Abstract
Introduction:
Oral submucous fibrosis (OSF) is a chronic, insidious disease that is associated with significant functional morbidity and an increased risk for malignancy. Turmeric and its active ingredient “curcumin” are being studied upon as chemopreventive agents in various diseases. The present study aims to determine the efficacy of curcumin in the treatment of OSF.
Materials and Methods:
Thirty clinically diagnosed OSF patients were divided into two groups, 15 patients in each group from the Outpatient Department. Test group patients were treated with Longvida (curcumin) lozenges and control group with Tenovate ointment (clobetasol propionate (0.05%). The treatment was given for 3 months duration and follow-up was done for 6 months. Both the groups were advised for physiotherapy exercises by mouth exercise device. The baseline and follow-up results were compared for IIO (interincisal distance on maximum mouth opening), Visual Analogue Scale (VAS) for normal food and VAS for spicy food.
Results:
The test group showed 5.93 (±2.37) mm increase in mouth opening compared to 2.66 (±1.76) mm of the control group. In relation to VAS scale with spicy and normal food the average reduction was 64 (42–73) and 77 (70.5–82) as compared to 34 (14.5–64.5) and 64 (46–75.5) respectively in control group. The test group results achieved in the treatment span was sustained in the follow-up (P < 0.05) compared to control group which showed statistically significant (P < 0.05) relapse.
Conclusion:
It can be concluded that combination strategies for the management of OSF which include the stoppage of causative ill habits, appropriate medicinal and physiotherapy management is more efficient than single therapeutic modality. It is evident from the study that curcumin holds good promise in the treatment of OSF in future.
Keywords: Clobetasol propionate, Curcumin, oral submucous fibrosis
INTRODUCTION
Oral submucous fibrosis (OSF) is a chronic, insidious disease that is associated with significant functional morbidity and an increased risk for malignancy. It initially affects the lamina propria of the oral mucosa and as the disease progresses, it involves the submucosa and the deeper tissues including muscles of the oral cavity with resulting loss of fibroelasticity.[1]
The treatment of OSF especially medicinal remains enigma. Corticosteroids have been found to be the medicine of choice by professionals. Turmeric (Curcuma longa) is a medicinal plant extensively used in Ayurveda, Unani and Siddha medicine as a home remedy for various diseases.[2] A large number of studies have revealed that Curcumin has wide therapeutic actions such as antiinflammatory, antioxidant and anticancer properties.[3] Curcumin have been studied for treatment of OSF by Hastak et al.,[4] Chainani-Wu et al. (2008), Das et al.[5] and Rai et al.[6] The present study aims to determine the efficacy of curcumin in the treatment of OSF and compare this to one of the available standard noninvasive drug treatment as topical application of corticosteroid such as clobetasol propionate (0.05%).
MATERIALS AND METHODS
The randomized controlled clinical trial at dental college and hospital of 33 human subjects 30 males and three females was carried out. Informed consent was obtained from all the subjects who were included in the present study. The study was approved by Institutional Ethical Committee, Dental College and Hospital as per Maharashtra University of Health Sciences, Maharashtra, India (ECM/GDCHN/SS/PG/ETHICS.COM MEET/7573-A/10, March 2011).
Selection criteria for samples
A total of 33 clinically diagnosed patients with OSF (diagnosed on the basis of reduction in interincisal distance on maximum mouth opening and palpable fibrous bands involving oral mucosa) within the age range of 18–50 years and interincisal opening in the range of 15–30 mm were selected (time period September 11–December 11). These patients were asked to discontinue all the habits of tobacco or areca usage 1-month prior to the commencement of the treatment. Out of total 33 patients, three were females and 30 were males. All the 33 patients were asked and it was made sure to discontinue all the habits related to usage of tobacco and areca and any treatment for OSF for 1-month before commencement of drug treatment for the study.
After 1-month of discontinuation of the treatment, 30 potential subjects were selected to continue the study and were evaluated for the baseline evaluation criteria (condition of the patient at the start of study) which would be re-evaluated in the subsequent study visits.
