Abstract
Introduction:
Oral submucous fibrosis (OSMF) is a precancerous condition predominantly seen in people of Asian descent. About 7–12% OSMF patients develop oral squamous cell carcinoma (OSCC). Morphological features of OSMF especially fibrosis suggests a possibility of the hypoxic environment in diseased tissues. Oral cancer usually develops from hyperplasia through dysplasia to carcinoma. Neovascularization and increased glycolysis, represent adaptations to a hypoxic microenvironment that are correlated with tumor invasion and metastasis. The adaptation of cells to hypoxia appears to be mediated via hypoxia inducible factor-1α (HIF-1α). HIF-1α is said to be associated with malignant transformation of epithelium in other sites. It appears that HIF-1α plays a significant role in both prostate and cervical carcinogenesis at early stages. We hypothesize that progression of OSMF and malignant transformation in the background of fibrosis mediates via HIF-1α either by up- or down-regulation of various such molecules. Therefore, the main objective of this study was to investigate the relationship between the expression of HIF-1α in OSMF, OSCC and OSCC with OSMF.
Aim:
To investigate the relationship between the expression of HIF-1α in OSMF, OSCC and OSCC with OSMF.
Materials and Methods:
The study group consists of histopathologically diagnosed 20 cases of OSCC, oral submucous fibrosis and OSCC with OSMF each. The immunohistochemistry was carried out on neutral buffered formalin-fixed paraffin-embedded tissue sections by using the monoclonal antibody of HIF-1α.
Results:
A rise in the expression of HIF-1α from OSMF to OSCC to OSCC with OSMF is observed.
Keywords: Angiogenesis, hypoxia inducible factor-1α, oral squamous cell carcinoma, oral submucous fibrosis
INTRODUCTION
Oral submucous fibrosis (OSMF) is a chronic, insidious and progressive oral mucosal disease that primarily affects any part of the oral cavity. It is characterized by a juxta-epithelial inflammatory reaction followed by progressive fibrosis of the lamina propria and the underlying submucosal layer, with associated epithelial atrophy. Although the etiology of OSF is obscure, evidence has shown that it is a precancerous disorder related to the habit of chewing areca nut, either alone or as a component of betel quid.[1] OSF carries a high risk of transition to oral cancer. In an epidemiologic study in India, the malignant transformation rate was 7.6% to 12 over a period of 17 years.[2]
Cancer induction is a multi-stage, multi-step process and includes multiple molecular and cellular events to transform a normal cell into a malignant neoplastic cell. However, three general steps can be identified in carcinogenesis; initiation, promotion and progression.[3]
Recently it has been proposed that oral cancers arising in OSF constitute a clinicpathologically distinct disease, the differences of which is believed to arise from differential mechanisms of areca nut carcinogenesis. A study recognized that most of these patients are younger males with better prognostic factors such as better grade of tumor differentiation, a lesser incidence of nodal metastases and extracapsular spread.[4] Another retrospective study has reported contradictory data. They state that oral squamous cell carcinoma (OSCC) can originate from OSMF and is clinically more invasive and also exhibits higher metastasis and recurrence rate than OSCC that has not originated from OSF.[5]
We hypothesize that progression of OSMF and malignant transformation in the background of fibrosis mediates via hypoxia inducible factor-1α (HIF-1α) either by up- or down-regulation of various such molecules.

Thus, this study aims at evaluating and comparing the expression of HIF-1α and mean blood vessel density in OSMF, OSCC and OSCC with OSMF patients.
MATERIALS AND METHODS
Twenty blocks of formalin-fixed and paraffin-embedded tissues of each OSMF, OSCC and OSCC with OSMF cases were randomly selected from the archives of the Department of Oral Pathology and Microbiology. All 60 cases and 10 normal oral mucosal biopsies were used to investigate the expression levels of HIF-1α by immunohistochemistry. Ethical approval and informed consent from patients were obtained for this study.
