Abstract
Context:
Oral lichen planus (OLP) is a chronic inflammatory mucocutaneous disease with female predominance with the potential for malignant transformation. Human papillomavirus (HPV) is associated with both malignant and benign disease in the head and neck region.
Aims:
The present study assesses the prevalence of high-risk HPV-16 and HPV-18 in tissue and saliva samples from an Iranian population diagnosed with OLP.
Subjects and Methods:
Deoxyribonucleic acid was extracted and investigated using polymerase chain reaction from tissue and saliva samples of the same individuals from 40 OLP cases and saliva samples of 40 healthy controls.
Statistical Analysis:
The prevalence of data was compared using the Chi-square test and inter-group differences were analyzed using Fisher's exact test.
Results:
OLP specimens were HPV-positive in 11 of 40 (27.5%) cases compared with three of 40 (7.5%) saliva specimens, representing a statistically significant difference (P = 0.0367). HPV-16 and HPV-18 were positive in eight of 40 (20%) OLP tissues and three of 40 (7.5%) saliva samples. Five of the 40 healthy saliva samples were positive for HPV-16. In the OLP patients with dysplasia, four of seven tissue samples were HPV-positive; threeof these four were HPV-16- positive in comparision with seven of 33 HPV-positive samples from OLP patients without dysplasia.
Conclusions:
Biopsies were more accurate than saliva analysis for evaluating HPV prevalence in OLP patients. HPV prevalence was higher in dysplastic than nondysplastic OLP lesions in this Iranian cohort.
Keywords: Human papilloma virus, human papillomavirus-16, oral lichen planus, saliva
INTRODUCTION
Oral lichen planus (OLP) is a chronic inflammatory mucocutaneous disease: Several types of OLPs are described, the two main clinical presentations being the reticular and erosive forms.[1] Reticular OLP is usually asymptomatic and can be present as white interlacing and keratotic lines (Wickham striae) with an erythematous border involving the buccal mucosa, tongue and gingiva.[1] OLP is a common disorder estimated to affect 0.5–2% of the general population and in a study from Iran, 0.5% of 1167 investigated Iranian textile workers had lichen planus.[1,2] It is seen worldwide, mostly in the fifth to sixth decades of life and is twice as common in women as in men.[3] The etiology and pathogenesis of OLP are unknown though several molecular hypotheses have been presented. The etiology of OLP involves the degeneration of the epithelial basal cell layer, induced by cell-mediated immunologic reactions.[4] Speculated causative factors such as stress, diabetes, hepatitis C, trauma and hypersensitivity to drugs and metals have different degrees of support.[5]
In 1997, the World Health Organization classified OLP as potentially malignant disorder with unspecified malignant transformation risk and suggested close monitoring of OLP patients.[6,7] An association between chronic inflammation and various cancers has been demonstrated.[6] OLP is a potentially malignant disorder in which human papilloma virus deoxyribonucleic acid (HPV DNA) is significantly and more likely to be detected than in normal oral mucosa, the highest prevalence of HPV DNA being found in atrophic variants of OLP.[4,6] The HPV viral protein, E6, increases the degradation of p53 and the viral protein E7 inactivates the retinoblastoma protein. This leads to cell cycle dysregulation and may eventually lead to malignant transformation.[8] In two large review studies, which included meta-analysis, Syrjänen et al.,[9] and Gorsky and Epstein,[10] suggested a potentially important association between HPV, oral squamous cell carcinoma and OLP.
Epidemiologic evidence suggests that HPV may be an independent risk factor for oropharyngeal cancer, being found three times more in many precancerous oral lesions and almost five times more in many oropharyngeal cancers as compared to normal oral mucosa.[11] In Iran, Seifi et al.[12] found the frequency of oral HPV in healthy individuals to be 6.1% with HPV-18 to be the most frequent type. Of all the HPV types, the high-risk (HR) strains such as HPV-16 and to a lesser extent HPV-18 are most commonly identified in oral biopsies.[13] In a study from Iran, Razavi et al.,[14] suggested that there might be a co-incidence of HPV-18 and OLP. Whole saliva has been successfully used in detecting proinflammatory cytokines and adhesion molecules in several immunologically mediated diseases.[15] It is important to evaluate the practical application of salivary biomarker analysis in the clinical management of OLP.[16]
The present study sought to investigate the prevalence of HPV DNA, HPV-16 and HPV-18 in tissue biopsies and salivary rinses in patients with OLP and in salivary rinses from healthy controls in an Iranian cohort.