Fifteen clinically diagnosed OSF patients were recruited for the test (curcumin) group. The patients were evaluated for the baseline examination before commencement of treatment. The test (curcumin) group were given Longvida lozenges (Mfg Lic.: GA/1482) (400 mg lozenges) manufactured by Pharmanza Herbal Pvt. Ltd. The total daily dose decided was 2 g of Longvida lozenges. This dose was within the safety limits for curcuma longa.[7]
Fifteen clinically diagnosed OSF patients were given topical steroid application and were considered under the control group. The patients were evaluated for the baseline examination before commencement of treatment (topical clobetasol propionate – Tenovate™, Mfg. Lic. No.: 361), manufactured by Encube Ethicals Pvt. Ltd. All the patients were instructed to apply the cream 3 times daily. One tube approximately contains the amount of the cream that can be applied for 1-week. Thus, the total dose of corticosteroid used for treatment was below the safety limit.[8]
The physiotherapy was indicated for both the groups by mouth exercise device (MED). The patients were instructed to exercise for 20 min (10 min on each side) with the help of the MED three times a day for 3 months.
Baseline and follow-up clinical evaluation
The baseline and recall evaluations were done on the basis of:
Objective criteria: Measurement of interincisal distance on maximum mouth opening over two fixed reference points with the help of digital vernier caliper. (Forbes Gokak Ltd. Model no. 111-322) The observations of which are depicted in Figure 1 (test group) and Figure 2 (control group)
Subjective criteria: Visual Analogue Scale (VAS) for burning sensation with normal and spicy food respectively.
Figure 1.
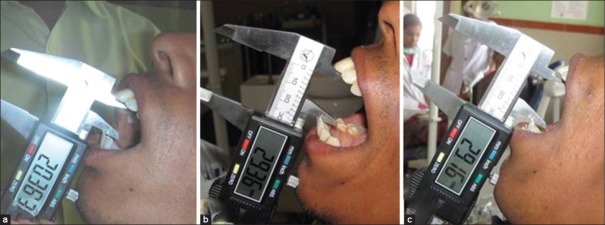
(a) Baseline evaluation of patient in test (curcumin) group (b) post treatment (3 months) evaluation of patient in test (curcumin) group (c) follow-up (6 months) evaluation of patient in test (curcumin) group
Figure 2.
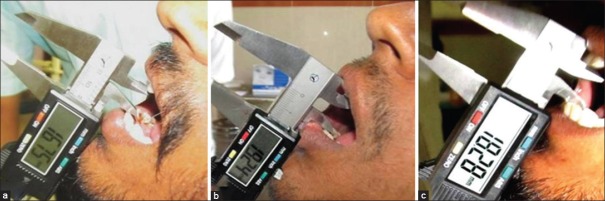
(a) Baseline evaluation of patient in control (steroid) group (b) post treatment (3 months) evaluation of patient in control (steroid) group (c) follow-up (6 months) evaluation of patient in control (steroid) group
A VAS was a 10 cm line without calibrations drawn with one extreme negative and one extreme positive end. The patient was instructed to mark one point on the VAS depicting his/her current status of burning sensation. On further recalls, the previous markings on VAS scale were not shown to the patient to prevent bias on subsequent examinations. The normal food included chapatti, rice and plain dal which should not include the addition of distinct amount of chilies. The criteria for spicy food included any food item with distinct addition of chilies. All the above criteria's were evaluated for baseline (objective and subjective criteria before the start of study), every month till 3 months. Then the treatment was stopped. But further recall follow-up was done at 6th month and 9th month interval.
Statistical analysis was done using statistical software SPSS 10.0© (statistical package for social science by International Business Machines Corporation) to evaluate the study for statistical significance. After clinical observations, the data collected was tabulated and all observed results were then subjected to various statistical analyses as per requirement. The test used were paired t-test to determine the efficacy of the individual drug by calculating the significance in the observations of baseline, 3, 6 and 9 months in relation to the interincisal distance on maximum mouth opening. T-test for independent samples test was used to determine the significance in the observations between study and control group at the baseline and follow-up for the interincisal distance on maximum mouth opening. Wilcoxon rank-sum test to determine significance in the observations between study and control group at the baseline for VAS for burning sensation with normal food and spicy food in study and control group. Wilcoxon signed-rank test to determine the efficacy of the individual drug by calculating the significance in the observations of baseline, 3, 6 and 9 months in relation to VAS for burning sensation with normal and spicy food. Significance was determined at 0.05 level of confidence.
