Abstract
We describe a case of a 21-year-old primigravida at 36 weeks’ gestation who was admitted to a local hospital because of abdominal pain. She was prescribed a total of six doses of diclofenac 50 mg over 2 days. One day later, there was difficulty registering the fetal heartbeats on cardiotocography. Ultrasound examination revealed a fetus with ascites and pathological flow over the tricuspid valve. The patient was referred to a tertiary centre for fetal medicine. Fetal echocardiography revealed, in addition to ascites and tricuspid regurgitation, a constricted ductus arteriosus, dilated right ventricle and reduced flow in the pulmonary artery. Immediate caesarean section resulted in an excellent neonatal outcome.
Background
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used in the general population. Some of these drugs can be bought without prescription, and are mostly used for mild-to-moderate pain and inflammation. NSAIDs are a group of medicaments that reduce the production of prostaglandin by direct inhibition of the enzyme cyclooxygenase.1 NSAIDs have previously been widely used in pregnancy-related conditions including polyhydramnios and to prevent preterm labour, because of its tocolytic properties.2 However, administration in pregnancy may cause adverse fetal effects as they cross the placenta,3 4 and prostaglandin inhibition has several effects on the fetus.2 5 Prostaglandin induces relaxation of systemic and pulmonary vessels, including the ductus arteriosus.6 Intake of NSAIDs limits the production of prostaglandin and may lead to constriction or closure of the ductus arteriosus, causing pulmonary hypertension and, eventually, fetal death. We report a case of premature constriction of the ductus arteriosus following maternal treatment with diclofenac at 36 weeks’ gestation.
Case presentation
A 21-year-old primigravida was admitted twice to a local hospital because of abdominal pain. The first and second trimester had been uneventful. Her only previous medication had been Symbicort (formoterol and budenoside) because of asthma. She presented at 34 weeks of gestation with suspected pyelonephritis and was treated with antibiotics (mecillinam). At 36 weeks’ gestation, she was readmitted with severe right-sided abdominal pain. Diclofenac and paracetamol were given three times daily for 2 days, and she received several doses of morphine intravenously to relieve the pain. Medical examination, including blood samples, abdominal maternal ultrasound and urine test strip, were normal. In total, the patient received six doses of diclofenac 50 mg during the 2 days. Before discharge, there was difficulty registering the fetal heartbeats on cardiotocography. Fetal ultrasound examination the next day showed a fetus with ascites and pathological flow over the tricuspid valve. The patient was referred to the National Center for Fetal Medicine at St Olavs Hospital, Trondheim University Hospital, for a second opinion. Fetal echocardiography revealed a dilated right ventricle, fetal ascites and oedema. There was no detectable flow, with normal colour flow settings, in the pulmonary trunk, sparse filling of the right atrium, significant tricuspid regurgitation and reduced contractility in the right ventricle (video 1). The ductus arteriosus was constricted and showed increased velocity (figure 1). Emergency caesarean section was performed and a female neonate was delivered at 36+6 weeks of gestation. The baby cried 10 s after birth and her heart rate was >100 bpm when she was examined at the resuscitation table immediately thereafter. She had facial oedema and was cyanotic, with an oxygen saturation of approximately 70%. Oxygen saturation gradually increased with supplemental oxygen. After 40 min, the oxygen saturation stabilised above 95%. The baby's weight was 3580 g, and umbilical pH was normal (7.34). Echocardiography just after the delivery showed a dilated right ventricle with reduced contractility (video 2). However, the right ventricular function was improved compared to the findings on intrauterine ultrasound before delivery. There was a right-to-left shunt over the foramen ovale, causing cyanosis, and findings of suprasystemic pulmonary pressure (estimated pulmonary pressure was 70 mm Hg).
Figure 1.
