Abstract
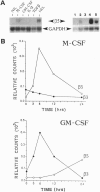
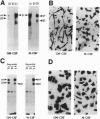
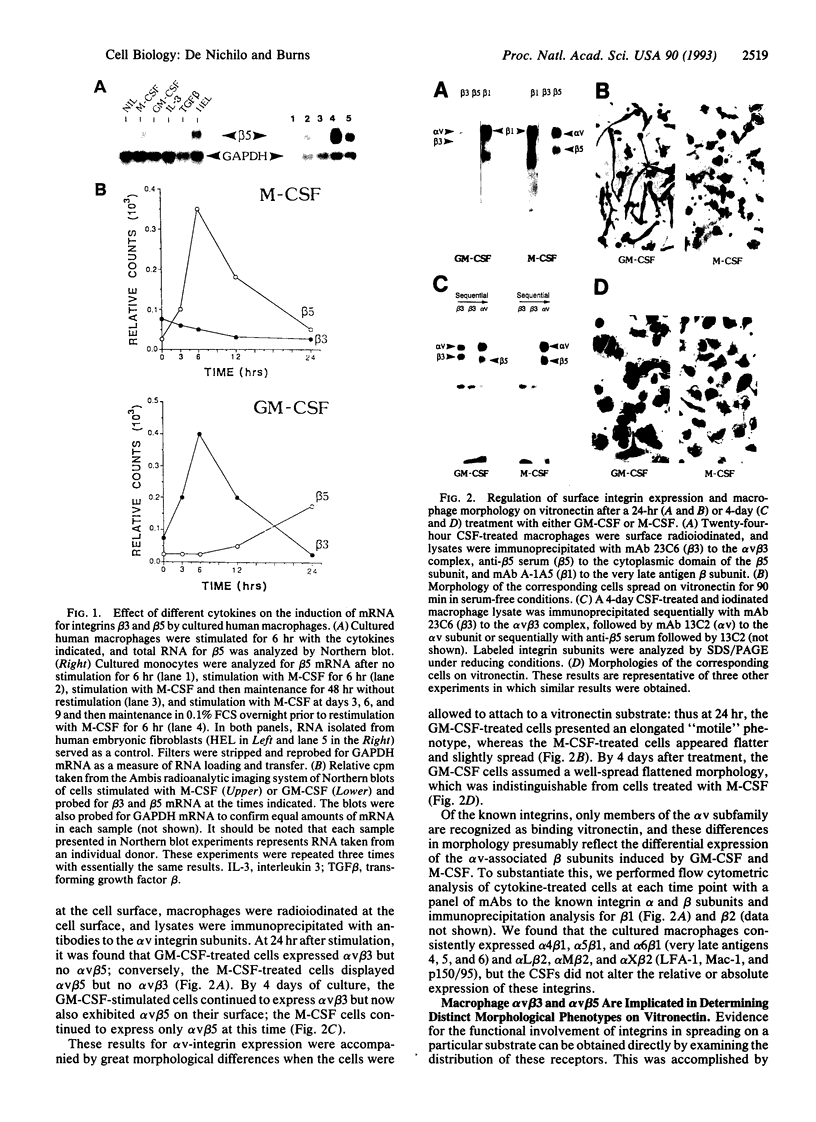
The colony-stimulating factors (CSFs) greatly influence mature macrophage function in vitro: macrophage (M)-CSF induces maturation of monocytes and enhances differentiated cell function; granulocyte-macrophage (GM)-CSF stimulates a variety of antimicrobial functions. In vivo M-CSF is thought to promote differentiation, and GM-CSF is thought to potentiate the inflammatory response. One mechanism by which these differential effects may be achieved is through the receptor-mediated interaction of macrophages with their extracellular matrix. Here we show that M-CSF induces specifically the expression of the alpha v beta 5 integrin receptor, whereas GM-CSF rapidly induces mRNA and surface expression of the alpha v beta 3 integrin. The M-CSF-treated cells acquire a flattened epitheloid phenotype, and on vitronectin the alpha v beta 5 is located in adhesion plaques. These cells do not bind collagen or laminin. In contrast, cells treated with GM-CSF adopt an elongated phenotype on a number of substrates, including collagen and laminin, and express alpha v beta 3 at the leading edge of cells on vitronectin. These results suggest that a primary means by which the CSFs exert their individual effects on mature cells may be through regulating integrin expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Cytokines in chronic inflammatory arthritis. IV. Granulocyte/macrophage colony-stimulating factor-mediated induction of class II MHC antigen on human monocytes: a possible role in rheumatoid arthritis. J Exp Med. 1989 Sep 1;170(3):865–875. doi: 10.1084/jem.170.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R. C., Rankin L. M., Lucas C. M., Scott J. L., Krissansen G. W., Burns G. F. Individual embryonic fibroblasts express multiple beta chains in association with the alpha v integrin subunit. Loss of beta 3 expression with cell confluence. J Biol Chem. 1991 Oct 5;266(28):18593–18599. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Mauray F., Kramer R. H. Beta 1 and beta 3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp Cell Res. 1992 Jun;200(2):272–284. doi: 10.1016/0014-4827(92)90173-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNichilo M. O., Stewart A. G., Vadas M. A., Lopez A. F. Granulocyte-macrophage colony-stimulating factor is a stimulant of platelet-activating factor and superoxide anion generation by human neutrophils. J Biol Chem. 1991 Mar 15;266(8):4896–4902. [PubMed] [Google Scholar]
- Dedhar S., Mitchell M. D., Pierschbacher M. D. The osteoblast-like differentiated phenotype of a variant of MG-63 osteosarcoma cell line correlated with altered adhesive properties. Connect Tissue Res. 1989;20(1-4):49–61. doi: 10.3109/03008208909023874. [DOI] [PubMed] [Google Scholar]
- Dedhar S. Regulation of expression of the cell adhesion receptors, integrins, by recombinant human interleukin-1 beta in human osteosarcoma cells: inhibition of cell proliferation and stimulation of alkaline phosphatase activity. J Cell Physiol. 1989 Feb;138(2):291–299. doi: 10.1002/jcp.1041380210. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Cleland L. G., Gamble J. R., Lopez A. F. IL-3 and granulocyte-macrophage colony-stimulating factor stimulate two distinct phases of adhesion in human monocytes. J Immunol. 1990 Jul 1;145(1):167–176. [PubMed] [Google Scholar]
