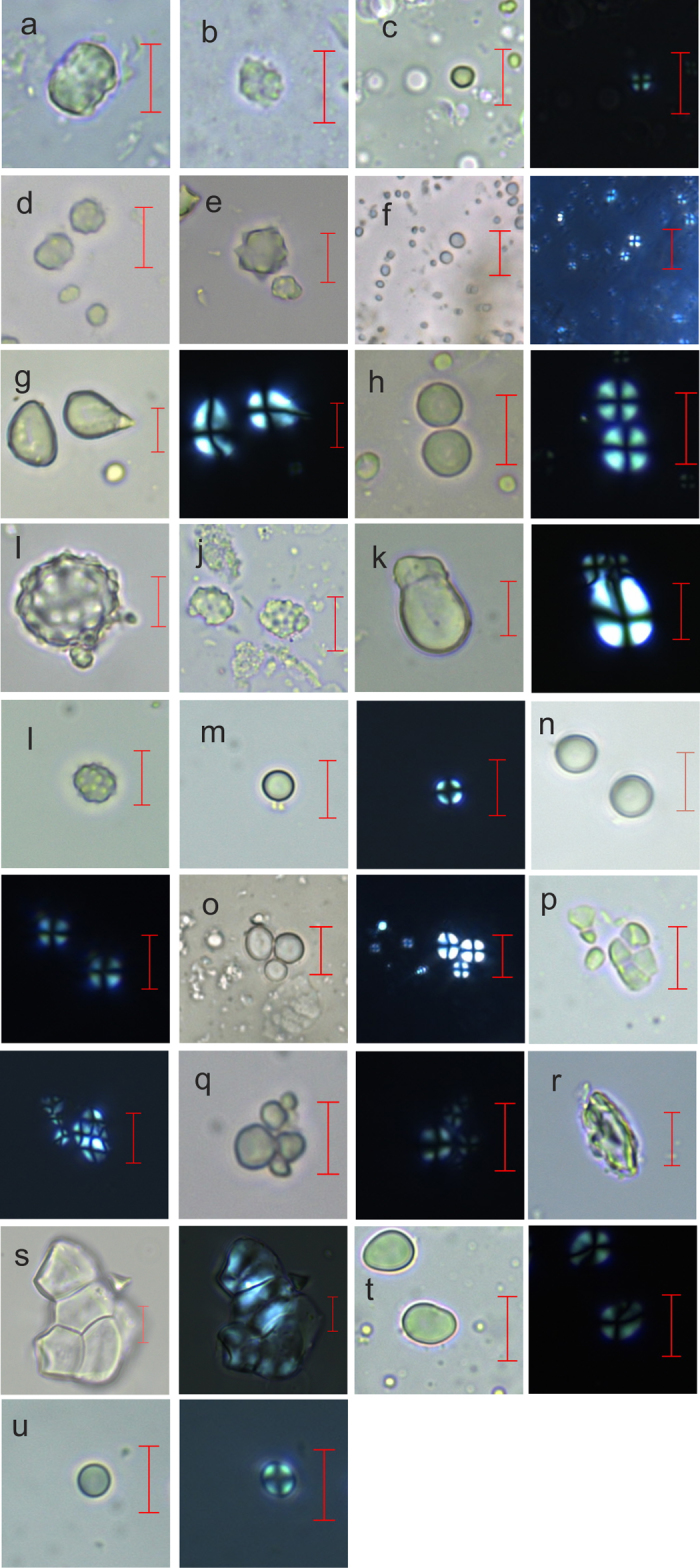
Each scale bar represents 10 μm. (a) Aframomum sceptrum seed phytolith, (b) Aframomum excarpum leaf phytolith, (c) Aframomum excarpum seed starch under normal (left) and cross polarized light (right), (d) Laccosperma opacum pith phytolith, (e) Laccosperma secondiflorum seed phytolith, (f) Calpocalyx sp. fruit starch under normal (left) and cross polarized light (right), (g) Cola nitida seed starch under normal (left) and cross polarized light (right), (h) Coula edulis seed starch under normal (left) and cross polarized light (right), (i) Elaeis guineensis fruit phytolith, (j) Elaeis guineensis leaf phytolith, (k) Gilbertiodendron splendidum seed starch under normal (left) and Cross polarized light (right), (l) Eremospatha macrocarpa pith phytolith, (m) Eremospatha macrocarpa pith starch under normal (left) and cross polarized light (right), (n) Napoleona leonensis seed starch under normal (upper right) and cross polarized light (lower left), (o) Panda olesosa seed starch, (p) Piper guineense seed starch under normal (upper right) and cross polarized light (lower left), (q) Sacoglottis gabonensis fruit starch under normal (left) and cross polarized light (right), (r) Sarcophrynium prionogonium fruit phytolith, (s) Sarcophrynium prionogonium fruit starch under normal (left) and cross polarized light (right), (t) Treculia africana seed starch under normal (left) and cross polarized light (right), (u) Xylia evansii seed starch.
Of the 24 chimpanzee calculus samples, we found starches in 17 of the samples, and phytoliths in 20 (Figs 2 and 3; Supplementary Table 3; Supplementary Data 3). We also found unidentified phytoliths, unsilicified plant fragments, diatoms, pollen and insects, but these were not identified to taxon (Supplementary Data 3). In ambiguous cases microremains were classified as possible starches and specifically stated, but were not used for statistical genera identification. Most definite starches and phytoliths that were free from damage (234 starches and 1035 phytoliths) were identified to genus using the random forest model, which assigned each unknown microremain to a genus and provided a certainty score that indicated the confidence with which that assignment was made. A microremain was considered to be damaged if it showed pitting, ruptured surfaces or other major irregularities. The highest certainty score for each individual microremain depended heavily on each genus identification rate (as described above), but generally ranged between 0.25 and 0.95.
Figure 2. Unsilicified microremains, starches (definite and possible) and phytoliths recovered in Taï Chimpanzee dental calculus with chimpanzee age at death (in years) and approximate age of the cessation of weaning highlighted.