RESULTS
The baseline observations of IIO (P = 0.0610), VAS for normal food (P = 0.0678) and spicy food (P = 0.0927), of patients enrolled in the two study groups were compared. The difference between the mean ages of two groups was statistically not significant using t-test for independent samples and Wilcoxon rank-sum test.
Comparative statistics for IIO for both the study groups
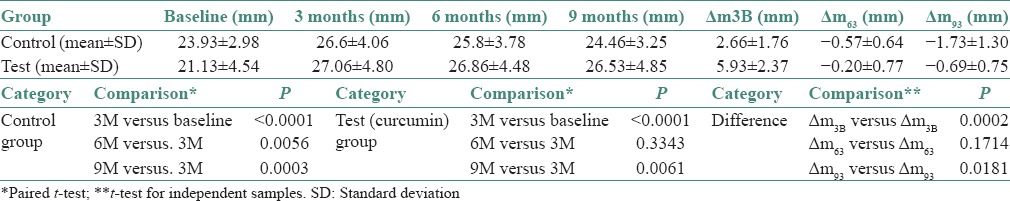
The IIO for the control group at baseline was 23.93 ± 2.98 mm, at 3 months was 26.6 ± 4.06 mm, at 6 months was 25.8 ± 3.78 mm and at 9 months was 24.46 ± 3.25 mm. The IIO for the control group at baseline was 21.13 ± 4.54 mm, at 3 months was 27.06 ± 4.80 mm, at 6 months was 26.86 ± 4.48 mm and at 9 months was 26.53 ± 4.85 mm. The control group shows the mean difference in the baseline and 3 months IIO is 2.66 ± 1.76 mm, 3 and 6 months is 0.57 ± 0.64 mm and 3 and 9 months is 1.73 ± 1.30 mm. The P value for test Δ m3B versus control Δ m3B was found to be 0.0002, which is statistically significant.
IIO in test and control group individually at baseline, 3, 6 and 9 months was compared by paired t-test. The difference (Δm) in interincisal distance on maximum mouth opening (IIO) in test and control group was compared and the data was subjected to t-test for independent samples. The P value for test of the difference in the pre and postop interincisal opening between the test (curcumin) and control group was found to be 0.0002, which is statistically significant. Thus, it can be concluded from the statistical analysis that test and control group shows a significant increase in IIO from baseline to 3 months. Posttreatment follow-ups for control group showed a statistically significant decrease in mouth opening after comparing 6 monthly and 9 monthly observations with 3 months observations (posttreatment). The observations are tabulated in Table 1 and Graph 1.
Table 1.
Descriptive and comparative statistics for interincisal distance on maximum mouth opening (IIO) in test and control group
Graph 1.
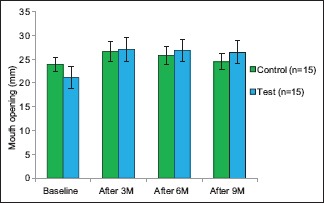
Interincisal distance on maximum mouth opening in test and control group
Thus, it can be concluded that the test (curcumin) group shows statistically significant difference in the IIO as compared to what was achieved with the control group in the treatment span. While in the posttreatment follow-up the relapse in relation to IIO in the control group is statistically significant after 9 months when compared to IIO achieved after 3 months.
Comparative statistics for Visual Analogue Scale for normal food for both the study groups
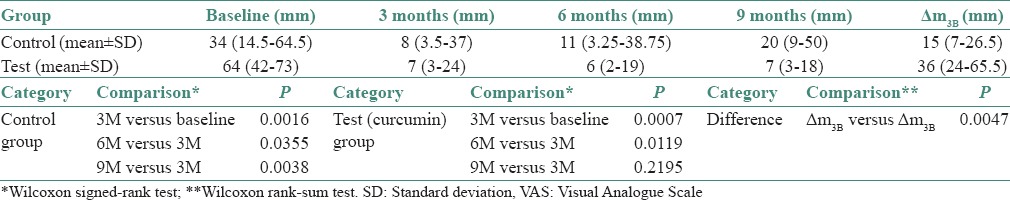
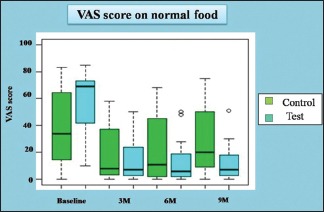
The VAS for normal food in control group at baseline was 34 (14.5–64.5), at 3 months was 8 (3.5–37), at 6 months was 11 (3.25–38.75) and at 9 months was 20 (9–50). The VAS for normal food in test group at baseline was 64 (42–73), at 3 months was 7 (3–24), at 6 months was 6 (2–19) and at 9 months was 7 (3–18). VAS with normal food in test and control group individually at baseline, 3, 6 and 9 months was evaluated by Wilcoxon signed-rank test.
