Abstract
Aim:
To investigate and record the palatal changes in individuals habituated to reverse chutta smoking in rural coastal Andhra population.
Materials and Methods:
Sixty individuals out of whom 47 females and 13 males habituated to reverse smoking with no other tobacco and alcohol habits and no other systemic disturbances were selected. The palatal changes were recorded by six examiners. Database were searched for the following terms “reverse smokers,” “nicotina palatini” and “palatal lesions.”
Results:
The mean and percentage prevalence of the each lesion recorded and agreed by six examiners among 60 subjects showed presence of 87.77% hyperpigmented areas, 64.44% depigmented areas, 51.66% excrescences, 32.22% potentially malignant lesions and 9.72% frank ulcerations.
Conclusion:
Reverse smoking is an endemic tobacco habit still practiced in the coastal rural Andhra Pradesh. It is a well-established and socially acceptable habit among adult females. The changes recorded clinically shows characteristic features that are unique among this population group.
Keywords: Palatal changes, reverse smokers, rural coastal Andhra population, nicotina palatini
INTRODUCTION
Tobacco smoking has a wide regional variation in India, not only in the prevalence but also in the method and use of the smoking device. Reverse smoking is smoking with the lightened end inside the mouth,[1] is a peculiar habit mostly prevalent among adult fishermen of coastal rural Andhra Pradesh, India. The practice of this habit in the local language is called as “Adda Poga.” The smoking device is a homemade cigar made by crudely rolling few semi dried tobacco twigs, called as “chutta.” The word chutta is derived from the Tamil word “churutu” which means “to roll or fold”. Each chutta weighs about 5–7.5 g. On an average, each adult may smoke 3–4 chuttas per day and each chutta lasts for about 15–30 min. Few individuals smoke complete chutta at once or one chutta in 2–3 intervals; each interval lasts about 5–10 min. The characteristic feature of this habit is that the lit extreme of the chutta is kept inside the mouth and then closed, lips seal the mouth thereby allowing slow inhalation of smoke from chutta and the smoke is let out from the unlit end.
This habit of reverse smoking is frequently observed in females than males. According to Gavarasana and Susarla, the frequency of reverse smoking was 6.23 times higher in females than males.[2] The prevalence of this habit among females has been attributed to various reasons like: 1. Females started reverse smoking because they wanted to keep their smoking habit a secret from their husbands and parents. 2. Because of strong winds and splashing water during household work and fishing, the lit end of the chutta would get extinguished if used in the conventional method. 3. Also, fisherwomen during weaving wanted to prevent the hot ashes from falling on to the fishing net to prevent its damage. There are few taboos involved such as: The habit was practiced to reduce the tooth ache, mask halitosis and children were forcefully made to smoke chutta in reverse to get rid of various diseases.
Reverse smoking was also reported in various other populations like rural barangays in Cabanatuan City, Philippines and also from few communities in South America, The Caribbean, The Netherlands, Columbia, Sardinia etc.[1,3,4,5,6] In India, this habit of reverse smoking is predominantly seen in regions of Andhra Pradesh like Srikakulam District, East Godavari District and in states of Orissa and Goa.[2,7,8,9] The female predominance has been consistently reported among all the above communities except in Sardinia.[1]
The clinical manifestations of the oral mucosa in patients with reverse smoking habit are reported to vary from conventional smokers and nonsmoking individuals. The commonly affected areas in reverse smokers are palate and tongue.[10] Lesions associated with this habit range from palatal keratosis, excrescences, leukoplakia, ulcerations to frank malignancy.[11] According to Ortiz 96.7% of Fillipino women with reverse smoking habit exhibited palatal mucosal changes including leukoplakia, mucosal thickening, fissuring, pigmentation, nodularity, erythema and ulceration whereas 26.7% of conventional smokers exhibited mucosal changes predominantly focal pigmentation and mild erythema.[10] According to Van Der EB et al., 33% of total north rural coastal areas of Andhra population practised reverse smoking out of that the prevalence rate of palatal lesions was 55% of the population.[12]
Earlier, these changes induced by reverse smoking were called as nicotinic stomatitis. This led to a controversy as it is well-known that nicotine alone is not the etiological factor. There is no report about the actual terminology yet, the term “palatal changes associated with reverse smoking” or “palatal keratosis associated with reverse smoking” is well-accepted.[6,13] Gupta et al. in 1984 performed a 10-year follow-up study of tobacco usage and oral disease in a random sample of 10,169 individuals of Srikakulam district between 1967 and 1976, they reported that both in men, as well as in women the age-adjusted mortality rates were nearly twice for reverse smokers than those of other tobacco users.[14] We had a unique opportunity to observe and record these palatal mucosal changes from the population of reverse smokers in coastal rural Andhra Pradesh.