SUBJECTS AND METHODS
Subjects
Biopsy samples and salivary samples were collected in twelve months from 40 OLP patients (eight male, 32 female with a mean age of 53 ± 12.5 years), all were histopathologically confirmed cases. As controls, salivary samples were collected from 40 healthy individuals (eight male and 32 female), age- and sex-matched to the OLP patients. The OLP specimens were examined by an experienced oral pathologist. When the typical criteria for OLP were fulfilled without any histopathological signs of dysplasia, the specimen was included in the group “OLP without dysplasia.” If the OLP specimen had any dysplastic changes (slight to severe), it was included in the group “OLP with dysplasia.”
The clinical procedures were performed at the Department of Oral and Maxillofacial Medicine. The material was obtained from the patients after obtaining informed consent and in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Tehran University.
Saliva collection
After full examination and diagnosis, the patients were asked to rinse orally for 30s with 5 mL of saline solution and then the entire contents was collected inan Eppendorf tube. For DNA extraction, saliva was stored at −70°C.
Tissue collection
Local anesthesia was used while obtaining the biopsy specimens, which were taken from oral mucosa at the OLP lesional site; the biopsy specimens were immediately rinsed twice in buffered saline. The specimens were placed in 99% alcohol and kept at room temperature for 24 h before being stored at −20°C until being analyzed. The specimens were histopathologically investigated at the Department of Microbiology.
Deoxyribonucleic acid extraction
DNA was extracted from the tissue and saliva samples using a PSP Spin Stool DNA Plus DNA extraction kit (Cat. No. 1038110200; STRATEC Molecular GmbH, Berlin, Germany) using the standard protocol. The tissue samples were dissolved in 150 mL of TE buffer before the kit was used. Purified DNA was quantified using a Biochrom WPA Biowave DNA Life Science Spectrophotometer (Biochrom Ltd., Cambridge, UK).
Single polymerase chain reaction assay
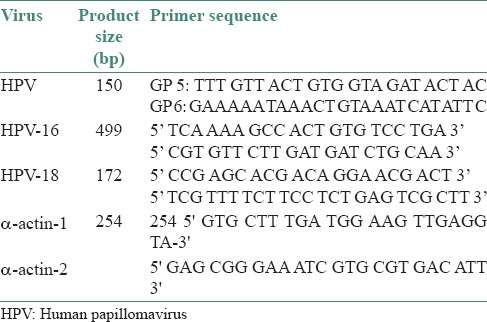
For the detection of target HPV DNA and typing of HPV-16 and HPV-18, a single polymerase chain reaction (PCR) assay was used. Each PCR reaction mixture contained 2.5 μL of 10 times PCR buffer, 0.6 μL of 10 times dNTP mix, 2.5 μL of MgCl2 (25 mM), 0.3 μL of oligonucleotide primer F (100 mM), 0.3 μL of oligonucleotide primer R (100 mM) and 14.2 μL of H2 O, making the total amount of each reaction to 21.4 μL. We then added 3.5 μL of the sample and 0.15 μL of Taq-polymerase (0.5 U) to the reaction mixture. Each cycle consisted of 1 min at 95°C, 1 min at 55°C and 2 min at 72°C. After the amplification, the mixture was heated at 72°C for 5 min. The primer sequences used in the PCR reactions are shown in Table 1. A single PCR assay was used to detect HPV DNA. Before testing the samples, the specificity of the method was examined using HeLa cell lines as a positive control and sterile water as a negative control. To ensure that the DNA extracts were PCR amplifiable, β-actin (254 base pair) was included as a household gene and analyzed in all the samples. Total tissue and saliva DNA samples were tested for HPV DNA by PCR using the general primers GP5/GP6 (150 bp) and the positive samples were investigated using HPV-16 primers (499 bp) and HPV-18 primers (172 bp) [Table 1].
Table 1.