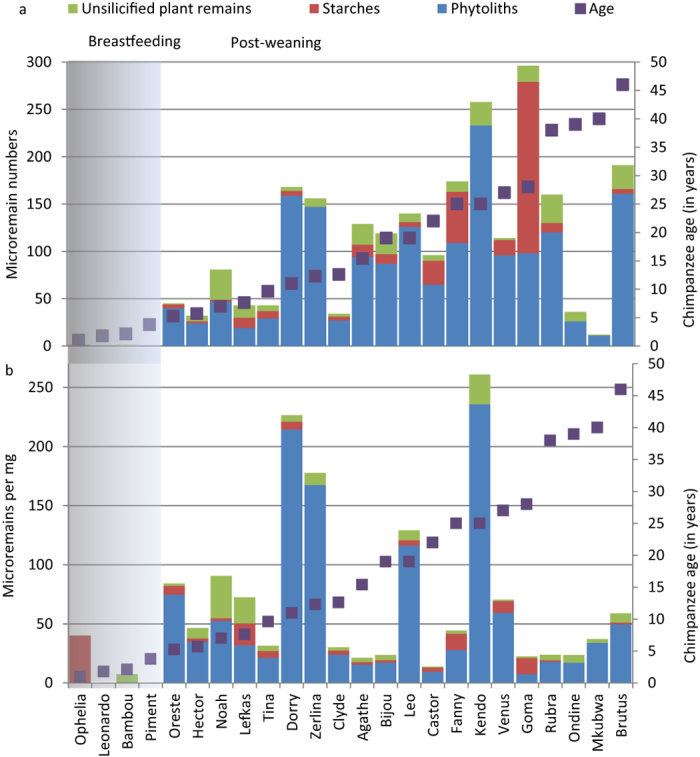
(a) Total counts and (b) counts per milligram of calculus. The number of microremains per mg in Ophelia was affected by an unusually small amount of calculus in the sample.
Figure 3. Microremain assemblages recovered in calculus.
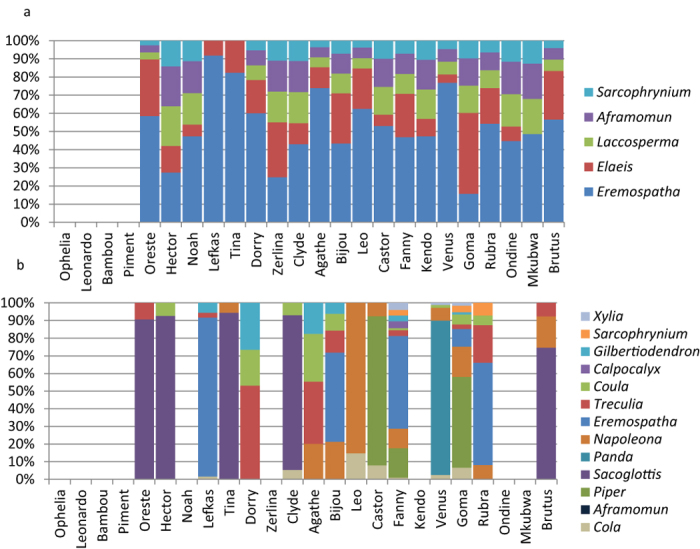
(a) Bar chart of the composition of the phytolith assemblage recovered from calculus. (b) Bar chart of the composition of the starch assemblage recovered from calculus. The individuals are ordered by age from youngest to oldest.
Assessment of biases in our data
First of all, it was important to ascertain if the treatment of the skeletal material to prevent the spread of disease (including one year of burial, and various chemical treatments) had impacted microremain preservation in the calculus. After 2004, chorine and formalin was used to clean skeletal material. Boiling may have been used on some skeletons to clean them and remove Ebola pathogens between the Autumn of 1994 and the Spring of 1996 (Supplementary Table 3; Supplementary Fig. 1). To test if the three types of treatments significantly influenced starch preservation we used a Kruskal-Wallis test on starch per mg on samples from each period (H = 3.7633, df = 2, p-value = 0.1523). We included microremains classified as possible starches in the starch per mg count. Due to the small sample size, we calculated a Kruskal-Wallis p-value based on 999 random permutations. This indicated no differences between the three groups (Permutation H = 7.1215, df = 2, p = 0.159). Previous studies of other organic material (bone collagen) in the Taï skeletal collection have indicated no significant post-mortem alteration36,39. While collagen does not necessarily behave in the same manner as plant microremains, it is likely that the comparable hydroxyapatite mineral matrices of bone and calculus have a similar protective effect on the organic materials trapped within them40.
Before comparing the calculus results to the observational records, we wanted to see if there was excessive variation in plant representation among the calculus samples. Phytoliths from four of the five phytolith-producing genera were found on most, but not all, of the calculus samples, suggesting that there is not much variability among these calculus samples. Some genera are found in each sample (Eremospatha and Elaeis) while others, like Sarcophrynium, were rare. However, the starch record varies significantly among individuals, with most of the thirteen starch-producing genera seldom found. This probably reflects the far lower numbers of starches compared with phytoliths. Several genera dominate the starch record, namely Gilbertiodendron, Coula, Eremospatha, Treculia and Cola (Fig. 3; Supplementary Table 4). Most microremains were isolated, but three calculus samples had four starch aggregates from Piper; each starch in the aggregate was counted as an individual starch granule as counting each was not possible and thus constitutes a large proportion of the total number of the recovered starches. This potentially biases the starch assemblage’s dietary representativeness (Figs 3 and 4). In sum, it seems that there is not much variation in the phytolith record of our chimpanzee samples, but the starch record is less homogeneous.
Figure 4. Microremain assemblages recovered in calculus.