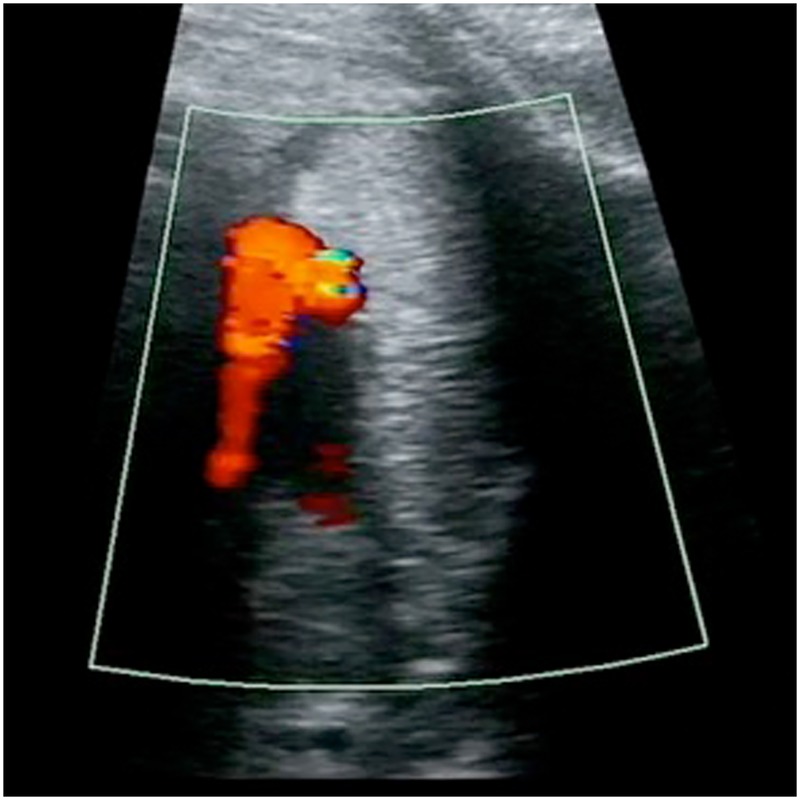
Prenatally: constricted ductus arteriosus with increased velocity. No visible blood flow in the pulmonary artery.
Video 1.
Prenatally: hypertrophic right ventricle with reduced contractility, reduced filling and tricuspid regurgitation.
Video 2.
Postnatally, 30 min after birth; improved, but reduced contractility of the right ventricle yet remaining. Right ventricle hypertrophy and tricuspid regurgitation.
Outcome and follow-up
The baby needed supplemental oxygen the first week and was feeding well from day 8 (total weight loss 13.7% of birth weight). Echocardiography during hospitalisation showed gradual improvement of the right ventricular function, and pulmonary pressure was in the upper normal level from day 7. Diuresis and creatinine levels were normal. The baby was discharged after 10 days and follow-up at 5 weeks of age revealed normal findings on echocardiography, except for a small physiological left-to-right shunt over the foramen ovale, which, at last follow-up at 2 years of age, had closed spontaneously.
Discussion
Constriction or closure of the ductus arteriosus is a rare condition that can occur spontaneously or be secondary to maternal medication. The ductus arteriosus is a blood vessel connecting the pulmonary artery and the descending aorta, allowing the blood to bypass the high-resistance pulmonary vessels. It is crucial for the fetal circulation by shunting most of the blood volume from the right ventricle to the aorta, and it is essential for normal fetal development.7 The duct normally closes spontaneously after birth and most often there is a functional closure 24 h after birth. However, the complete anatomical occlusion of the lumen of the duct may take days or weeks.7 8 A Doppler study on human fetus showed that 78% of the right cardiac output and 46% of the total cardiac output crosses the ductus arteriosus.9 If the duct closes in utero, all the blood is ejected into the pulmonary circulation, leading to hypertrophy of the medial layer of the pulmonary arteries, and pulmonary hypertension. This can have serious impact on the fetus, such as congestive heart failure or fetal hydrops, and may even lead to intrauterine death.10 In our case, a pregnant woman at 36 weeks’ gestation was given six doses of diclofenac 50 mg during 48 h, for abdominal pain. Fetal echocardiography revealed a constricted ductus arteriosus with consequential acute pulmonary hypertension and right-sided heart failure, fetal ascites and generalised oedema.