Sequences of HPV general and type-specific primers and oral human pap
Gel electrophoresis
Aliquots of 15 μL of the PCR product were analyzed on 3% (HPV) Agarose gel (DNA Agar; Marine Bio Products Inc., Delta, BC, Canada) containing 0.5 g/mol of ethidium bromide (Merck KGaA, Darmstadt, Germany) and visualized under ultraviolet light. The size of the amplified product was determined by comparison with base-pair ladder size markers for HPV DNA, HPV-16 DNA and HPV-18 DNA (Gene Ruler, 100 bp DNA Ladder Plus; Fermentas, St. Leon-Rot, Germany) [Figure 1].
Figure 1.
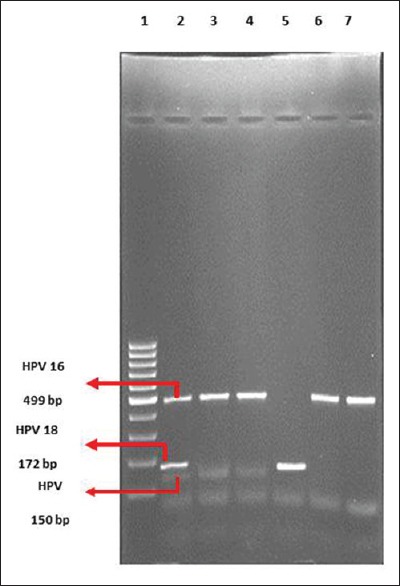
Deoxyribonucleic acid (DNA) Agarose gel (3%) electrophoresis of human papilloma virus deoxyribonucleic acid polymerase chain reaction products. Column 1: Molecular weight marker 100 bp. Column 2: Positive sample for human papillomavirus-16 and human papillomavirus-18. Columns 3–7: Patient samples
Statistical analysis
The prevalence of data was statistically compared using the Chi-square test and inter-group differences were analyzed using Fisher's exact test. SPSS 11.5 software was used for all the statistical analyses. P values <0.05 was considered as statistically significant.
RESULTS
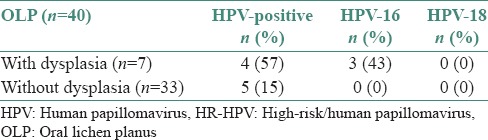
To test the accuracy of saliva samples as test material for HPV detection, we compared the results for tissue and saliva from the OLP patients. Eleven of 40 (27.5%) OLP tissue samples were HPV- positive compared with three of 40 (7.5%) HPV-positive saliva samples. This difference was statistically significant (P = 0.0367). We also found that five of 40 (12.5%) OLP tissue samples were HPV-16-positive and three of 40 (7.5%) were HPV-18-positive. In the saliva samples from the OLP patients, one of 40 (2.5%) was HPV-16-positive and two of 40 (5.0%) were HPV-18-positive [Table 2 and Figure 2]. Comparing the saliva samples of the two groups, we found that three of 40 (7.5%) OLP patients were HPV-positive in comparison to five of 40 (12.5%) healthy controls. This difference was not statistically significant (P = 0.7119) [Table 2]. HPV DNA was found in four of seven (57%) OLP specimens with dysplasia, three of which (43%) were HPV-16- positive. HPV was detected in five of 33 (15%) OLP specimens without dysplasia with none positive for HPV-16 or HPV-18 [Table 3].
Table 2.
Presence of HPV DNA, HPV-16 and HPV-18 in the tissue and salivary samples of OLP patients and healthy controls
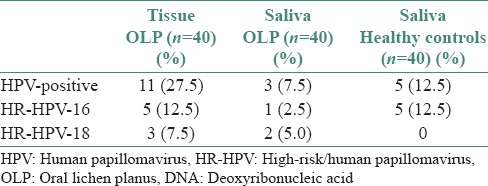
Figure 2.
