Abstract
Objectives:
The prevalence of obesity is rapidly increasing among Indian children, who, in general, are more prone to develop metabolic complications at an early age. Valproate and phenytoin are commonly used antiepileptic drugs in children. This study aimed to assess the parameters of the metabolic syndrome in Indian children with epilepsy on valproate or phenytoin monotherapy.
Methods:
This cross-sectional study recruited children from the Pediatric Epilepsy Clinic, Department of Pediatrics, Kalawati Saran Children Hospital, New Delhi from March 2012 to September 2012. All consecutive children diagnosed with epilepsy as per International League Against Epilepsy definition aged 3–18 years on valproate or phenytoin monotherapy for at least 6 months were enrolled at a tertiary care children's hospital in Northern India. After clinical and anthropometric evaluation (including body mass index [BMI] and waist circumference), the blood samples were analyzed for fasting serum glucose, total cholesterol, high-density lipoprotein-cholesterol, and serum triglyceride.
Results:
Children with BMI >95th centile and waist circumference >90th centile were not significantly different among children on valproate and phenytoin monotherapy. Children on valproate had significantly higher mean serum triglyceride (96.9 mg/dL vs. 77.6 mg/dL; P < 0.001) and total cholesterol (148.3 mg/dL vs. 132.8 mg/dL; P = 0.002) levels as compared to children on phenytoin monotherapy.
Conclusions:
The lipid abnormalities may be observed in children on valproate or phenytoin therapy and may warrant periodic screening.
Keywords: Cholesterol, metabolic syndrome, pediatric epilepsy, phenytoin, triglycerides, valproate
Introduction
Valproic acid is a broad-spectrum antiepileptic drug which is commonly used to treat many childhood epilepsies. It is a known enzyme inhibitor and may affect hepatic function. Valproate adversely affects the bone, lipid, and gonadal steroid metabolism. The mechanisms responsible for the lipid abnormalities observed on valproate treatment remain unclear. Although many studies have described the lipid abnormalities in children on valproate,[1,2,3,4,5,6,7,8,9,10] the results are conflicting with regard to the variable trends observed in the lipid parameters. The lipid abnormalities with phenytoin have been described infrequently.[11,12]
This study aimed to describe the parameters of the metabolic syndrome observed in Indian children with epilepsy on valproate monotherapy and to compare them with those observed in children on phenytoin monotherapy.
Methods
This cross-sectional study recruited children from the Pediatric Epilepsy Clinic of a Tertiary Care Pediatric Hospital in North India from March 2012 to September 2012. All consecutive children diagnosed with Epilepsy as per International League Against Epilepsy definition[13,14] aged 3–18 years on valproate or phenytoin monotherapy for at least 6 months were enrolled. The children who were on drugs which may alter the lipid profile or blood glucose such as steroids, insulin, and statins; children having thyroid disorder or other endocrinopathies, chronic liver, heart or renal disease, progressive neurological or psychiatric illness and those whose guardians refused consent were excluded. The ethical approval was taken from the Institute's Ethics Committee.
The study population was divided into two groups: Group I-children on valproate monotherapy and Group II-children on phenytoin monotherapy.
After obtaining the written and informed consent from the guardian of the children, a clinical evaluation was performed as per a predesigned proforma. The information on the age, sex, type of seizures, duration of the antiepileptic drug monotherapy, and dose of the antiepileptic drug was collected followed by a detailed systemic examination.
Anthropometric data were obtained for each child. Standing height (cm) was measured to the nearest 0.1 cm with a standard calibrated stadiometer, and the body weight (kg) was noted on a standard weighing scale to the nearest 0.1 kg with children dressed in minimal clothing. The body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m). The waist circumference was noted midway between the pubic symphysis and the xiphisternum using a measuring tape for each child. Obesity was defined as BMI >95th percentile for age and sex according to the standard WHO growth charts or waist circumference ≥90th percentile for age and gender.[15] The blood pressure was obtained from the right arm using an appropriate size cuff with the child in sitting position and three readings were taken to avoid error. They were then averaged. The age-, sex-, and height-specific percentile for BP were calculated according to WHO reference data.[16]
A blood sample (2 ml) was drawn after an overnight fast of at least 10 h for serum glucose, total cholesterol, high-density lipoprotein (HDL)-cholesterol, and serum triglyceride estimation. Serum samples were kept at −20°C until analyzed using modular biochemistry analyzer using the protocol described by the manufacturer. The laboratory personnel was blinded to the group of the enrolled study participant.