Grading and stage of the disease
Routine hematoxylin and eosin stained sections were prepared from all the cases. The histological grading of OSMF was carried out. Also, the squamous cell carcinomas were graded as well, moderate and poor according to the Broder's and Bryne's grading system[6,7,8] [Figure 1].
Figure 1.
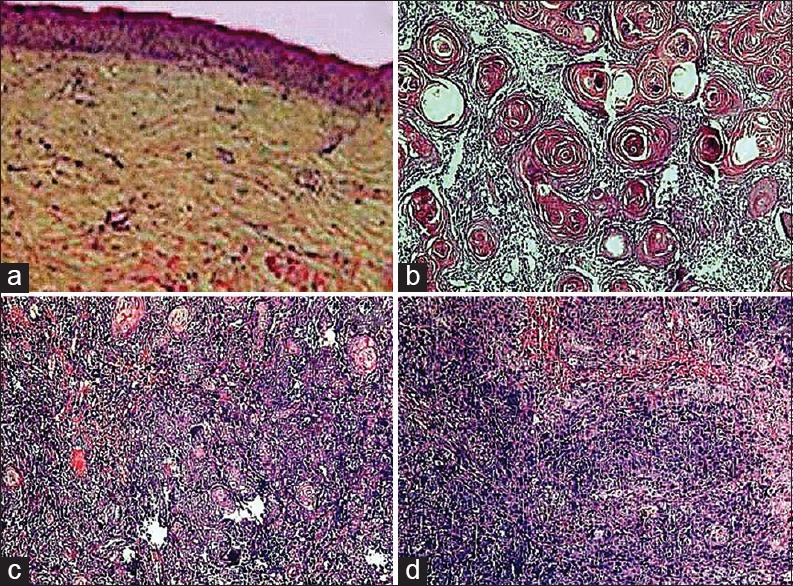
Photomicrographs of tissue sections for histopathological diagnosis (a) OSMF (Van-Gieson stain, x40), (b) Well differentiated squamous cell carcinoma (H&E stain, x100), (c) Moderately differentiated squamous cell carcinoma (H&E stain, x40), (d) Poorly differentiated squamous cell carcinoma (H&E stain, x40)
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections were cut to 5 μm thickness. Silane coated slides were used for the proper adherence of tissue sections to the glass slides. The immunohistochemical staining for HIF-1α using Universal Immuno-Enzyme Polymer Technique was carried out in the Department of Oral Pathology and Microbiology. Sections were hydrated with increased grades of alcohol and brought to distilled water and treated with hydrogen peroxide to eliminate endogenous peroxidase activity. Then antigen retrieval with tri-sodium citrate for HIF-1α was carried out. The tissue was incubated sequentially with:
Primary antibodies, that is, HIF-1α
DAKO Envision system HRP labeled polymer detection system
3,3 Diaminobenzidine substrate solution – This results in formation of a colored precipitate at the tissue antigen sites. Visualization was aided by counterstaining with hematoxylin.
Assessment of the hypoxia inducible factor-1α staining intensity
Section was considered either as negative or positive according to the absence or presence of brown staining in epithelial or stromal cells. The positive cases were graded into three categories depending on the intensity of staining. Homogenous dark brown staining was considered as strong (+++) and light faint staining was considered as mild (+) and cases in between the two extremes were graded into the moderate (++) category[2] [Figure 2].
Figure 2.
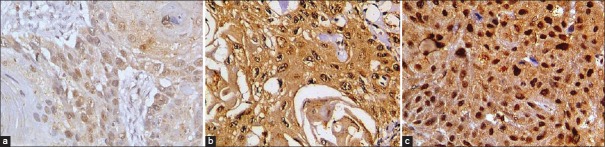
Tissue sections stained for hypoxia inducible factor-1α showing grading (a) mild, (b) moderate, (c) severe (IHC stain, x400)
Assessment of mean blood vessel density
The most intense vascular area was found by light microscope in Ö40. Five such fields were selected under Ö40 for each slide, with slide moving in clockwise direction. The area representing vascular tissue in the three digital images were imported to image analysis software and the area counted. This indicated vascular tissue area. The area representing total tissue in the three digital images were imported to image analysis software and the area counted. This indicated total tissue area. Mean vessel density (MVD): The ratio of the vascular tissue area to total tissue area in the digital images imported to image analyzer software.