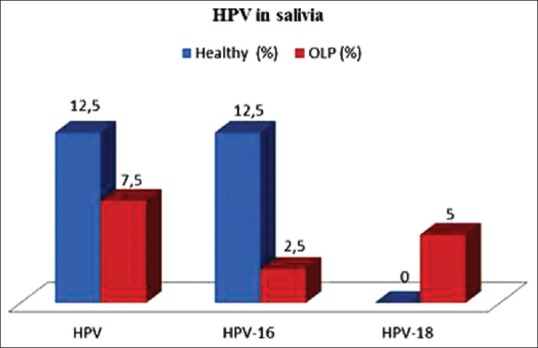
Presence of human papilloma virus deoxyribonucleic acid, human papilloma virus-16 and human papilloma virus-18 in the saliva of oral lichen planus patients and healthy controls
Table 3.
Presence of HPV-positive, HR-HPV-16 and HRHPV-18 in OLP patients with and without dysplasia
DISCUSSION
OLP affects 1.27% of the global population with prevalence varying according to the geographic location.[17] Efforts to explore the correlation between HPV and OLP have mainly comprised epidemiological studies of various populations.
In developing a screening procedure for oral precancer and cancer in Iran, we investigated HPV DNA in both tissue specimens and saliva samples from the patients diagnosed with OLP. In the present study, we found a statistically significant difference (P = 0.0367) in HPV prevalence in OLP patients, with 27.5% being positive for HPV in tissues versus 7.5% positive in saliva. We found biopsy to be a more reliable method than salivary analysis for determining HPV status.
There are profound variations in HPV prevalence among geographically different populations.[18] In two studies from the USA, the authors found no relationship between HPV and OLP.[19,20] In contrast, patients with OLP in European countries have been reported to have an HPV prevalence of 11.8–100%.[9,21,22,23,24,25] A study from Italy detected HPV DNA in 12 of 49 (25%) OLP lesions, with HPV-18 being the most frequent genotype.[22] HR-HPV was found in three of seven (42%) cases of OLP in Germany and six of 22 (27%) in Sweden.[26] The present study supports the finding of a previous report on HPV prevalence in OLP tissue specimens by Sand et al.,[27] who reported HPV-positive status in six of 22 (27.3%) OLP patients; five (83%) of whom were HPV-18 -positive, in a Swedish cohort. A study from India showed that HPV-16 may play a role in the pathogenesis of OLP.[28]
HPV-16 is an established risk factor in oral cancer development.[13] Previously, in a review article by Sand and Jalouli,[28] they described that HPV-16 produces two oncoproteins, E6 and E7, which are necessary for viral replication. In our study, the prevalence of HPV DNA-positive samples in OLP patients with dysplasia (57%) was significantly higher than in OLP patients without dysplasia (15%) (P = 0.0337). Furthermore, the prevalence of HPV-16 in OLP patients with dysplasia (43%) was significantly higher than in OLP patients without dysplasia (0%) (P = 0.0035). These results are comparable to those of a study from Ireland, in which HPV was detected in six of 38 (26%) patients (all HPV-16) with OLP.[29] In an Eastern Hungarian population, HPV prevalence differed significantly between OLP lesions associated with higher malignancy risk versus OLP lesions with lower malignancy risk (42.6% vs. 22.4%).[30] However, there are few current investigations of HPV as a risk factor for malignant transformation in the Iranian population.
The variance in HPV DNA prevalence among these studies may be attributed to the differences in sensitivity of the applied molecular methods, sampling methodologies, patient characteristics and the types of studied specimens (e.g., saliva, oral mucosal scrapings or biopsy tissue).[31] Further, oral rinse samples may provide a cost-effective approach for disease monitoring and screening of large populations.[32] In the present study, we used PCR, which is a sensitive method, considered as the “gold standard.” PCR for this application has been tested in previous studies and one analysis of healthy oral mucosa for HPV DNA using both Southern blot hybridization and PCR resulted in positive findings in 3.8% and 29.4% of the samples, respectively.[33]
Our results provide new information about oral HPV status in the tissue and saliva of OLP patients from Iran, helping to contextualize results of other studies that demonstrate oral precancerous rates to have risen in some geographic areas.