The difference (ΔVAS) in VAS score for normal food in the test (curcumin) and control group in relation to baseline and posttreatment 3 months was compared by Wilcoxon rank-sum test. The P value for the difference between the VAS score of the test (curcumin) and control group was found to be 0.0047, which is statistically significant.
Thus, it can be concluded from the statistical analysis that test (curcumin) group and control group shows a statistically significant decrease in VAS score for normal food from baseline to 3 months. Posttreatment follow-ups show, statistically significant increase in VAS score found after comparing 6 monthly and 9 monthly observations with 3 months observations (posttreatment) than that in the control group, indicative of relapse. The observations are tabulated in Table 2 and Graph 2.
Table 2.
Descriptive and comparative statistics for VAS with normal food in test and control group
Graph 2.
Visual Analogue Scale score on normal food in test and control group
Comparative statistics for Visual Analogue Scale for spicy food for both the study groups
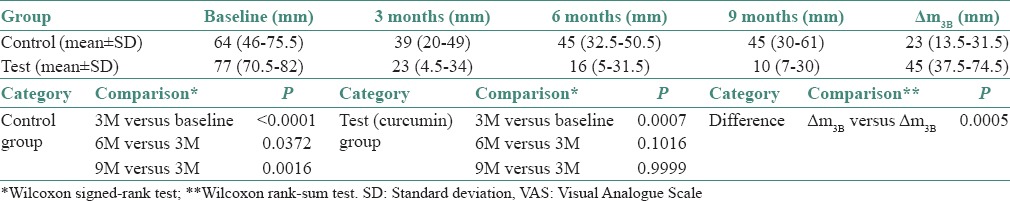
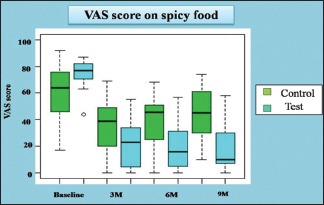
The VAS for spicy food in control group at baseline was 64 (46–75.5), at 3 months was 39 (20–49), at 6 months was 45 (32.5–50.5) and at 9 months was 45 (30–61). The VAS for normal food in test group at baseline was 77 (70.5–82), at 3 months was 23 (4.5–34), at 6 months was 16 (5–31.5) and at 9 months was 10 (7–30).
VAS with spicy food in test and control group individually at baseline, 3 months, 6 months and 9 months was evaluated and data analysed using by Wilcoxon signed-rank test. The statistical analysis showed that test (curcumin) and control group shows a statistically significant decrease in VAS score for spicy food from baseline to 3 months. Posttreatment follow-ups show, statistically significant increase in VAS score after comparing 6 monthly and 9 monthly observations with 3 months observations (posttreatment) than that in the test (curcumin) group, indicative of relapse. The observations are tabulated in Table 3 and Graph 3.
Table 3.
Descriptive and comparative statistics for VAS with spicy food in test and control
Graph 3.
Visual Analogue Scale score with spicy food
The difference (ΔVAS) in VAS score for spicy food in test and control group in relation to baseline and posttreatment (3 months) was compared by Wilcoxon rank-sum test. The P value for the difference between the VAS score of the test (curcumin) and control was found to be 0.0005, which is statistically significant. The mean decrease in the VAS score was statistically significant in the test (curcumin) group as compared to control group. The observations are tabulated in Table 3 and Graph 3.
DISCUSSION
Once OSF is induced by the habit of chewing areca nut, the reversal of the disease after cessation of the habit does not occur. In clinical practice, there are a number of treatments for OSF, ranging from medicinal and surgical interventions, physical therapy and ofcourse habit control (i.e., cessation of areca nut use). Often a combination of strategies is used.[1]
Patients with OSF characteristically complain of two problems:
Inability to open their mouths and function normally
Burning sensation and intolerance to spicy foods (that are often the mainstay of the Asian diet), leaving an individual disadvantaged, both physically and psychologically.