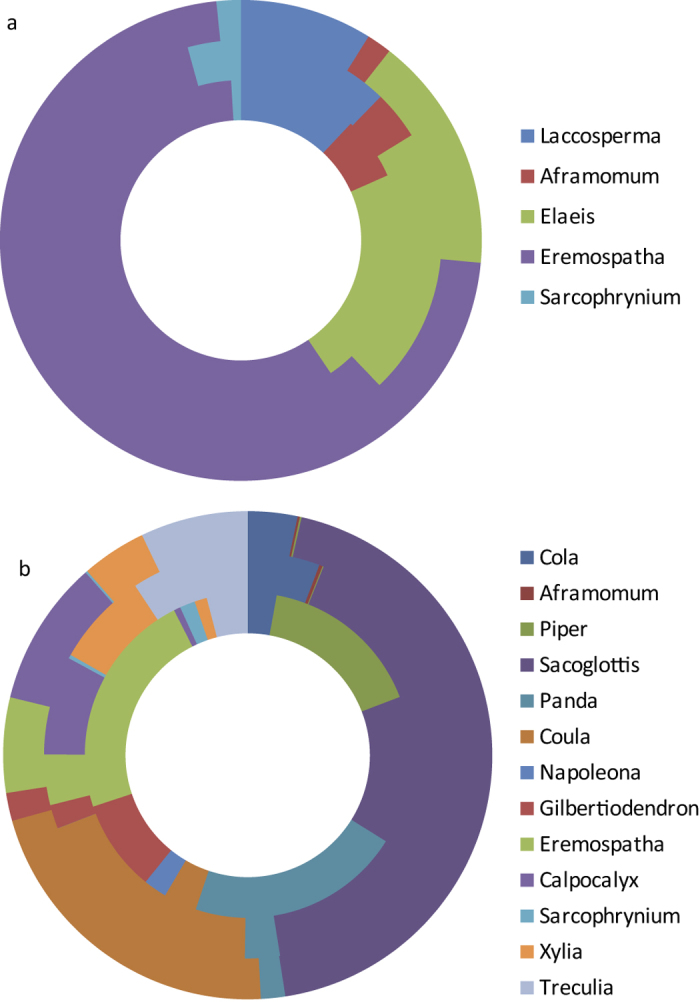
Microremain counts are normalised by dividing counts by the percent content of dry plant weight of starches and phytoliths among different genera. (a) Phytolith counts compared with feeding records. Outermost ring = proportions of minutes spent consuming each genus averaged across the feeding records of sampled 24 sampled chimpanzees, middle ring = proportions of minutes spent consuming each genus averaged across the feeding records of all 128 chimpanzees, innermost ring = phytolith counts from the sampled 24 chimpanzees. (b) Starch counts compared with feeding records: outermost ring = proportions of minutes spent consuming each genus averaged across the feeding records of sampled 24 chimpanzees, middle ring = proportions of minutes spent consuming each genus averaged across the feeding records of all 128 chimpanzees, innermost ring = starch counts.
Another potential source of bias comes from the differential preservation of microremains relating to their inherent properties, like size and shape. We noted that our results were biased towards foods with larger sized microremains. Elaeis phytoliths and Cola starches, the largest microremains in the study (Figs 1 and 4), are disproportionately frequent across the assemblages even after controlling for the high concentration of microremains within these genera. They are frequently found, but are not dominant foods (Supplementary Fig. 2).
Microremain accumulation, chimpanzee age and sex
We predicted that number of microremains should increase with age, and might vary by sex. We tested this using a negative binomial regression, with microremain count as the response, and age and sex as predictors, weighing each observation by the weight of the calculus sample (see detailed methods below). We ran separate tests for phytoliths, starches and unsilicified remains. For phytoliths, the full model of age and sex significantly influenced the count of phytoliths (x2 = 11.794, df = 2, P = 0.0003), and the effect of age was also significant by itself (x2 = 12.753, df = 1, P = 0.0004) (Supplementary Table 5). Older chimpanzees generally have a higher abundance of phytoliths. However, sex by itself did not explain the abundance of phytoliths we found (x2 = 0.028, df = 1, P = 0.866). For unsilicified microremains, age and sex as the full model significantly influenced the microremain count (x2 = 10.067, df = 1, P = 0.015), the effect of age alone was also significant (x2 = 9.202, df = 1, P = 0.0015), but not sex by itself (x2 = 0.59, df = 1, P = 0.806). Starch abundance was significantly determined by age and sex together (x2 = 23.994, df = 2, p = 6.1622e–06). Older chimpanzees generally have a higher abundance of starches (x2 = 3.559, df = 1, p = 0.0592). Unlike with phytoliths and unsilicified remains, sex strongly influenced the abundance of starch (x2 = 17.301, df = 1, p = 3.1897e–05), with female chimpanzees having more starches (Supplementary Table 5).
Microremain dietary picture and observational feeding records
We predicted that more frequently consumed plants should be highly represented in the chimpanzee calculus. To test this, we used an observational random effect Poisson model (Supplementary Text 5). The count of identified classes of microremains (phytoliths and starches) belonging to a particular genus was our response variable, and the fixed predictors were: (a) minutes spent consuming each genus, and (b) chimpanzee age in months. Sex was included as a control predictor, and both calculus sample weight and successful identification rate of each genus were included as weights. We used counts of each genus predicted to be present with the total minutes spent consuming each genus. The chimpanzee individual was included as a random slope term, while year of death, tooth and food type were treated as random intercept terms (see methods below for more details).
When comparing the genera proportions present in the diet (calculated as the number of minutes spent foraging on a genus) with the recovered phytolith assemblages, we found a clear relationship. The amount of minutes spent consuming a given plant genus influences its phytolith count in the calculus assemblage (Figs 4 and 5), even when accounting for the effects of sex, the tooth we sampled, variation in phytolith production between different plants, and the year the individual died. More specifically, an increased reliance on a genus leads to an increase in its representation in calculus (x2 = 4.048, df = 1, P = 0.045; Supplementary Table 5). The age of the chimpanzees did not influence how well it matches group diet (x2 = 0.356, df = 1, P = 0.55 Supplementary Table 5).
Figure 5. Plot of Mixed Poisson regression model.
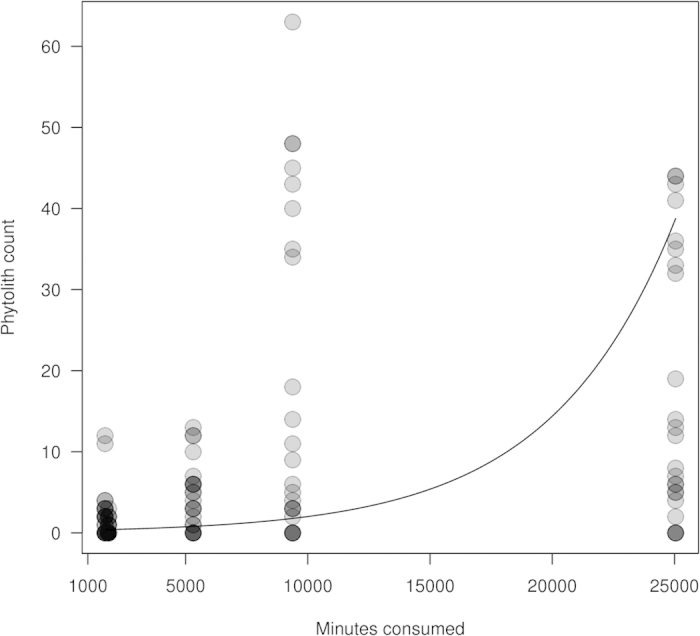
The number of phytoliths from a genus increased as the minutes spent consuming this plant resource increased. Darker circles reflect overlapping values.