Though there is a lack of a single universally acceptable definition of metabolic syndrome in children, we have used guidelines from International Diabetes Federation and American Heart Association to interpret our observations.[17,18] Abdominal obesity was defined as BMI ≥95th percentile for age and gender or waist circumference ≥90th percentile for age and gender; hypertension as systolic/diastolic blood pressure ≥95th percentile adjusted for age, sex, and height; fasting glucose >100 mg/dL was considered impaired, and high serum triglyceride was defined as >90th percentile for age.
Statistical analysis
Descriptive statistics were used to report the baseline characteristics and various laboratory parameters. The continuous data were compared between the two groups using t-test for normal data and Rank-Sum test for the skewed data. The qualitative data were compared using Chi-square test or Fischer's exact test. P < 0.05 was considered statistically significant. The analysis was done using StataCorp, 4905 Lakeway Drive, College Station, Texas 77845 USA which is a standard analytical software.
Results
One hundred and seventeen children were screened for enrollment. Seven children were excluded as the parents refused consent. Thus, 110 children were subsequently analyzed; 57 children on valproate monotherapy (Group 1) and 53 on phenytoin monotherapy (Group 2). The baseline characteristics of the two groups are shown in Table 1.
Table 1.
Baseline characteristics of the study participants
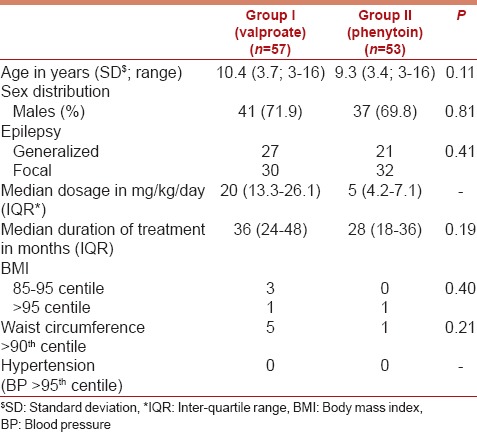
One in each group had BMI >95th centile. Five children on valproate monotherapy and one child on phenytoin monotherapy had waist circumference >90th centile (P = 0.21). None of the children was found to have hypertension in either group.
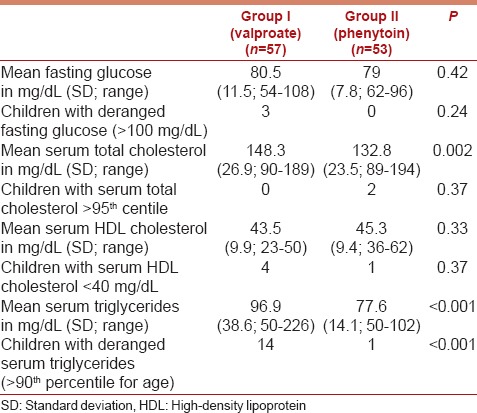
Children on valproate had significantly higher mean serum triglyceride (P < 0.001) and total cholesterol (P = 0.002) levels as compared to children on phenytoin monotherapy [Table 2]. Fourteen patients (24.6%) on valproate had deranged serum triglycerides while only one child on phenytoin monotherapy showed elevated serum triglycerides. The mean serum HDL-cholesterol (P = 0.33) and fasting glucose (P = 0.42) were similar in the two groups.
Table 2.