The aims of study are therefore, to evaluate the safety of the medicines and the efficacy in the form of pre and postoperative difference in the interincisal distance, difference in the VAS score for normal and spicy food.
Safety of the drugs
Human clinical trials have indicated that curcumin has no toxicity when administered at doses of 10 g/day.[7] Curcumin lozenges were well tolerated by all the patients (n = 15).[5] Only three patients reported yellowish coating over the teeth and dorsum of the tongue, which was removed by oral prophylaxis procedures and oral hygiene maintenance by patients. The total dosage of topical corticosteroid should not exceed 50 g/week because of the ability of the drug to suppress the hypothalamic-pituitary-adrenal axis.[8] One tube of clobetasol propionate (0.05%) approximately contains the amount of the cream to be applied for 1-week. That would make a total dosage of 15 g/week. None of the patients reported any gastrointestinal disturbances, rashes or allergic reactions.
The clinical evaluation
IIO for test and control group
The test and control group shows a significant increase in IIO from baseline to 3 months. Posttreatment follow-ups for control group showed a statistically significant decrease in mouth opening after comparing 6 monthly and 9 monthly observations with 3 months observations (posttreatment) than that seen in the test (curcumin) group.
The test (curcumin) group showed 5.93 (±2.37) mm increase in mouth opening compared to 2.66 (±1.76) mm of the control group. Thus, it can be concluded that the test (curcumin) group shows high statistical significant difference in IIO as compared to what was achieved with the control group in the treatment span. While in the posttreatment follow-up the relapse in relation to IIO in the control group is statistically significant after 9 months when compared to what was achieved after 3 months. The difference (Δm) in interincisal distance on maximum mouth opening (IIO) in test and control group was compared and the data was subjected to t-test for independent samples. The results are tabulated in Table 1 and Graph 1.
Curcumin has been reported to possess fibrinolytic action in liver and lung fibrosis in studies conducted by Kuttan et al.[9] Turmeric and curcumin are regarded as fibrinolytic agents in Chinese medicine.[9] Li et al.[10] has attributed the fibrinolytic action of curcumin to its three properties namely inhibition of lipid peroxidation, checking cellular proliferation and inhibition of collagen synthesis.[10] Hastak et al.[4] in the study found that turmeric oil treated patients show highest efficacy with 35% of patients showing marked improvement (>5 mm). While patients treated by turmeric oleoresin and turmeric extracts showed maximum results with moderate (3–5 mm) and mild improvement (2 mm) of increase in mouth opening respectivley.[4] Das et al.[5] in the study reported statistically significant and equal increase in the mouth opening of patients in Groups I (curcumin capsules) and II (turmeric oil) after 1-month and 3 months of treatment and also after the follow-up period. The mean increase was 0.87 cm in both the groups which was significant when compared with the other groups. Rai et al.[6] in their study reported that in patients with submucous fibrosis, mouth opening recovered significantly (P < 0.05) after 6 months of the treatment.[6]
The corticosteroids differ from almost all other anti-inflammatory drugs in that they are capable of inhibiting virtually all the components of inflammation.[11] Steroids are well known to act as immunosuppressive agents for prevention or suppression of the fibroproductive inflammation found in OSF lesions, thus, ameliorating this fibrocollagenous condition,[12,13] Lai et al.[14] and Borle et al.[12] in their study reported no improvement of the trismus at any stage of the treatment when treated by topical triamcinolone acetonide (0.1%) on mucosal ulcers at bedtime as one of the medicinal regimen along with a subset of group A patients who were administered Vitamin B complex tablets orally in a dose of 200 mg twice a day, buflomedil hydrochloride as three tablets (450 mg) per day and topical betamethasone (0.5%) respectively as one of the component of medicinal treatment. The medicinal treatment included Vitamin A 50,000 IU in the form of chewable tablets once daily, oral ferrous fumarate tablets in a dose of 200 mg once daily and topical betamethasone drops (0.5 mg/mL) to be used every 6 h for 3 weeks in one of the study group respectively.[12,14] The disease invariably became reactivated within 3–4 months, the eruptive phase reappeared and the trismus became more pronounced. The increase in the interincisal distance in case of control group might have been attributed to use of clobetasol propionate (0.05%) which is more potent than triamcinolone acetonide. It can also be attributed to the use of physiotherapy exercises that patients were asked to perform. Cox and Zoellner[15] and Patil and Patil,[16] reported that physiotherapy improved mouth opening.