The number of blood vessels was counted in five high-power fields using the “HOT SPOT” method. The hotspot areas in the tumor tissues are those that show the greatest microvessel density as defined by Weidner in 1991. Briefly, microvessel density was determined by light microscopy in areas of invasive tumor containing the highest numbers of capillaries and small venules (microvessels) per area (i.e., areas of the most intense vascularization). We found these vascular “hotspots” by scanning the tumor sections at low power (×40 and ×100) and identifying those areas of invasive carcinoma having the greatest numbers of distinct HIF staining microvessels per area [Figure 3]. The hotspots could occur anywhere within an invasive rumor but most frequently appeared at the margins of the carcinoma.[9] Then the mean was calculated as:
Figure 3.
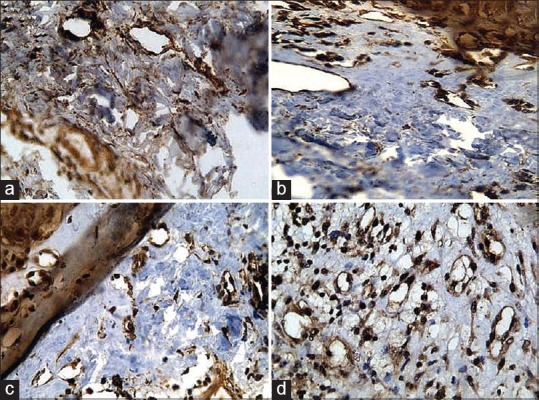
Tissue sections showing various levels of mean vessel density (a) 4–5/High power field, (b) 7–8/High power field, (c) 10–12/High power field, (d) 15–17/High power field (IHC stain, x100)
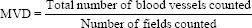
Statistical analysis
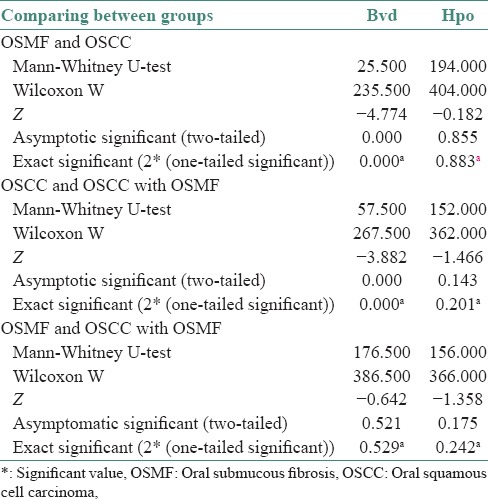
The correlation between MVD and HIF-1α staining was established using the Mann–Whitney test in all the three study groups. The intergroup correlation was also established.[10]
RESULTS
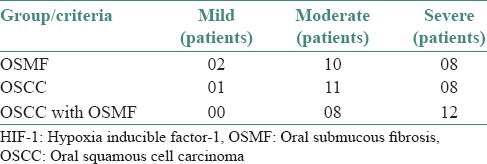
On comparing HIF-1α expression in OSMF, OSCC and OSCC with OSMF, a gradual increase was found in its intensity [Table 1].
Table 1.
Level of HIF-1 staining intensity in all the three groups
The blood vessel density was found to be the highest in OSCC with OSMF, intermediate for OSCC and the lowest for OSMF [Table 2]. The HIF-1α expression and blood vessel density were positively correlated in all the three groups that are OSMF, OSCC and OSCC with OSMF [Table 3].
Table 2.