In contrast to phytoliths, there was no significant effect of consumption time on starch numbers (x2 = 1.95, df = 2, P = 0.376). The number of minutes this group spent eating a specific genus of starchy foods does not influence its frequency in dental calculus. Yet there is an element of uncertainty because starches vary more among individuals than do phytoliths, as described above, and do not seem to be as good a record of dietary behaviour. Figures 3 and 4 show the discrepancy between the consistency of starch and phytolith results clearly. These results may be a product of post-mortem diagenesis that impacted our chimpanzee samples, including burial to deflesh the remains (Supplementary Table 3). Because these processes are likely unique to our sample, we cannot assume that the same confounding factors will always be present in calculus from other groups.
Weaning and other behavioural signatures in calculus
The microremains in the Taï calculus record other aspects of their behaviour. First, microremains were strikingly rare in samples from individuals less than 5.3 years old (Figs 2 and 6; Table 2). The calculus deposits were sparse on these individuals, but despite the small volume of calculus, it was notable that only a single starch and an unsilicified plant fragment were found in these samples. Chimpanzees more than 5.3 years old typically show high numbers of microremains, regardless of the size of the calculus deposit.
Figure 6. The occurrence of Coula nut starches with chimpanzee age at death (in years).
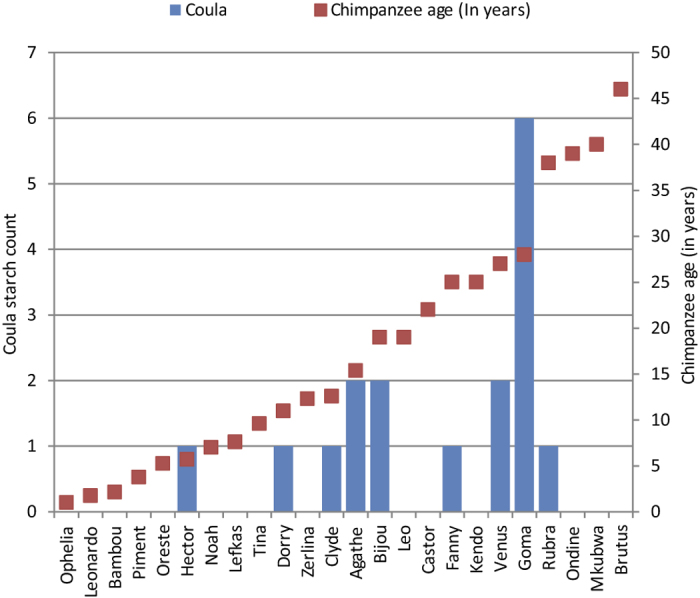
Coula nut consumption requires nut cracking and its presence implies nut cracking and tool use or food sharing. The individuals are ordered by age from youngest to oldest.
Table 2. All chimpanzee dental calculus samples analysed.
| Name | ID | Tooth | Sex | Weight (mg) of calculus sample | Age at death |
|
|---|---|---|---|---|---|---|
| In years | In months | |||||
| Ophelia | 14993 | Lower Left DM1 | Female | 0.025 | 1 | 12 |
| Leonardo | 13432 | Upper DM2 | Male | 0.329 | 1.92 | 23 |
| Bambou | 11777 | Lower Left DM1 | Male | 0.135 | 2.08 | 25 |
| Piment | 11788 | Lower Right DM1 | Female | 0.27 | 3.58 | 43 |
| Oreste | 14995 | Lower Left M1 | Male | 0.536 | 5.25 | 63 |
| Hector | 12175 | Upper Right M1 | Male | 0.689 | 5.67 | 68 |
| Noah | 15011 | Lower M1 | Male | 1.165 | 7 | 84 |
| Lefkas | 13433 | Upper Left M2 | Male | 0.595 | 7.58 | 91 |
| Tina | 11790 | Lower Right M1 | Female | 1.36 | 9.08 | 109 |
| Dorry | 15020 | Lower Right M2 | Female | 0.742 | 11 | 132 |
| Zerlina | 11792 | Lower Left M3 | Female | 0.878 | 12.3 | 144 |
| Clyde | 11779 | Lower Right M1 | Male | 1.131 | 13 | 156 |
| Agathe | 11775 | Lower Right M2 | Female | 6.076 | 16 | 192 |
| Leo | 15012 | Lower Right M3 | Male | 1.085 | 19 | 228 |
| Bijou | 11778 | Lower Left M2 | Female | 5.041 | 19 | 228 |
| Castor | 13439 | Lower Left M1 | Female | 6.982 | 22 | 264 |
| Kendo | 11781 | Lower Left M2 | Male | 2.895 | 25 | 300 |
| Fanny | 11780 | Lower Left M3 | Female | 3.915 | 25 | 300 |
| Venus | 15001 | Upper Right M1 | Female | 1.133 | 27 | 324 |
| Goma | 15004 | Upper Right M3 | Male | 13.208 | 28 | 336 |
| Rubra | 15023 | Lower Left M2 | Female | 6.751 | 38 | 456 |
| Ondine | 11786 | Lower Left M1 | Female | 1.529 | 39 | 468 |
| Mkubwa | 13435 | Lower Right M3 | Male | 0.324 | 40 | 480 |
| Brutus | 15029 | Upper Left M3 | Male | 3.246 | 46 | 552 |
Second, the exact plants that were recovered in the calculus provide an interesting view of an important learned behaviour. In our sample, many calculus samples had starches from the Coula nut, which is mainly consumed once chimpanzees learn to crack open these nuts. Coula nut starches were found in samples from individuals across all age ranges (except those under 5.7 years) (Fig. 6). Although common, Coula nut appears to be underrepresented in our sample. It was found only in nine calculus samples, despite this plant being a major food source, comprising of 4.7% of the total Taï diet.
Discussion
Much of the chimpanzee calculus is relatively rich in plant microremains compared with what has been reported in previous studies of human calculus11,16,18. This is not entirely unexpected for several reasons. First, our samples are modern, and post-mortem microremain diagenesis is therefore less acute than in ancient remains. Secondly, Taï Chimpanzee diet is plant-dominated and voluminous (Supplementary Fig. 1). Thirdly, and in contrast to humans, chimpanzees consume a large amount of phytolith-rich material. This richness in microremains is largely confined to phytoliths. Starch abundance falls within ranges observed elsewhere12,16,18.