Visual Analogue Scale for normal and spicy food respectively for both the study groups
The VAS for normal food in the control group at baseline was 34 (14.5–64.5) and 64 (42–73), for the test (curcumin) group at 3 months. Both the test (curcumin) group and control group shows statistically significant decrease in VAS score for normal and spicy food from baseline to 3 months. Posttreatment follow-ups show, statistically significant increase in VAS score as found after comparing 6 monthly and 9 monthly observations with 3 months observations (posttreatment) indicative of relapse when compared with the test (curcumin) group.
The difference (ΔVAS) in VAS score for normal and spicy food in test and control group in relation to baseline and posttreatment (3 months) was 64 (42–73) and 77 (70.5–82) for the test (curcumin) group compared to 34 (14.5–64.5) and 64 (46–75.5) for the control group respectively by Wilcoxon rank-sum test. The observations are tabulated in Table 2 and Graph 2. The mean decrease in the VAS score was statistically significant [(P = 0.0047 for normal food) and (P = 0.0005 for spicy food)] in the test (curcumin) group as compared to control group.
Curcumin offers anti-inflammatory effect through inhibition of NF-kB activation.[2,13,17] Curcumin blocks the IK-mediated phosphorylation and degradation of IBα, thus, NF-kB remains bound to IkBα in the cytoplasm and is not able to enter the nucleus to activate transcription.[18] Rao et al.[19] have demonstrated the scavenging effect of curcumin on superoxide radicals, hydroxyl radicals and lipid peroxidation.[19] This can be the reason of reduction in the burning sensation with normal and spicy food. Hastak et al.[4] in his study to evaluate the efficacy of turmeric extract, turmeric oil and turmeric oleoresin reported all the patients showed clinical improvement in burning sensation to various degrees out of which turmeric oil showed optimum effect.[4] Das et al.[5] in the study reported that patients in Groups I and II (curcumin capsules and turmeric oil) presented with statistically significant reduction in burning sensation and intolerance to spicy food after 3 months of treatment and follow-up, when compared to those in Group III (placebo group). However, both the articles did not mention the method by which burning sensation was evaluated (Das et al., 2010).[5]
Corticosteroids partially relieved patients of their symptoms at an early stage of OSF, as confirmed in many studies. They were less useful in reversing the abnormal deposition of fibrotic tissues and recovering the suppleness of the mucosa and thus this treatment was always associated with a high incidence of relapse. Moreover, prolonged use or overdose invariably produced unwanted side effects.[20] Borle et al.[12] and Lai et al.[14] in their study reported that the majority of patients treated had symptomatic relief. The burning sensation, feeling of stiffness and vesicles disappeared. The disease invariably became reactivated within 4–6 months. The eruptive phase reappeared and the trismus became more pronounced.[12,14]
CONCLUSION
The test (curcumin) group showed 5.93 (±2.37) mm increase in mouth opening compared to 2.66 (±1.76) mm of the control group. In relation to VAS scale with spicy and normal food the average reduction was 64 (42–73) and 77 (70.5–82) as compared to 34 (14.5–64.5) and 64 (46–75.5) respectively in control group. The test (curcumin) group results achieved in the treatment span was sustained in the follow-up (P < 0.05) compared to the control group showed statistically significant (P < 0.05) relapse.
It can be concluded that combination strategies for the management of OSF which include the stoppage of causative ill habits, appropriate medicinal and physiotherapy management is more efficient than single therapeutic modality.
In the short treatment span and small sample size, curcumin, a active ingredient in turmeric which is extensively used as medicinal plant in Ayurveda, Unani and Siddha medicine as home remedy for various diseases, has shown long term beneficial effects. Curcumin being antioxidant and antiinflammatory may enhance the neoangiogenic and antifibrotic potential of the sufferers. To our knowledge, till date no human randomized clinical trials have been reported in the literature to evaluate the efficacy of curcumin over any standard medicinal management modality for OSF. It is evident from the study that curcumin holds good promise in the treatment of OSF in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge the contribution by:
Dr. Prof. Saman Warnakulasuriya, Department of Oral Medicine, King's College London Dental Institute at Guy's, King's and St Thomas’ Hospitals and the WHO Collaborating Centre for Oral Cancer and Precancer, Denmark Hill Campus, Caldecot Road, London, UK; for his guidance
Dr. Lal Hingorani, PhD, Managing director Pharmanza Herbal Pvt. Ltd. 214, Borsad Tarapur Road, Keniya, Gujrat - 388435, for the supply of drug Longvida for the trial.