CONCLUSIONS
We found tissue biopsies to be more reliable than saliva analysis in detecting HPV presence. We also found that dysplastic OLP lesions had a higher HPV prevalence than nondysplastic OLP lesions and that HPV-16 is more frequently present in dysplastic OLP lesions. Further studies are clearly needed to assess the potential role of HPV and specifically HPV-16, in dysplastic OLP lesions in the Iranian population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bobbio A, Vescovi P, Ampollini L, Rusca M. Oral erosive lichen planus regression after thymoma resection. Ann Thorac Surg. 2007;83:1197–9. doi: 10.1016/j.athoracsur.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Jahanbani J. Prevalence of oral leukoplakia and lichen planus in 1167 Iranian textile workers. Oral Dis. 2003;9:302–4. doi: 10.1034/j.1601-0825.2003.00967.x. [DOI] [PubMed] [Google Scholar]
- 3.Scully C, Carrozzo M. Oral mucosal disease: Lichen planus. Br J Oral Maxillofac Surg. 2008;46:15–21. doi: 10.1016/j.bjoms.2007.07.199. [DOI] [PubMed] [Google Scholar]
- 4.Mattila R, Rautava J, Syrjänen S. Human papillomavirus in oral atrophic lichen planus lesions. Oral Oncol. 2012;48:980–4. doi: 10.1016/j.oraloncology.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Zhou G. Green tea consumption: An alternative approach to managing oral lichen planus. Inflamm Res. 2012;61:535–9. doi: 10.1007/s00011-012-0440-z. [DOI] [PubMed] [Google Scholar]
- 6.Ebrahimi M, Nylander K, van der Waal I. Oral lichen planus and the p53 family: What do we know? J Oral Pathol Med. 2011;40:281–5. doi: 10.1111/j.1600-0714.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- 7.Georgakopoulou EA, Achtari MD, Achtaris M, Foukas PG, Kotsinas A. Oral lichen planus as a preneoplastic inflammatory model. J Biomed Biotechnol 2012. 2012 doi: 10.1155/2012/759626. 759626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SY, Cho NH, Choi EC, Baek SJ, Kim WS, Shin DH, et al. Relevance of human papilloma virus (HPV) infection to carcinogenesis of oral tongue cancer. Int J Oral Maxillofac Surg. 2010;39:678–83. doi: 10.1016/j.ijom.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Syrjänen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011;17(Suppl 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 10.Gorsky M, Epstein JB. Oral lichen planus: Malignant transformation and human papilloma virus: A review of potential clinical implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:461–4. doi: 10.1016/j.tripleo.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Wong DT, Münger K. Association of human papillomaviruses with a subgroup of head and neck squamous cell carcinomas. J Natl Cancer Inst. 2000;92:675–7. doi: 10.1093/jnci/92.9.675. [DOI] [PubMed] [Google Scholar]
- 12.Seifi S, AsvadiKermani I, Dolatkhah R, AsvadiKermani A, Sakhinia E, Asgarzadeh M, et al. Prevalence of oral human papilloma virus in healthy individuals in East Azerbaijan province of Iran. Iran J Public Health. 2013;42:79–85. [PMC free article] [PubMed] [Google Scholar]
- 13.Goon PK, Stanley MA, Ebmeyer J, Steinsträsser L, Upile T, Jerjes W, et al. HPV and head and neck cancer: A descriptive update. Head Neck Oncol. 2009;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razavi SM, Ghalayani P, Salehi MR, Attarzadeh H, Shahmoradi M. Human papilloma virus as a possible factor in the pathogenesis of oral lichen planus. Dent Res J (Isfahan) 2009;6:82–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Ghallab N, Shaker O. Salivary-soluble CD44 levels in smokers and non-smokers with chronic periodontitis: A pilot study. J Periodontol. 2010;81:710–7. doi: 10.1902/jop.2010.090630. [DOI] [PubMed] [Google Scholar]