Distribution of the laboratory parameters in the study participants in the two groups
Discussion
Rapidly changing dietary practices accompanied by an increasingly sedentary lifestyle predispose to nutrition-related noncommunicable diseases, including childhood obesity.[19] Obesity is the important forerunner of insulin resistance and metabolic syndrome and their cardiovascular complications.[20] The rates of atherogenic dyslipidemia, glucose intolerance, thrombotic tendency, subclinical inflammation, and endothelial dysfunction are higher in Asians. Many of these manifestations are more severe even at an early age in South Asians than white Caucasians. Metabolic and cardiovascular risks in South Asians are also heightened by their higher body fat, truncal subcutaneous fat, intra-abdominal fat, and ectopic fat deposition (liver fat, muscle fat, etc.). Further, cardiovascular risk cluster manifests at a lower level of adiposity and abdominal obesity.[21] Hence, Indian children are likely to be vulnerable to any potential factor unfavorably affecting their metabolic status.
Over the last 5 years, reports from several developing countries indicate prevalence rates of obesity (inclusive of overweight) >15% in children and adolescents aged 5-19 years; Mexico 41.8%, Brazil 22.1%, and India 22.0%. Childhood obesity tracks into adulthood, thus increasing the risk for conditions like the metabolic syndrome, type 2 diabetes mellitus, polycystic ovarian syndrome, hypertension, dyslipidemia, and coronary artery disease later in life.[19] One among every 25 children in India satisfies the complete definition of metabolic syndrome.[20] These facts prompted us to make an effort to clarify the status of commonly used agents like valproate and phenytoin with regard to their potential to adversely affect the metabolic status of children.
Although the effects of cytochrome-P450 enzyme-inducing antiepileptics such as phenobarbital and carbamazepine, on lipid levels have been well-studied and are consistent, the effect of valproic acid and phenytoin is still unclear. This cross-sectional study showed that children with epilepsy on long-term valproate monotherapy showed significantly higher mean serum cholesterol and triglyceride levels among Indian children.
The mechanisms responsible for the lipid abnormalities observed on valproate treatment remain unknown. The suggested mechanisms include valproate-induced hyperinsulinemia leading to insulin resistance[22] and hyperleptinemia leading to leptin resistance.[4,23] Insulin and leptin act together to balance the food intake and energy expenditure. Insulin has a lipogenic effect. It stimulates fatty acid and triacylglycerol synthesis and suppresses fatty acid oxidation.[24,25] Leptin has a lipolytic effect. It antagonizes insulin action.[26]
The results of previous studies exploring the effects of valproate on lipid metabolism are inconsistent. The studies have shown either increased,[7] decreased,[1,2,4,5,9] or no change[3,8,27] in the levels of serum cholesterol or triglycerides in children on valproate monotherapy.
Our study showed significantly higher serum cholesterol and triglycerides in children on valproate monotherapy after a median of 36 months of treatment. The majority of the previous studies reported results after 12 months of valproate therapy. Verrotti et al.[4] and Tekgul et al.[9] reported lipid abnormalities after at least 2.5 years and 2 years, respectively. Both studies, however, showed lower serum cholesterol[4] and triglycerides[4,9] at the end of the study periods. These results are conflicting with our results.
The development of metabolic syndrome has been described in adults on valproate monotherapy.[28,29] Obese patients treated with valproate may have a higher risk of developing metabolic syndrome.[29] The definition of metabolic syndrome in children is still evolving. Verrotti et al.[30] reported metabolic syndrome in 46.5% obese older children on valproate monotherapy.
There is a paucity of studies exploring the effects of phenytoin on lipid profiles in children. Dewan et al.[11] reported higher total cholesterol in children on phenytoin when compared to valproate and controls.
The limitations of the cross-sectional study included small sample size, unavailability of the baseline lipid profiles and anthropometric parameters, lack of serial measurements, lack of measurement of other lipid components, insulin, parameters of insulin resistance and leptin, and absence of standard definitions for pediatric metabolic syndrome. There was also the unavailability of dietary habits of the enrolled children. However, this study showed higher levels of cholesterol and triglycerides in children on long-term valproate monotherapy and explored the prevalence of metabolic syndrome in this population. Better designed cohort studies are required to ascertain the lipid abnormalities seen in children on valproate or phenytoin, and the underlying mechanisms and the long-term cardiovascular risk imposed.