It is evident that starches are underrepresented, and in some samples are even totally absent. In addition, phytoliths present a far more uniform picture of diet between different chimpanzees. This may be due to diagenesis occurring during the preparation of the skeletons for the osteology collection that preferentially alters or removes starches from the calculus record. It is known that all skeletons were buried for short periods of time during the defleshing process (Supplementary Table 3). These processes may preferentially alter or remove starches from the calculus record that are not sufficiently trapped and sealed, while leaving the phytoliths relatively unaltered. Fortunately, our Kruskal-Wallis test indicates cleaning processes have not influenced starch numbers.
Additionally, we found that the microremain record was likely biased by the differential survivability of microremains from different plants. The plants with the largest starches and phytoliths were overrepresented in our sample, possibly due to the larger surface area. This ties in with research that shows that phytolith and starch morphology and surface area is linked to long term stability41,42. Larger blocky microremains may be preferentially preserved.
Overall, our results verify that the calculus record can be accumulative by showing that older individuals present more microremains. Sex may be a factor to take into consideration, and seems to influence the accumulation of starches, but not phytoliths or unsilicified remains. It may reflect higher consumption of starches by female chimpanzees, or sex differences in amylase production or calculus formation, as has been suggested for humans43. We do not currently have the ability to distinguish among these possibilities. The increase in microremains with age and possibly sex implies that microremain accumulation is bound up in aspects of diet that regulate calculus formation. Thus, microremain presence and proportions are likely effected or confounded by all the factors that influence dental plaque and calculus (i.e. intake of protein, smoking, polysilicic acid and silica)44,45,46. Calculus clearly can approach a long-term dietary signal, although the timespan involved is not yet clarified.
Our results strongly suggest that care must be taken when interpreting the microremains record preserved in dental calculus, particularly the starch grain record. However, our results also indicate that microremains in calculus can be used to recover important information about diet, behaviour and life history. For example, we observed a lack of microremains from deciduous teeth of chimpanzees less than 5.3 years old. This pattern matches what is generally reported for age of weaning by using other measures. Much information on the age of chimpanzee weaning is estimated from inter-birth interval (IBI)39. IBI estimates of weaning ages vary from 4.5 years at Gombe to 5.75 years at Taï47, to 6 years at Mahale48. Yet IBI is an indirect measure as it includes more than simply suckling duration. Isotopic based data indicates weaning at Taï commences at 2 years and carries on till 3–4.5 years, varying by factors such as sex of the offspring.
Thus, microremain assemblages could indicate a rapid accumulation of microremains as solid food dominates the diet (Fig. 2). If we combine this trend together with the verification of the accumulative nature of the microremain assemblages, we can conclude calculus reflects information on the weaning transition that may be useful for studying unhabituated populations.
Furthermore, though the starch dietary record appears more stochastic than that of phytoliths, starches can still provide useful information about behaviour. Many of our starches come from the Coula nut (Fig. 6). Among chimpanzees, Coula consumption requires a learned behaviour: nut cracking with a hammer and anvil. This behaviour is restricted to a limited area of the chimpanzee range in West Africa1. The presence of Coula starches (Figs 3 and 6) shows calculus can reveal nut-cracking behaviour in a group. The fact that tool-use in a group is discernible is relevant for dental calculus studies in both primatology and hominin evolution. The use of Coula nut is influenced by age and sex differences in nut cracking2,49, and, as expected, Coula starches are absent in youngest chimpanzees who are not yet weaned. Even after weaning infant nut consumption is low and is derived from nuts cracked by the mother as it takes years to learn how to crack nuts2. Beyond this, we do not have enough calculus samples to examine if there are sex or age differences in the calculus record of nut cracking.
In summary, the study verifies the relevance of dental calculus for investigations on diet, food acquisition behaviour and life history. It is the first to link dental calculus with foods that entered the oral cavity in quantified abundances. The data also provide valuable information on the commencement of plant food consumption in wild chimpanzees, and confirm the consumption of solid foodstuffs from at least 5.3 years in life. Our study suggests that calculus analysis provides a rich but wavering insight into complex dietary structure, and that phytoliths, when present in calculus and in diet, may provide a more reliable record of chimpanzee diet than starches.
Materials and Methods
Taï Forest reference material
A reference collection with 91 genera (113 species) of the most frequently consumed chimpanzee plant foods in the Taï Forest was collected and examined for phytoliths and starches (Supplementary Table 6). Phytoliths and starches were isolated from reference plants using conventional approaches50. We selected thirteen starch- and seven phytolith-producing genera from the 91 we analysed for the identification model (Supplementary Text 3).
Calculus sampling
The calculus samples used for our analysis come from permanent and deciduous molars of 24 chimpanzee individuals from the Taï Chimpanzee Osteology Collection at Max Planck Institute for Evolutionary Anthropology (MPI-EVA) with varied life histories (Supplementary Text 1, 2; Supplementary Table 1). We selected only molars to standardise the sampling, and chose teeth that were encrusted with a prominent band of supragingival calculus (calculus present above the original gumline) on the enamel crown. Deposits of supragingival calculus were present on all individuals ≥1 year old. Subgingival calculus was also present but was not sampled since it occurs below the former gums and it is unclear if it preserves food remains. Calculus on the teeth was documented with photography before sampling, and the colour noted with how each skeleton was treated before our sampling (Supplementary Table 1). Packing material was sampled as a control. An unidentified adhesive used in the curation of some specimens was removed before sampling. A dental scalar was then used to remove small areas of calculus. The amount of calculus sampled had no relationship with the amount of calculus present on the teeth except in the youngest chimpanzees (<5.3 years), where calculus was rare and almost entirely collected. We sampled in clean conditions in a laminar flow cabinet at positive-pressure at the MPI-EVA. We then weighed each of the samples and transferred them to microcentrifuge tubes. After sampling, the teeth and surviving calculus were photographed.
Some studies have highlighted the risks of laboratory contamination from modern plant microremains51,52,53. To address the possibility of contamination, we conducted a regime of weekly laboratory cleaning to remove contamination. All work surfaces were wiped with hot water, washed with starch-free soap and wiped with 5% sodium hydroxide (NaOH). We additionally performed wipe tests before and after weekly cleaning to quantify starch contamination and assess contaminating types. Wipe tests retrieved settled particles of the surface area (74 × 43 cm2) of the laboratory positive-pressure laminar flow hood used for mounting. Results of these intensive contamination control tests are in Supplementary Data 4.