A PubMed search from 1998 until the present, revealed six other case reports describing constriction or closure of the ductus arteriosus following maternal ingestion of diclofenac.11–16 It has also been described after maternal use of topical diclofenac in two case reports.17 18 Diclofenac is a NSAID that is widely used; it is commonly prescribed to treat various diseases and symptoms such as inflammation and mild-to-moderate pain. There is an increased risk of premature closure of the ductus arteriosus if NSAIDs are used after 28 weeks of gestation, and the risk increases with advancing gestational age.19 Ductal closure is described both after a single dose and after long term use of NSAIDs in pregnancy. The neonate described in this case improved during the first days after delivery and echocardiography was normal at 5 weeks of age. There are a variety of neonatal outcomes described in the literature.
The renal function was not impaired in our case. However, NSAIDs can affect fetal and neonatal renal function. NSAIDs can cause reduced renal perfusion, which leads to reduced urine production and oligohydramnios. The effect can be transient, but there are cases describing end-stage renal failure.20–23 The main problems with administration of NSAIDs during pregnancy are adverse effects on the ductus arteriosus and fetal renal function. However, other adverse neonatal effects are also described, such as intraventricular haemorrhage, necrotising enterocolitis, periventricular leucomalacia, respiratory distress syndrome and bronchopulmonary dysplasia.5 24 25
NSAIDs are widely used among fertile women and can be bought without prescription. It is therefore important to inform pregnant women of the potential fetal and neonatal complications following maternal ingestion, and that these drugs should be avoided as self-medication especially in the third trimester. There is an ongoing debate whether NSAIDs should be reintroduced as tocolytic therapy since their effect on delaying preterm delivery is high.26 27 However, the potential negative side effects, most importantly premature closure of the ductus arteriosus and fetal renal impairment, have to be balanced, and treatment should only be introduced after thorough consideration and with caution.
Fetal monitoring should be performed if any of these drugs have been taken in late pregnancy. Ductal constriction is usually, but not always, reversible after withdrawing the inciting agent.28 29 If the pregnant woman is term or near-term and the duct has closed, immediate delivery is recommended. As in our case, the right heart failure usually improves if the disorder is diagnosed and the neonate is delivered promptly.
Patient's perspective.
The mother's thoughts:
I find it very difficult to think about what happened. Everything happened so fast and I did not really understand what took place. I was in pain, but the doctors did not know why. They gave me tablets to relieve the pain, and I trusted that they knew what they were doing. I was angry and sad over the way I was treated. I felt that something was wrong. I was told that the baby was fine even though I was in extreme pain. Before I was discharged, they wanted to do another CTG-registration and they did another ultrasound examination, which showed fluid in the baby's abdomen. I was referred to the National Center for Fetal Medicine and got an appointment for the next day. I do not understand why they did not send me the same day. When I came to the National Center for Fetal Medicine, the diagnosis came as a shock. We waited for many hours before I was examined. Three or four medical doctors came to look at the ultrasound pictures. I lay there and smiled, not understanding the medical language they were using. Another doctor came and said that the baby had to come out. Immediately… totally unexpected. I was angry and sad when I finally understood what had happened to me. I had been given Voltaren in the last trimester.
After this I no longer trust medical doctors. I had postnatal depression and it has taken a long time to accept what happened. I want another baby, but do not dare have one. I am still afraid. I try to console myself that things could have been worse and that I was lucky to get the help I did. My daughter is now healthy and I am happy…
Learning points.
Non-steroidal anti-inflammatory drugs (NSAIDs) should only be prescribed after thorough consideration and with caution in pregnant women, especially during the third trimester.
NSAIDs may be associated with premature closure of the ductus arteriosus.