Thus, the lipid abnormalities may be encountered in children on valproate or phenytoin therapy. Periodic screening and counseling for lifestyle modifications may be warranted. Their use should be cautious in those with preexisting risk factors for metabolic syndrome such as family history, obesity, dyslipidemia, hypertension, or insulin resistance. Whenever used, the children should get periodic screening for cardio-metabolic risk factors. The protocol for initial and periodic screening for metabolic derangements in children on long-term valproate or phenytoin therapy is the area for further research proposed by our observations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Eirís JM, Lojo S, Del Río MC, Novo I, Bravo M, Pavón P, et al. Effects of long-term treatment with antiepileptic drugs on serum lipid levels in children with epilepsy. Neurology. 1995;45:1155–7. doi: 10.1212/wnl.45.6.1155. [DOI] [PubMed] [Google Scholar]
- 2.Eirís J, Novo-Rodríguez MI, Del Río M, Meseguer P, Del Río MC, Castro-Gago M. The effects on lipid and apolipoprotein serum levels of long-term carbamazepine, valproic acid and phenobarbital therapy in children with epilepsy. Epilepsy Res. 2000;41:1–7. doi: 10.1016/s0920-1211(00)00119-4. [DOI] [PubMed] [Google Scholar]
- 3.Demircioglu S, Soylu A, Dirik E. Carbamazepine and valproic acid: Effects on the serum lipids and liver functions in children. Pediatr Neurol. 2000;23:142–6. doi: 10.1016/s0887-8994(00)00175-2. [DOI] [PubMed] [Google Scholar]
- 4.Verrotti A, Domizio S, Angelozzi B, Sabatino G, Morgese G, Chiarelli F. Changes in serum lipids and lipoproteins in epileptic children treated with anticonvulsants. J Paediatr Child Health. 1997;33:242–5. doi: 10.1111/j.1440-1754.1997.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 5.Voudris KA, Attilakos A, Katsarou E, Drakatos A, Dimou S, Mastroyianni S, et al. Early and persistent increase in serum lipoprotein (a) concentrations in epileptic children treated with carbamazepine and sodium valproate monotherapy. Epilepsy Res. 2006;70:211–7. doi: 10.1016/j.eplepsyres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Rauchenzauner M, Haberlandt E, Scholl-Bürgi S, Karall D, Schoenherr E, Tatarczyk T, et al. Effect of valproic acid treatment on body composition, leptin and the soluble leptin receptor in epileptic children. Epilepsy Res. 2008;80:142–9. doi: 10.1016/j.eplepsyres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Abaci A, Saygi M, Yis U, Demir K, Dirik E, Bober E. Metabolic alterations during valproic acid treatment: A prospective study. Pediatr Neurol. 2009;41:435–9. doi: 10.1016/j.pediatrneurol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz E, Dosan Y, Gürgöze MK, Güngör S. Serum lipid changes during anticonvulsive treatment serum lipids in epileptic children. Acta Neurol Belg. 2001;101:217–20. [PubMed] [Google Scholar]
- 9.Tekgul H, Demir N, Gokben S. Serum lipid profile in children receiving anti-epileptic drug monotherapy: Is it atherogenic? J Pediatr Endocrinol Metab. 2006;19:1151–5. doi: 10.1515/jpem.2006.19.9.1151. [DOI] [PubMed] [Google Scholar]
- 10.Hamed SA, Fida NM, Hamed EA. States of serum leptin and insulin in children with epilepsy: Risk predictors of weight gain. Eur J Paediatr Neurol. 2009;13:261–8. doi: 10.1016/j.ejpn.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Dewan P, Aggarwal A, Faridi MM. Effect of phenytoin and valproic acid therapy on serum lipid levels and liver function tests. Indian Pediatr. 2008;45:855–8. [PubMed] [Google Scholar]