Optical microscopy
Optical microscopy was performed at the Plant Foods in Hominin Dietary Ecology laboratory in the MPI-EVA (for reference collection microscopy see Supplementary Text 3 and Supplementary Tables 4, 7). We added 150 μl of 10% hydrochloric acid to the calculus samples for one to three hours. The samples were then centrifuged at 1691 × g (Heraeus MEGAFUGE 16 with a microcentrifuge rotor) for 10 minutes and then about 100 μl of supernatant was decanted and replaced with distilled water. This was repeated three times to remove the hydrochloric acid. After the second decanting, they were refilled with a 25% glycerine solution. The samples were then ground in the solution in the 1.5 ml Eppendorf microcentrifuge tube to reduce sample loss due to static electricity. The samples then were centrifuged again at the same speed, and about 1 ml of supernatant was decanted. We mounted 20 μl per slide on as many slides as needed in order to examine the entire sample. Microscopy was used as in conventional phytolith and starch studies12,54. We examined each slide under brightfield and cross-polarized light on a Zeiss Axioscope microscope at 400× magnification. We photographed each microremain and described each with the international microremain nomenclature including the International Code of Phytolith Nomenclature55. In some cases starch aggregates were identified in calculus. In this case, each component granule of each aggregate was counted as an individual starch.
Microremain identification
We identified microremains with a reference collection using multivariate analysis with a random forest algorithm. We collected five general microremain measurements, four specific to phytoliths and six specific to starches from a total of 900 reference microremains (Supplementary Table 7; Supplementary Data 1 and 2). With this reference collection we generated an identification certainty score for each microremain. The validity was tested through cross-validation with a subset of reference data (Table 1; Supplementary Table 1, 2, 8, 9; Supplementary Data 5). We identified the microremain as coming from the genus with the highest certainty score (Supplementary Data 5 and 6).
Behavioural records
The chimpanzees of the Taï Forest have been studied since the commencement of the Taï Chimpanzee Project in 19792. The detailed recorded behaviour of the group included observation of feeding time and food item consumed. The feeding records used in our study span the period between 1992 and 2014 (Supplementary Fig. 1). The database includes 1,165,150 million behavioural observations of about 128 chimpanzees, with a total of 417,628 dietary observations (2,380,202 minutes). However, only roughly 30,000 observations come from chimpanzees available for sampling at the osteology collection. Furthermore, most of these chimpanzees have only sporadic coverage of their life history. Therefore, instead of using dietary records of individual chimpanzees or the collated records of the 24 chimpanzees we sampled, we chose to combine dietary records from all 128 chimpanzees to best represent the average Taï Forest diet.
The feeding record includes the times when a chimpanzee started and finished eating, and the food consumed. We chose only those feeding records where the genus of the plant food eaten was documented, and calculated the total amount of time spent consuming each resource. Behavioural records do not account for variations in the volume of food consumed in given amount of minutes. In addition, although some observations record the specific plant part that was eaten, most do not, so we do not include this information.
Statistical analysis
To test for the effects of age on microremains we used a negative binomial regression (log link) with a count of each microremain class treated as a response (phytoliths, starches and other unsilicified plant fragments) using a likelihood ratio test in R 3.1.056. We ran the regression using the glm.nb function of R package MASS57. The full model included the fixed effects of age and sex (Supplementary Text 5). The mg weight of each calculus sample was used to weigh the model to account for larger samples likely being more representative of overall diet due to the potential of microremains to have a clustered distribution in the calculus matrix. Controlling for weight, heavier samples have less variable microremain counts (Compare Table 2 with Supplementary Table 1, 2). The full model was compared with a null model using an ANOVA. We used likelihood ratio tests comparing the full models with reduced models in which each fixed effect was dropped, one at a time. Model assumptions were met. Collinearity was not an issue (largest Variance Inflation Factor = 1.001) and leverage values, as well as DFBeta values, indicated no obvious highly influential cases.
To explore the relationship between diet and the phytolith microremains found in dental calculus, we tested an Observational random effect Poisson model with likelihood ratio tests. We used counts of each genus predicted to be present with the total minutes spent consuming each genus. For this, we used the glmer function of the R package lme458. If any genus was not predicted to be present in a chimpanzee sample, they were included as a 0 value. Our full model included minutes and chimpanzee age in months as fixed effects, and sex as a control predictor. The model included the weight of each calculus sample and the successful identification rate of each type of genera as model weights, and used microremain content as an offset to factor in significant differences in content between different genera. To prepare the data, we z-transformed the minutes and age variables. The chimpanzee individual was included as a random slope term while year of death, tooth and food type were treated as random intercept terms. An id was assigned to each observation, and this was also included as a random intercept, thus reducing overdispersion to (x2 = 13.369, df = 116, dispersion parameter = 0.115) in the phytolith model. To test the significance of the full model it was compared with a null model excluding fixed effects of minutes of observation and age. Variance inflation factors (VIF)59 were derived to assess collinearity using the function vif of the R-package car, from a standard linear model minus random effects, as offsets and weights60. Variance inflation factors indicated collinearity to not be an issue (largest VIF = 1.02). We tested model stability by excluding each random effect one by one from the data set, running the full model and comparing the results with those from the original model that suggest no highly influential cases.
To explore the relationship between diet and the starch microremains, we could not use the same approach due to high zero inflation present in the starch data. To overcome this we implemented a Mixed effects logistic regression using the same terms, random effects, weighs and offset as the phytolith Poisson model. This required the count data (the response) to be treated as presence and absence data resulting in some loss of data. Variance inflation factors59 were derived to access collinearity using the function vif of the R-package car, from a standard linear model minus random effects as well as offsets and weights60. Variance inflation factors indicated collinearity to not be an issue (largest VIF = 1.018).
Additional Information
How to cite this article: Power, R. C. et al. Dental calculus evidence of Taï Forest Chimpanzee plant consumption and life history transitions. Sci. Rep. 5, 15161; doi: 10.1038/srep15161 (2015).