If any NSAIDs have been taken in late pregnancy, it is important to monitor the fetus closely to detect signs of ductal constriction.
Immediate delivery is recommended if the duct has closed and the pregnant woman is term or near-term.
Acknowledgments
The authors thank Wendy Williams for copy editing the case report.
Footnotes
Contributors: KA conducted the literature search, participated in data collection, and drafted and critically revised the manuscript. AB conducted intrauterine diagnostics, conducted literature searches, and participated in drafting and critical evaluation of the manuscript. SAN contributed to intrauterine diagnostics, conducted postnatal treatment and follow-up of the patient, and participated in drafting and critical evaluation of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971;231:232–5. 10.1038/newbio231232a0 [DOI] [PubMed] [Google Scholar]
- 2.Østensen ME. Safety of non-steroidal anti-inflammatory drugs during pregnancy and lactation. Inflammopharmacology 1996;4:31–41. 10.1007/BF02735557 [DOI] [Google Scholar]
- 3.Siu SS, Yeung JH, Lau TK. A study on placental transfer of diclofenac in first trimester of human pregnancy. Hum Reprod 2000;15:2423–5. 10.1093/humrep/15.11.2423 [DOI] [PubMed] [Google Scholar]
- 4.Antonucci R, Zaffanello M, Puxeddu E et al. Use of non-steroidal anti-inflammatory drugs in pregnancy: impact on the fetus and newborn. Curr Drug Metab 2012;13:474–90. 10.2174/138920012800166607 [DOI] [PubMed] [Google Scholar]
- 5.Koren G, Florescu A, Costei AM et al. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysis. Ann Pharmacother 2006;40:824–9. 10.1345/aph.1G428 [DOI] [PubMed] [Google Scholar]
- 6.Olley PM, Coceani F. Prostaglandins and the ductus arteriosus. Annu Rev Med 1981;32:375–85. 10.1146/annurev.me.32.020181.002111 [DOI] [PubMed] [Google Scholar]
- 7.Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation 2006;114:1873–82. 10.1161/CIRCULATIONAHA.105.592063 [DOI] [PubMed] [Google Scholar]
- 8.Gournay V. The ductus arteriosus: physiology, regulation, and functional and congenital anomalies. Arch Cardiovasc Dis 2011;104:578–85. 10.1016/j.acvd.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 9.Mielke G, Benda N. Cardiac output and central distribution of blood flow in the human fetus. Circulation 2001;103:1662–8. 10.1161/01.CIR.103.12.1662 [DOI] [PubMed] [Google Scholar]
- 10.Menahem S. Administration of prostaglandin inhibitors to the mother; the potential risk to the fetus and neonate with duct-dependent circulation. Reprod Fertil Dev 1991;3:489–94. 10.1071/RD9910489 [DOI] [PubMed] [Google Scholar]
- 11.Shastri AT, Abdulkarim D, Clarke P. Maternal diclofenac medication in pregnancy causing in utero closure of the fetal ductus arteriosus and hydrops. Pediatr Cardiol 2013;34:1925–7. 10.1007/s00246-012-0461-y [DOI] [PubMed] [Google Scholar]
- 12.Zenker M, Klinge J, Kruger C et al. Severe pulmonary hypertension in a neonate caused by premature closure of the ductus arteriosus following maternal treatment with diclofenac: a case report. J Perinat Med 1998;26:231–4. [PubMed] [Google Scholar]
- 13.Mas C, Menahem S. Premature in utero closure of the ductus arteriosus following maternal ingestion of sodium diclofenac. Aust N Z J Obstet Gynaecol 1999;39:106–7. 10.1111/j.1479-828X.1999.tb03456.x [DOI] [PubMed] [Google Scholar]