REFERENCES
- 1.Kerr AR, Warnakulasuriya S, Mighell AJ, Dietrich T, Nasser M, Rimal J, et al. A systematic review of medical interventions for oral submucous fibrosis and future research opportunities. Oral Dis. 2011;17(Suppl 1):42–57. doi: 10.1111/j.1601-0825.2011.01791.x. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: Biologica actions and medicinal application. Curr Sci. 2004;87:10. [Google Scholar]
- 3.Kohli K, Ali J, Ansari MJ, Raheman Z. Educ Forum. Vol. 37. Curcumin; 2005. Curcumin: A natural antiinflammatory agent; pp. 141–7. [Google Scholar]
- 4.Hastak K, Jakhi SD, More C, John A, Ghaisas SD, Bhide SV. Therapeutic response to turmeric oil and turmeric oleoresin in oral submucous fibrosis patient. Amala Res Bull. 1998;18:23–8. [Google Scholar]
- 5.Das AD, Balan A, Sreelatha KT. Comparative study of the efficacy of curcumin and turmeric oil as chemopreventive agents in oral submucous fibrosis: A clinical and histopathological evaluation. J Indian Acad Oral Med Radiol. 2010;22:88–92. [Google Scholar]
- 6.Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci. 2010;52:251–6. doi: 10.2334/josnusd.52.251. [DOI] [PubMed] [Google Scholar]
- 7.Joshi J, Ghaisas S, Vaidya A, Vaidya R, Kamat DV, Bhagwat AN, et al. Early human safety study of turmeric oil (Curcuma longa oil) administered orally in healthy volunteers. J Assoc Physicians India. 2003;51:1055–60. [PubMed] [Google Scholar]
- 8.Steroids-Topical Steroid Side Effects from Heather Brannon, MD, Former. About.com Guide. [Last updated on 2007 May 20]. Avaliable from: http://dermatology.about.com/cs/medications/a/steroidgroups.htm .
- 9.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;2:28–9. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 10.Li CJ, Zhang LJ, Dezube BJ, Crumpacker CS, Pardee AB. Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication. Proc Natl Acad Sci U S A. 1993;90:1839–42. doi: 10.1073/pnas.90.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes PJ. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin Sci (Lond) 1998;94:557–72. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 12.Borle RM, Borle SR. Management of oral submucous fibrosis: A conservative approach. J Oral Maxillofac Surg. 1991;49:788–91. doi: 10.1016/0278-2391(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 13.Lin CL, Lin JK. Curcumin: A potential cancer chemopreventive agent through suppressing NF-κB signaling. J Cancer Mol. 2008;4:1–6. [Google Scholar]
- 14.Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med. 1995;24:402–6. doi: 10.1111/j.1600-0714.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 15.Cox S, Zoellner H. Physiotherapeutic treatment improves oral opening in oral submucous fibrosis. J Oral Pathol Med. 2009;38:220–6. doi: 10.1111/j.1600-0714.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- 16.Patil PG, Patil SP. Novel mouth-exercising device for oral submucous fibrosis. J Prosthodont. 2012;21:556–60. doi: 10.1111/j.1532-849X.2012.00874.x. [DOI] [PubMed] [Google Scholar]
- 17.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–9. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haque MF, Meghji S, Khitab U, Harris M. Oral submucous fibrosis patients have altered levels of cytokine production. J Oral Pathol Med. 2000;29:123–8. doi: 10.1034/j.1600-0714.2000.290304.x. [DOI] [PubMed] [Google Scholar]
- 19.Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. J Nutr. 1970;100:1307–15. doi: 10.1093/jn/100.11.1307. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Hu J. Drug treatment of oral submucous fibrosis: A review of the literature. J Oral Maxillofac Surg. 2009;67:1510–5. doi: 10.1016/j.joms.2008.12.056. [DOI] [PubMed] [Google Scholar]