The mean blood vessel density found in various groups as defined by the HIF-1 staining intensity
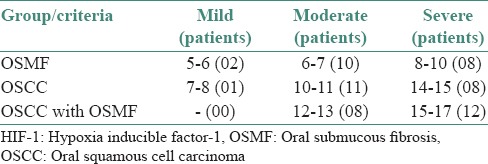
Table 3.
Results of Mann-Whitney U-test
DISCUSSION
OSMF is histopathologically characterized by fibrosis of subepithelial connective tissue. Collagens are the major structural component of extracellular matrix, hence precise regulation of collagen metabolism is essential to maintain the normal integrity of connective tissue.[5] With the progression of the disease process of OSMF, the production of collagen type 1 is increased and the degradation of collagen is reduced by up to 75%. Extensive fibrosis of the connective tissue causes reduction of vascularity, resulting in subsequent hypoxia in both fibroblasts and surface epithelium. Hypoxia causes atrophy and ulceration of the epithelium by inducing apoptosis. In addition, the overexpression of hypoxia-induced factor-1α is seen in OSMF, which indicates changes in cell proliferation, maturation and metabolic adaptation, increasing the possibility of malignant transformation.[11] The cellular response to hypoxic stress is controlled by a family of prolyl hydroxylases (PHD). In the presence of adequate oxygen, PHDs hydroxylate HIF-1α at conserved proline residues within the oxygen-dependent degradation domain. Once hydroxylated, HIF-1α becomes a substrate for von Hippel-Lindau-mediated ubiquitination and degradation. Upon degradation, HIF-1 becomes inactive and the cells lacking oxygen supply are unable to survive and undergo apoptosis. However, under hypoxic conditions as in OSMF, PHDs are inactive and HIF-1α is stabilized and on stabilization it translocates to the nucleus where it forms the functional transcription factor HIF-1. The functional HIF-1 helps in cell survival under hypoxia and thus helps cell proliferation promoting tumorigenesis.[12] HIF-1 is rarely expressed in the normal oral mucosa. However, a significant HIF-1 expression was found in OSMF in the basal and suprabasal layers of epithelium. This indicates the role of hypoxia in malignant transformation of OSMF. Thus, the upregulation of HIF-1α is an early event in carcinogenesis. The rise in the HIF-1 in the absence of epithelial proliferation can be attributed to this pathway.
Physiologists and clinicians define hypoxia as a state of reduced O2 availability or decreased O2 partial pressures below critical thresholds, thus restricting or even abolishing the function of organs, tissues or cells. In solid tumors, oxygen delivery to the respiring neoplastic and stromal cells is frequently reduced or even abolished by deteriorating diffusion geometry, severe structural abnormalities of tumor microvessels and disturbed microcirculation.[13] Development of hypoxic microenvironment is caused by the imbalance between oxygen consumption and oxygen delivery. The rapidly proliferating head and neck squamous cell carcinoma has insufficient vascularization with poor blood supply. Limiting blood supply network in the rapidly proliferating tumor region limits oxygen diffusion, resulting in the development of the hypoxic region.[14] These areas with very low (down to zero) oxygen partial pressures exist in solid tumors, occurring either acutely or chronically. These microregions are heterogeneously distributed within the tumor mass and may be located adjacent to regions with normal O2 partial pressures.[13] The hypoxic stress stimulates solid tumor to upregulate expression of a variety of oncogenes such as HIF and vascular endothelial growth factor, which enhance irregular vascular endothelial cell proliferation and differentiation.[14]