Supplementary Material
Acknowledgments
The authors wish to thank Roger Mundry and Colleen Stephens for invaluable support on statistics. We also thank the following for comments and support: Antje Hutschenreuther, Jörg Watzke, Geraldine Fahy, Julia Riedel, Chelsea Leonard, Layne Vashro, Simone Schmidt, Thomas Büdel, Nadia Scott, Gottfried Hohmann, Viviana Toro Ibacache, Ammie Kalan, and Ana Karen Negrete García. We thank Roger Kami Nabo and other field assistants at Taï National Park for collection of plant samples. We also wish to acknowledge the Ministère de la Recherche Scientifique and the Ministère de l’Environnement et des Eaux et Forêts of Côte d’Ivoire, the Office Ivorien des Parcs et Reserves, the Director of the Taï National Park for permission to conduct this research. We are grateful for support by the Centre Suisse de Recherches Scientifiques at Abidjan, Côte d’Ivoire. DCSG acknowledges support from the Generalitat Valenciana (VALi+d APOSTD/2014/123 and GV/2015/060), the BBVA Foundation (I Ayudas a investigadores, innovadores y creadores culturales) and the European Union (FP7/2007-2013 - MSCA-COFUND, n°–245743 via a Braudel-IFER-FMSH). This research was funded by the Max Planck Society.
Footnotes
Author Contributions R.C.P. designed the research with input from A.G.H. and D.C.S.G., R.C.P. performed analysis, R.C.P. analysed data, R.M.W. and M.F. provided materials and resources, A.G.H. and D.C.S.G. supervised the project, and R.C.P. wrote the paper with input from the other co-authors.
References
- Boesch C., Marchesi P., Marchesi N., Fruth B. & Joulian F. Is nut cracking in wild chimpanzees a cultural behaviour? J. Hum. Evol. 26, 325–338 (1994). [Google Scholar]
- Boesch C. & Boesch-Achermann H. The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution. Oxford Univ. Press. Oxford 326 p (2000). [Google Scholar]
- Teaford M. F. & Ungar P. S. Diet and the evolution of the earliest human ancestors. Proc. Natl. Acad. Sci. USA. 97, 13506–13511 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. Primate life histories. Evol. Anthropol. 6, 54–63 (1998). [Google Scholar]
- Phillips C. & Lancelotti C. Chimpanzee diet: phytolithic analysis of feces. Am. J. Primatol. 76, 757–73 (2014). [DOI] [PubMed] [Google Scholar]
- Fiorenza L. et al. To meat or not to meat? New perspectives on Neanderthal ecology. Am. J. Phys. Anthropol. 156 Suppl, 43–71 (2015). [DOI] [PubMed] [Google Scholar]
- Scott R. S., Teaford M. F. & Ungar P. S. Dental microwear texture and anthropoid diets. Am. J. Phys. Anthropol. 147, 551–79 (2012). [DOI] [PubMed] [Google Scholar]
- Grine F. E. Dental evidence for dietary differences in Australopithecus and Paranthropus: a quantitative analysis of permanent molar microwear. J. Hum. Evol. 15, 783–822 (1986). [Google Scholar]
- Armitage P. L. The extraction and identification of opal phytoliths from the teeth of ungulates. J. Archaeol. Sci. 2, 450–455 (1975). [Google Scholar]
- Adler C. J. et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet. 45, 450–5, 455e1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera M., Pany-Kucera D., Boyadjian C. H., Reinhard K. & Eggers S. Efficient but destructive: A test of the dental wash technique using secondary electron microscopy. J. Archaeol. Sci. 38, 129–135 (2011). [Google Scholar]
- Power R. C., Salazar-García D. C., Wittig R. M. & Henry A. G. Assessing use and suitability of scanning electron microscopy in the analysis of micro remains in dental calculus. J. Archaeol. Sci. 49, 160–169 (2014). [Google Scholar]
- Salazar-García D. C. et al. Neanderthal diets in central and southeastern Mediterranean Iberia. Quat. Int. 318, 3–18 (2013). [Google Scholar]
- Warinner C. et al. Pathogens and host immunity in the ancient human oral cavity. Nat. Genet. 46, 336–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R. C., Salazar-García D. C., Straus L. G., González Morales M. R. & Henry A. G. Microremains from El Mirón on Cave human dental calculus suggest a mixed plant/animal subsistence economy during the Magdalenian in Northern Iberia. J. Archaeol. Sci. 60, 39–46 (2015). [Google Scholar]
- Leonard C., Vashro L., O’Connell J. F. & Henry A. G. Plant microremains in dental calculus as a record of plant consumption: A test with Twe forager-horticulturalists. J. Archaeol. Sci. Reports 2, 449–457 (2015). [Google Scholar]
- Boyadjian C. H. C., Eggers S. & Reinhard K. Dental wash: a problematic method for extracting microfossils from teeth. J. Archaeol. Sci. 34, 1622–1628 (2007). [Google Scholar]
- Mickleburgh H. L. & Pagán-Jiménez J. R. New insights into the consumption of maize and other food plants in the pre-Columbian Caribbean from starch grains trapped in human dental calculus. J. Archaeol. Sci. 39, 2468–2478 (2012). [Google Scholar]
- Lalueza-Fox C., Juan J. & Albert R. M. Phytolith analysis on dental calculus, enamel surface, and burial soil: Information about diet and paleoenvironment. Am. J. Phys. Anthropol. 101, 101–113 (1996). [DOI] [PubMed] [Google Scholar]
- Gobetz K. E. & Bozarth S. R. Implications for Late Pleistocene mastodon diet from opal phytoliths in tooth calculus. Quat. Res. 55, 115–122 (2001). [Google Scholar]
- Buckley S., Usai D., Jakob T., Radini A. & Hardy K. Dental calculus reveals unique insights into food items, cooking and plant processing in prehistoric central Sudan. PLoS One 9, e100808 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry A. G. et al. The diet of Australopithecus sediba. Nature 487, 90–3 (2012). [DOI] [PubMed] [Google Scholar]
- Lazzati A. M. B. et al. The Diet of Three Medieval Individuals from Caravate (Varese, Italy). Combined Results of ICP-MS Analysis of Trace Elements and Phytolith Analysis Conducted on Their Dental Calculus. Int. J. Osteoarchaeol (2015), 10.1002/oa.2458. [DOI] [Google Scholar]
- Buck L. T. & Stringer C. B. Having the stomach for it: A contribution to Neanderthal diets? Quat. Sci. Rev. 96, 161–167 (2014). [Google Scholar]
- Dudgeon J. V. & Tromp M. Diet, Geography and Drinking Water in Polynesia: Microfossil Research from Archaeological Human Dental Calculus, Rapa Nui (Easter Island). Int. J. Osteoarchaeol. 24, 634–648 (2014). [Google Scholar]