- 14.Rein AJ, Nadjari M, Elchalal U et al. Contraction of the fetal ductus arteriosus induced by diclofenac. Case report. Fetal Diagn Ther 1999;14:24–5. doi:20882 [DOI] [PubMed] [Google Scholar]
- 15.Siu KL, Lee WH. Maternal diclofenac sodium ingestion and severe neonatal pulmonary hypertension. J Paediatr Child Health 2004;40:152–3. 10.1111/j.1440-1754.2004.00319.x [DOI] [PubMed] [Google Scholar]
- 16.Karadeniz C, Ozdemir R, Kurtulmus S et al. Diclofenac-induced intrauterine ductal closure. Fetal Diagn Ther 2013;34:133–4. 10.1159/000350979 [DOI] [PubMed] [Google Scholar]
- 17.Torloni MR, Cordioli E, Zamith MM et al. Reversible constriction of the fetal ductus arteriosus after maternal use of topical diclofenac and methyl salicylate. Ultrasound Obstet Gynecol 2006;27:227–9. 10.1002/uog.2647 [DOI] [PubMed] [Google Scholar]
- 18.Auer M, Brezinka C, Eller P et al. Prenatal diagnosis of intrauterine premature closure of the ductus arteriosus following maternal diclofenac application. Ultrasound Obstet Gynecol 2004;23:513–16. 10.1002/uog.1038 [DOI] [PubMed] [Google Scholar]
- 19.Vermillion ST, Scardo JA, Lashus AG et al. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol 1997;177:256–9; discussion 59–61 10.1016/S0002-9378(97)70184-4 [DOI] [PubMed] [Google Scholar]
- 20.Phadke V, Bhardwaj S, Sahoo B et al. Maternal ingestion of diclofenac leading to renal failure in newborns. Pediatr Nephrol 2012;27:1033–6. 10.1007/s00467-012-2114-z [DOI] [PubMed] [Google Scholar]
- 21.Benini D, Fanos V, Cuzzolin L et al. In utero exposure to nonsteroidal anti-inflammatory drugs: neonatal renal failure. Pediatr Nephrol 2004;19:232–4. 10.1007/s00467-003-1338-3 [DOI] [PubMed] [Google Scholar]
- 22.Østensen ME, Skomsvoll JF. Anti-inflammatory pharmacotherapy during pregnancy. Expert Opin Pharmacother 2004;5:571–80. 10.1517/14656566.5.3.571 [DOI] [PubMed] [Google Scholar]
- 23.Bloor M, Paech M. Nonsteroidal anti-inflammatory drugs during pregnancy and the initiation of lactation. Anesth Analg 2013;116:1063–75. 10.1213/ANE.0b013e31828a4b54 [DOI] [PubMed] [Google Scholar]
- 24.Hammers AL, Sanchez-Ramos L, Kaunitz AM. Antenatal exposure to indomethacin increases the risk of severe intraventricular hemorrhage, necrotizing enterocolitis, and periventricular leukomalacia: a systematic review with metaanalysis. Am J Obstet Gynecol 2015;212:505.e1–13. [DOI] [PubMed] [Google Scholar]
- 25.Amin SB, Sinkin RA, Glantz JC. Metaanalysis of the effect of antenatal indomethacin on neonatal outcomes. Am J Obstet Gynecol 2007;197:486.e1–10. 10.1016/j.ajog.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinebrant HE, Pileggi-Castro C, Romero CL et al. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Syst Rev 2015;6:CD001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas DM, Caldwell DM, Kirkpatrick P et al. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ 2012;345:e6226 10.1136/bmj.e6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huhta JC, Moise KJ, Fisher DJ et al. Detection and quantitation of constriction of the fetal ductus arteriosus by Doppler echocardiography. Circulation 1987;75:406–12. 10.1161/01.CIR.75.2.406 [DOI] [PubMed] [Google Scholar]
- 29.Hofstadler G, Tulzer G, Altmann R et al. Spontaneous closure of the human fetal ductus arteriosus—a cause of fetal congestive heart failure. Am J Obstet Gynecol 1996;174:879–83. 10.1016/S0002-9378(96)70317-4 [DOI] [PubMed] [Google Scholar]