The hypoxic and anaerobic conditions found at the center of the solid tumor would require cells present there to rely on glycolysis. As the O2 levels decreased, the generation of adenosine triphosphate (ATP) shifted from oxidative phosphorylation (OXPHOS) to glycolysis (Pasteur effect). OXPHOS is more efficient in generating ATP than glycolysis; the oxidation of one molecule of glucose gives a net yield of 30 ATPs via OXPHOS and 2 ATPs via glycolysis. It is comprehensible, therefore, that cells generate ATP through OXPHOS when they have enough O2 levels.[15]
A hallmark of malignant tumors is the elevated uptake of glucose even under normal oxygen conditions, known as aerobic glycolysis or the “Warburg effect.” The Warburg effect is the metabolic change observed in cancer cells from OXPHOS to glycolysis as the primary source of cellular energy.[16]
It has been proposed that cancer cells have increased glycolytic rates despite the presence of O2 because these cells have irreversible damages to OXPHOS. It has been reported that cells from the most common cancer types have decreased expression of ATP synthase, a protein complex required for OXPHOS. Also, mitochondrial mutations, which may lead to malfunction in OXPHOS, have been observed in tumor cells.[17] It has also been shown that inactivation of p53 – one of the most commonly mutated genes in cancer – may trigger the Warburg effect; p53 is involved in the activity of cytochrome c oxidase, a protein complex involved in OXPHOS.[18] These cellular responses have been shown to cause distinct transformations like the upregulation of proteins such as HIF-1α that help the tumor survive adverse conditions in which normal cells cannot persist.[19]
Thus both hypoxic and normoxic cells in OSCC show a rise in HIF-1α. The aerobic glycolysis stimulated by HIF-1α leads to a positive feedback loop in the cancer cells. Tumor cells in normoxic conditions show an over expression of HIF-1α when compared to normal cells in the same environment.[20] The greater rate of aerobic glycolysis and a lower rate of oxidative respiration in tumor cells leads to increased intracellular levels of glycolytic metabolites, specifically pyruvate.[21] Pyruvate promotes the production and stability of HIF-1α thus creating a positive feedback loop that bolsters the proliferation of cancer cells[22] [Figure 4].
Figure 4.
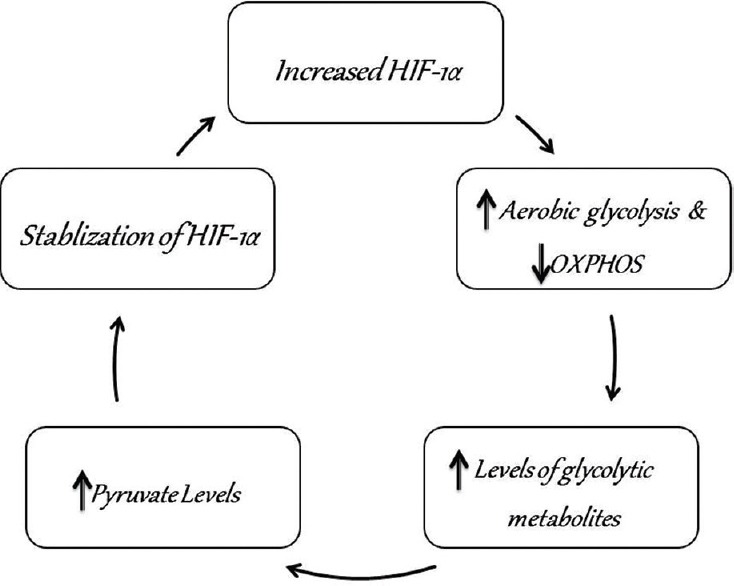
Flow chart depicting how aerobic glycolysis stimulated by hypoxia-inducible factor-1α leads to a positive feedback loop in the cancer cells that further enhances their proliferative capacity
Thus, the rise in HIF-1α expression in OSCC can be attributed to the genetic changes in the cancer cells along with the tumor hypoxia leading to stabilization and production of HIF-1α. We found that there was a gradual rise in the intensity of staining for HIF-1 among the three study groups. It was least for OSMF (mild-02, mod-10, sev-08 patients), intermediate for OSCC (mild-01, mod-11, sev-08 patients) and the highest for OSCC with OSMF (mod-08, sev-12 patients). This increased severity of HIF-1 expression in OSCC with OSMF can be due to the combined effect of these two pathways in OSCC with OSMF.