- Tromp M. & Dudgeon J. V. Differentiating dietary and non-dietary microfossils extracted from human dental calculus: the importance of sweet potato to ancient diet on Rapa Nui. J. Archaeol. Sci. 54, 54–63 (2015). [Google Scholar]
- Santos J. L. et al. Copy number polymorphism of the salivary amylase gene: implications in human nutrition research. J. Nutrigenet. Nutrigenomics 5, 117–31 (2012). [DOI] [PubMed] [Google Scholar]
- Perry G. H. et al. Diet and the evolution of human amylase gene copy number variation. Nat. Genet. 39, 1256–1260 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobney K. & Brothwell D. In Teeth and anthropology 55–82 (British Archaeological Reports, 1986). [Google Scholar]
- Breiman L. Random forests. Mach. Learn. 45, 5–32 (2001). [Google Scholar]
- Saul H. et al. A systematic approach to the recovery and identification of starches from carbonised deposits on ceramic vessels. J. Archaeol. Sci. 39, 3483–3492 (2012). [Google Scholar]
- Fenwick R. S. H., Lentfer C. J. & Weisler M. I. Palm reading: A pilot study to discriminate phytoliths of four Arecaceae (Palmae) taxa. J. Archaeol. Sci. 38, 2190–2199 (2011). [Google Scholar]
- Out W. A., Pertusa Grau J. F. & Madella M. A new method for morphometric analysis of opal phytoliths from plants. Microsc. Microanal. 20, 1876–87 (2014). [DOI] [PubMed] [Google Scholar]
- Out W. A. & Madella M. Morphometric distinction between bilobate phytoliths from Panicum miliaceum and Setaria italica leaves. Archaeol. Anthropol. Sci. (2015), 10.1007/s12520-015-0235-6. [DOI] [Google Scholar]
- Coster A. C. F. & Field J. H. What starch grain is that? – A geometric morphometric approach to determining plant species origin. J. Archaeol. Sci. 58, 9–25 (2015). [Google Scholar]
- Fahy G. E., Richards M. P., Riedel J., Hublin J.-J. & Boesch C. Stable isotope evidence of meat eating and hunting specialization in adult male chimpanzees. Proc. Natl. Acad. Sci. USA. 110, 5829–33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangham R. W. & Smuts B. B. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. J. Reprod. Fertil. Suppl. Suppl 28, 13–31 (1980). [PubMed] [Google Scholar]
- N’guessan A. K., Ortmann S. & Boesch C. Daily energy balance and protein gain among Pan troglodytes verus in the Taï National Park, Côte d’Ivoire. Int. J. Primatol. 30, 481–496 (2009). [Google Scholar]
- Fahy G. E. et al. Stable nitrogen isotope analysis of dentine serial sections elucidate sex differences in weaning patterns of wild chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 153, 635–642 (2014). [DOI] [PubMed] [Google Scholar]
- Nicholson R. A. Bone Degradation, Burial Medium and Species Representation: Debunking the Myths, an Experiment-based Approach. J. Archaeol. Sci. 23, 513–533 (1996). [Google Scholar]
- Cabanes D., Weiner S. & Shahack-Gross R. Stability of phytoliths in the archaeological record: a dissolution study of modern and fossil phytoliths. J. Archaeol. Sci. 38, 2480–2490 (2011). [Google Scholar]
- Haslam M. The decomposition of starch grains in soils: implications for archaeological residue analyses. J. Archaeol. Sci. 31, 1715–1734 (2004). [Google Scholar]
- Monteiro da Silva A. M., Newman H. N., Oakley D. A. & O’Leary R. Psychosocial factors, dental plaque levels and smoking in periodontitis patients. J. Clin. Periodontol. 25, 517–523 (1998). [DOI] [PubMed] [Google Scholar]
- Damen J. J. M. & Ten Cate J. M. The effect of silicic acid on calcium phosphate precipitation. J. Dent. Res. 68, 1355–1359 (1989). [DOI] [PubMed] [Google Scholar]
- Jin Y. & Yip H.-K. Supragingival calculus: formation and control. Crit. Rev. Oral Biol. Med. 13, 426–441 (2002). [DOI] [PubMed] [Google Scholar]
- Roberts-Harry E. A. & Clerehugh V. Subgingival calculus: where are we now? A comparative review. J. Dent. 28, 93–102 (2000). [DOI] [PubMed] [Google Scholar]
- Boesch C. Evidence for dominant wild female chimpanzees investing more in sons. Anim. Behav. 54, 811–815 (1997). [DOI] [PubMed] [Google Scholar]
- Nishida T. & Hasegawa T. In Topics in primatology (eds. Nishida T., Mcgrew W. C., Marler P., Pickford M. & de Waal M. F. B.) 159–174 (University of Tokyo Press, 1992). [Google Scholar]
- Boesch C. & Boesch H. Possible causes of sex differences in the use of natural hammers by wild chimpanzees. J. Hum. Evol. 13, 415–440 (1984). [Google Scholar]
- Piperno D. R. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists. (AltaMira, 2006). [Google Scholar]
- Crowther A., Haslam M., Oakden N., Walde D. & Mercader J. Documenting contamination in ancient starch laboratories. J. Archaeol. Sci. 49, 90–104 (2014). [Google Scholar]
- Weyrich L. S., Dobney K. & Cooper A. Ancient DNA analysis of dental calculus. J. Hum. Evol. 79, 119–24 (2014). [DOI] [PubMed] [Google Scholar]
- Barton H. & Torrence R. Cooking up recipes for ancient starch: assessing current methodologies and looking to the future. J. Archaeol. Sci. 56, 194–201 (2015). [Google Scholar]
- Power R. C., Rosen A. M. & Nadel D. The economic and ritual utilization of plants at the Raqefet Cave Natufian site: The evidence from phytoliths. J. Anthropol. Archaeol. 33, 49–65 (2014). [Google Scholar]
- Madella M., Alexandre A. & Ball T. International code for phytolith nomenclature 1.0. Ann. Bot. 96, 253–260 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team. R. R: A language and environment for statistical computing. R Found. Stat. Comput. Vienna, Austria URL http://www.R-project.org/. (2014).
- Venables W. N. & Ripley B. D. Modern Applied Statistics with S Fourth edition by. World 53, 86 (2002). [Google Scholar]
- Bates D., Maechler M. & Bolker B. lme4: Linear mixed-effects models using S4 classes. R Packag. version 0.999999-2. 999999 (2013), citeulike-article-id:1080437. [Google Scholar]
- Field A. Discovering Statistics Using SPSS. ISM Introducing Statistical Methods 2nd, (2005).
- Fox J. & Weisberg S. An R Companion to Applied Regression. Sage Publications (SAGE Publications, 2002), 10.1177/0049124105277200. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