- Gamble J. R., Elliott M. J., Jaipargas E., Lopez A. F., Vadas M. A. Regulation of human monocyte adherence by granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7169–7173. doi: 10.1073/pnas.86.18.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Veis N., Bordun A. M., Vairo G., Gonda T. J., Phillips W. A. Activation and proliferation signals in murine macrophages: relationships among c-fos and c-myc expression, phosphoinositide hydrolysis, superoxide formation, and DNA synthesis. J Cell Physiol. 1989 Dec;141(3):618–626. doi: 10.1002/jcp.1041410321. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Horiguchi J., Warren M. K., Kufe D. Expression of the macrophage-specific colony-stimulating factor in human monocytes treated with granulocyte-macrophage colony-stimulating factor. Blood. 1987 Apr;69(4):1259–1261. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Krissansen G. W., Elliott M. J., Lucas C. M., Stomski F. C., Berndt M. C., Cheresh D. A., Lopez A. F., Burns G. F. Identification of a novel integrin beta subunit expressed on cultured monocytes (macrophages). Evidence that one alpha subunit can associate with multiple beta subunits. J Biol Chem. 1990 Jan 15;265(2):823–830. [PubMed] [Google Scholar]
- Krissansen G. W., Lucas C. M., Stomski F. C., Elliott M. J., Berndt M. C., Boyd A. W., Horton M. A., Cheresh D. A., Vadas M. A., Burns G. F. Blood leukocytes bind platelet glycoprotein (IIb-IIIa)' but do not express the vitronectin receptor. Int Immunol. 1990;2(3):267–277. doi: 10.1093/intimm/2.3.267. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavesley D. I., Ferguson G. D., Wayner E. A., Cheresh D. A. Requirement of the integrin beta 3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992 Jun;117(5):1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Moore J. G. Divergent disease patterns in granulocyte-macrophage colony-stimulating factor transgenic mice associated with different transgene insertion sites. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7767–7771. doi: 10.1073/pnas.85.20.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Nicola N. A. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Ramaswamy H., Hemler M. E. Cloning, primary structure and properties of a novel human integrin beta subunit. EMBO J. 1990 May;9(5):1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Gehlsen K. R., Stetler-Stevenson W. G., Brown P. D., Ruoslahti E., Hendrix M. J. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D., Cohen D. S., Wang A., Busk M. Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem. 1992 Aug 25;267(24):17409–17414. [PubMed] [Google Scholar]
- Sherr C. J. Colony-stimulating factor-1 receptor. Blood. 1990 Jan 1;75(1):1–12. [PubMed] [Google Scholar]
- Streuli C. H., Bissell M. J. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990 Apr;110(4):1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Huang Z. S., Tanihara H. Cloning of an integrin beta subunit exhibiting high homology with integrin beta 3 subunit. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5354–5358. doi: 10.1073/pnas.87.14.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Vairo G., Cocks B. G., Cragoe E. J., Jr, Hamilton J. A. Selective suppression of growth factor-induced cell cycle gene expression by Na+/H+ antiport inhibitors. J Biol Chem. 1992 Sep 25;267(27):19043–19046. [PubMed] [Google Scholar]
- Vallés A. M., Tucker G. C., Thiery J. P., Boyer B. Alternative patterns of mitogenesis and cell scattering induced by acidic FGF as a function of cell density in a rat bladder carcinoma cell line. Cell Regul. 1990 Dec;1(13):975–988. doi: 10.1091/mbc.1.13.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Friedman H., Djeu J. Y. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J Immunol. 1989 Jul 15;143(2):671–677. [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- Young D. A., Lowe L. D., Clark S. C. Comparison of the effects of IL-3, granulocyte-macrophage colony-stimulating factor, and macrophage colony-stimulating factor in supporting monocyte differentiation in culture. Analysis of macrophage antibody-dependent cellular cytotoxicity. J Immunol. 1990 Jul 15;145(2):607–615. [PubMed] [Google Scholar]
- Yurochko A. D., Liu D. Y., Eierman D., Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]