Angiogenesis is an important mediator of tumor progression. As tumors expand, diffusion distances from the existing vascular supply increases resulting in hypoxia. Sustained expansion of a tumor mass requires new blood vessel formation to provide rapidly proliferating tumor cells with an adequate supply of oxygen and metabolites, failure to do so deprive it of energy and nutrients. O2 acts as a molecular signal and through its availability, coordinates blood vessel growth with the metabolic demands of growing tumor mass. A large number of genes involved in different steps of angiogenesis have been shown to increase by hypoxia. The key regulator of hypoxia-induced angiogenesis is the transcription factor HIF-1. Multiple HIF-1 target genes such as vascular endothelial growth factor (VEGF), angiopoietin and fibroblast growth factor have been shown to modulate angiogenesis by promoting the mitogenic and migratory activities of endothelial cells. Angiogenesis has been shown to increase by hypoxia. The key regulator of hypoxia-induced angiogenesis is the transcription factor HIF-1α.[23] Neo-angiogenesis is an essential step for many physiological processes such as growth, wound healing, organ regeneration and reproductive functions. Abnormal blood vessel growth occurs in several pathological conditions, including tumor growth and metastasis[10] [Figure 5].
Figure 5.
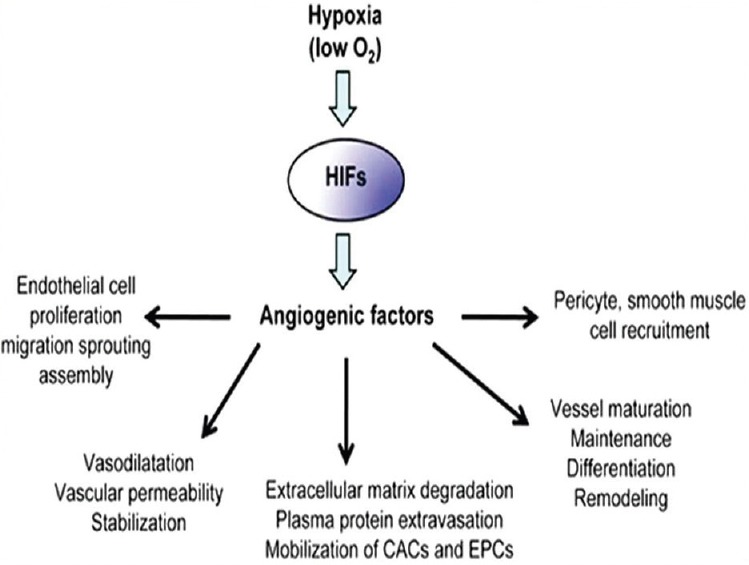
A flow chart showing the mechanisms by which hypoxia leads to neoangiogenesis in tumor tissues
There was a gradual rise in the mean blood vessel density from OSMF to OSCC and a further rise was seen in OSCC with OSMF. This can be attributed to the similarly rising levels of HIF-1α. Tumor angiogenesis is a critical step in the development, metastatic spread and regrowth of cancer. Tumors promote angiogenesis by secreting factors, including VEGF-A, basic fibroblast growth factor and transforming growth factor-β. Several reports suggest that an angiogenic switch may be activated in the premalignant stage of several human cancers. Thus, tumor angiogenesis is not necessarily a characteristic of the invasive tumor, as originally thought, but may be an early event during cancer development.
CONCLUSION
A progressive increase in the HIF-1 and mean blood vessel density was observed in OSMF to OSCC. The combination of the two (OSCC with OSMF) lead to a tissue phenotype that shows more hypoxia and angiogenesis. Thus, we postulate that on conversion of premalignancy to malignancy it leads to a more aggressive tumor phenotype. Hypoxia appears to be a key factor in regulating this domino effect.

Hence, the treatment modalities addressing it would be more beneficial to help prevent this conversion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank our institute for providing infrastructural facilities to carry out the research and the motivation for the present study. Also would like to thank Mr. Rupesh Maldhari for all the technical help.