- 12.Phabphal K, Geater A, Limapichart K, Sathirapanya P, Setthawatcharawanich S. Role of CYP2C9 polymorphism in phenytoin-related metabolic abnormalities and subclinical atherosclerosis in young adult epileptic patients. Seizure. 2013;22:103–8. doi: 10.1016/j.seizure.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 14.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE commission on classification and terminology, 2005-2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuriyan R, Thomas T, Lokesh DP, Sheth NR, Mahendra A, Joy R, et al. Waist circumference and waist for height percentiles in urban South Indian children aged 3-16 years. Indian Pediatr. 2011;48:765–71. doi: 10.1007/s13312-011-0126-6. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Li C. Defining the metabolic syndrome in children and adolescents: Will the real definition please stand up? J Pediatr. 2008;152:160–4. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 17.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents – An IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 18.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 19.Andrabi SM, Bhat MH, Andrabi SR, Kamili MM, Imran A, Nisar I, et al. Prevalence of metabolic syndrome in 8-18-year-old school-going children of Srinagar city of Kashmir India. Indian J Endocrinol Metab. 2013;17:95–100. doi: 10.4103/2230-8210.107812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N, Shah P, Nayyar S, Misra A. Childhood obesity and the metabolic syndrome in developing countries. Indian J Pediatr. 2013;80(Suppl 1):S28–37. doi: 10.1007/s12098-012-0923-5. [DOI] [PubMed] [Google Scholar]
- 21.Misra A, Bhardwaj S. Obesity and the metabolic syndrome in developing countries: Focus on South Asians. Nestle Nutr Inst Workshop Ser. 2014;78:133–40. doi: 10.1159/000354952. [DOI] [PubMed] [Google Scholar]
- 22.Luef GJ, Lechleitner M, Bauer G, Trinka E, Hengster P. Valproic acid modulates islet cell insulin secretion: a possible mechanism of weight gain in epilepsy patients. Epilepsy Res. 2003;55:53–8. doi: 10.1016/s0920-1211(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 23.Greco R, Latini G, Chiarelli F, Iannetti P, Verrotti A. Leptin, ghrelin, and adiponectin in epileptic patients treated with valproic acid. Neurology. 2005;65:1808–9. doi: 10.1212/01.wnl.0000187074.27586.d1. [DOI] [PubMed] [Google Scholar]
- 24.Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, Zhang Y, et al. Leptin expression in adipose tissue from obese humans: Depot-specific regulation by insulin and dexamethasone. Am J Physiol. 1998;275(3 Pt 1):E507–15. doi: 10.1152/ajpendo.1998.275.3.E507. [DOI] [PubMed] [Google Scholar]
- 25.Bai Y, Zhang S, Kim KS, Lee JK, Kim KH. Obese gene expression alters the ability of 30A5 preadipocytes to respond to lipogenic hormones. J Biol Chem. 1996;271:13939–42. doi: 10.1074/jbc.271.24.13939. [DOI] [PubMed] [Google Scholar]
- 26.Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145:2613–20. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- 27.Sonmez FM, Demir E, Orem A, Yildirmis S, Orhan F, Aslan A, et al. Effect of antiepileptic drugs on plasma lipids, lipoprotein (a), and liver enzymes. J Child Neurol. 2006;21:70–4. doi: 10.1177/08830738060210011301. [DOI] [PubMed] [Google Scholar]
- 28.Pylvänen V, Pakarinen A, Knip M, Isojärvi J. Insulin-related metabolic changes during treatment with valproate in patients with epilepsy. Epilepsy Behav. 2006;8:643–8. doi: 10.1016/j.yebeh.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Fang J, Chen S, Tong N, Chen L, An D, Mu J, et al. Metabolic syndrome among Chinese obese patients with epilepsy on sodium valproate. Seizure. 2012;21:578–82. doi: 10.1016/j.seizure.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Verrotti A, Manco R, Agostinelli S, Coppola G, Chiarelli F. The metabolic syndrome in overweight epileptic patients treated with valproic acid. Epilepsia. 2010;51:268–73. doi: 10.1111/j.1528-1167.2009.02206.x. [DOI] [PubMed] [Google Scholar]