- 16.Ghallab NA, el-Wakeel N, Shaker OG. Levels of salivary IFN-gamma, TNF-alfa and TNF receptor-2 as prognostic markers in (erosive) oral lichen planus. Mediators Inflamm. 2010;2010:847632. doi: 10.1155/2010/847632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCartan BE, Healy CM. The reported prevalence of oral lichen planus: A review and critique. J Oral Pathol Med. 2008;37:447–53. doi: 10.1111/j.1600-0714.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 18.Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K. Current controversies in oral lichen planus: Report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40–51. doi: 10.1016/j.tripleo.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 19.Young SK, Min KW. In situ DNA hybridization analysis of oral papillomas, leukoplakias and carcinomas for human papillomavirus. Oral Surg Oral Med Oral Pathol. 1991;71:726–9. doi: 10.1016/0030-4220(91)90282-h. [DOI] [PubMed] [Google Scholar]
- 20.Miller CS, White DK, Royse DD. In situ hybridization analysis of human papillomavirus in orofacial lesions using a consensus biotinylated probe. Am J Dermatopathol. 1993;15:256–9. doi: 10.1097/00000372-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Jontell M, Watts S, Wallström M, Levin L, Sloberg K. Human papilloma virus in erosive oral lichen planus. J Oral Pathol Med. 1990;19:273–7. doi: 10.1111/j.1600-0714.1990.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 22.Giovannelli L, Campisi G, Colella G, Capra G, Di Liberto C, Caleca MP, et al. Brushing of oral mucosa for diagnosis of HPV infection in patients with potentially malignant and malignant oral lesions. Mol Diagn Ther. 2006;10:49–55. doi: 10.1007/BF03256442. [DOI] [PubMed] [Google Scholar]
- 23.Maitland NJ, Bromidge T, Cox MF, Crane IJ, Prime SS, Scully C. Detection of human papillomavirus genes in human oral tissue biopsies and cultures by polymerase chain reaction. Br J Cancer. 1989;59:698–703. doi: 10.1038/bjc.1989.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox M, Maitland N, Scully C. Human herpes simplex-1 and papillomavirus type 16 homologous DNA sequences in normal, potentially malignant and malignant oral mucosa. Eur J Cancer B Oral Oncol. 1993;29B:215–9. doi: 10.1016/0964-1955(93)90025-a. [DOI] [PubMed] [Google Scholar]
- 25.González-Moles MA, Rodríguez-Archilla A, Ruiz Avila I, Esteban F, González-Moles S, Bravo M. Presence of HPV 16 sequences in oral lichen planus lesions. Bull Group Int Rech Sci Stomatol Odontol. 1998;40:92–7. [PubMed] [Google Scholar]
- 26.Vesper M, Reithdorf S, Christoph E, Ruthke A, Schmelzie R, Loning T. Detection of human papillomavirus (HPV)-DNA in oral manifestations of lichen planus. Mund Kiefer Gesichtschir. 2007;6:146–9. doi: 10.1007/BF03043534. [DOI] [PubMed] [Google Scholar]
- 27.Sand L, Jalouli J, Larsson PA, Hirsch JM. Human papilloma viruses in oral lesions. Anticancer Res. 2000;20:183–8. [PubMed] [Google Scholar]
- 28.Pol CA, Ghige SK, Gosavi SR. Role of human papilloma virus-16 in the pathogenesis of oral lichen planus– An immunohistochemical study. Int Dent J. 2015;65:11–14. doi: 10.1111/idj.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sand L, Jalouli J. Viruses and oral cancer. Is there a link? Microbes Infect. 2014;16:371–8. doi: 10.1016/j.micinf.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 30.OFlatharta C, Flint SR, Toner M, Butler D, Mabruk MJ. Investigation into a possible association between oral lichen planus, the human herpesviruses and the human papillomaviruses. MolDiagn. 2003;7:73–83. doi: 10.1007/BF03260023. [DOI] [PubMed] [Google Scholar]
- 31.Szarka K, Tar I, Fehér E, Gáll T, Kis A, Tóth ED, et al. Progressive increase of human papillomavirus carriage rates in potentially malignant and malignant oral disorders with increasing malignant potential. Oral Microbiol Immunol. 2009;24:314–8. doi: 10.1111/j.1399-302X.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 32.D’souza G, Sugar E, Ruby W, Gravitt P, Gillison M. Analysis of the effect of DNA purification on detection of human papillomavirus in oral rinse samples by PCR. J Clin Microbiol. 2005;43:5526–35. doi: 10.1128/JCM.43.11.5526-5535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majid S, Shafi M. Saliva as a diagnostic toll: A review. J Pharm Bio Sci. 2014;9:09–14. [Google Scholar]