REFERENCES
- 1.Li N, Jian X, Hu Y, Xu C, Yao Z, Zhong X. Discovery of novel biomarkers in oral submucous fibrosis by microarray analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:2249–59. doi: 10.1158/1055-9965.EPI-07-2908. [DOI] [PubMed] [Google Scholar]
- 2.Tilakaratne WM, Iqbal Z, Teh MT, Ariyawardana A, Pitiyage G, Cruchley A, et al. Upregulation of HIF-1alpha in malignant transformation of oral submucous fibrosis. J Oral Pathol Med. 2008;37:372–7. doi: 10.1111/j.1600-0714.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- 3.1st ed. Netherlands: Maastricht University; 2012. Schults, M.A.C. Doctoral thesis: Hypoxia, oxidative stress and benzo[a] pyrene induced carcinogenesis. [Google Scholar]
- 4.Chaturvedi P, Vaishampayan SS, Nair S, Nair D, Agarwal JP, Kane SV, et al. ral squamous cell carcinoma arising in background of oral submucous fibrosis: A clinicopathologically distinct disease. Head Neck. 2013;35(10):1404–9. doi: 10.1002/hed.23143. [DOI] [PubMed] [Google Scholar]
- 5.Ekanayaka RP, Tilakaratne WM. Oral submucous fibrosis: Review on mechanisms of pathogenesis and malignant transformation. [Last assessed on 2015 Aug 22];J Carcinogene Mutagene. 2013 S5:1–11. doi: 10.1016/j.oooo.2015.12.018. Avaliable from: http://dx.doi.org/4172/2157-2518.S5-002 . [DOI] [PubMed] [Google Scholar]
- 6.Broders AC. The microscopic grading of cancer. Surg Clin North Am. 1941;21:947–62. [Google Scholar]
- 7.Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375–81. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 8.Lindenblatt Rde C, Martinez GL, Silva LE, Faria PS, Camisasca DR, Lourenço Sde Q. Oral squamous cell carcinoma grading systems – Analysis of the best survival predictor. J Oral Pathol Med. 2012;41:34–9. doi: 10.1111/j.1600-0714.2011.01068.x. [DOI] [PubMed] [Google Scholar]
- 9.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–80. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 10.Alkhabuli JO. Significance of neo-angiogenesis and immuno-surveillance cells in squamous cell carcinoma of the tongue. Libyan J Med. 2007;2:30–9. doi: 10.4176/070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudarshan R, Annigeri RG, Vijayabala SG. Pathogenesis of oral submucous fibrosis: The past and current concepts. Int J Oral Maxillofac Pathol. 2012;3:27–36. [Google Scholar]
- 12.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 13.Höckel M, Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 14.Li JZ, Gao W, Chan JY, Ho WK, Wong TS. Hypoxia in head and neck squamous cell carcinoma. ISRN Otolaryngol 2012. 2012 doi: 10.5402/2012/708974. 708974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López-Lázaro M. The Warburg effect: Why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem. 2008;8:305–12. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- 16.Garber K. Energy boost: The Warburg effect returns in a new theory of cancer. J Natl Cancer Inst. 2004;96:1805–6. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- 17.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 18.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–3. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 19.Masakatsu F, Yoshihiro O, Hideaki S. The role of tumor microenvironment in oral cancer. In: Biswas S, editor. Tumor Microenvironment and Myelomonocytic Cells. 1st ed. Coratia (Europe): Intech; 2012. [Google Scholar]
- 20.Van Veelen CW, Rijksen G, Van Ketel BA, Staal GE. The pyruvate kinase isoenzyme shift in human gliomas: A potential marker in the treatment of gliomas. Br J Neurosurg. 1988;2:257–63. doi: 10.3109/02688698808992677. [DOI] [PubMed] [Google Scholar]
- 21.Varma SD, Devamanoharan PS, Ali AH. Formation of advanced glycation end (AGE) products in diabetes: Prevention by pyruvate and alpha-keto glutarate. Mol Cell Biochem. 1997;171:23–8. doi: 10.1023/a:1006846501081. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–5. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 23.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]