MATERIALS AND METHODS
Oral screening and oral health education camp was conducted in a village, Subbamapeta, East Godavari District with a population of 1700 individuals. A total of 60, of these 47 females and 13 males reported to be habituated to reverse smoking, with no other tobacco and alcohol habits and no systemic disturbances. Information regarding their habit history, systemic illness, medication causing pigmentation, family history and reasons for adopting this habit were collected from all 60 subjects with a habit of reverse smoking. Six observers viewed and recorded the mucosal changes. Photographs of palatal changes attributable to reverse smoking were taken using an Olympus digital camera.
An online search was performed, on “reverse smokers,” “nicotina palatini” and “palatal lesions” as search terms. All relevant online articles were collected and pursued for additional articles from the reference list. Articles before 1990's were not available online these articles were collected from Andhra Medical College Library, Vishakapatanam, Andhra Pradesh.
Statistical analysis
Palatal mucosal observations by six examiners were considered. The mean and percentage prevalence of lesions among 60 subjects were computed. Fisher's exact test was used to assess the association between the agreement of six examiners, a P value was calculated by two-tailed t-test. Logistic regression analysis model was used for co-efficient of prediction in presence or absence of a lesion.
RESULTS
Table 1 shows the presence of individual lesions on palatal mucosa among 60 reverse smokers recorded by six examiners. The mean and percentage prevalence of the each lesion recorded and agreed by six examiners among 60 subjects shows presence of 87.77% of hyperpigmented areas, 64.44% of depigmented areas, 51.66% of excrescences, 32.22% of potentially malignant lesions and 9.72% of frank ulcerations. Fisher's exact test was used to test the association between the agreement of six examiners, a P value was established at 0.7203. In the logistic regression analysis model the coefficient of the variable was set at 1, the confidence interval was found to be 95% and P < 0.005 was established.
Table 1.
Presence of individual lesions on palatal mucosa recorded by six observers (n=60)
DISCUSSION
Palatal mucosal changes in reverse smokers were of varying degrees ranging from adaptive changes to potentially malignant lesions and ulcerations. The adaptive changes were hyperpigmentation and excrescence. Depigmented areas are the transition regions between the adaptive and potentially malignant lesions. Potentially malignant lesions were leukoplakia and erythroplakia.
PALATAL CHANGES INDUCED BY REVERSE SMOKING
Hyperpigmentation
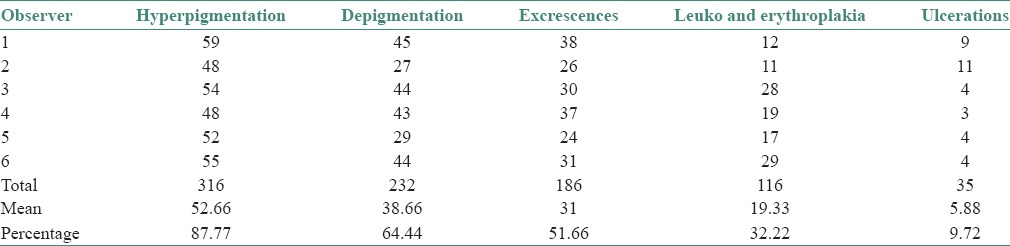
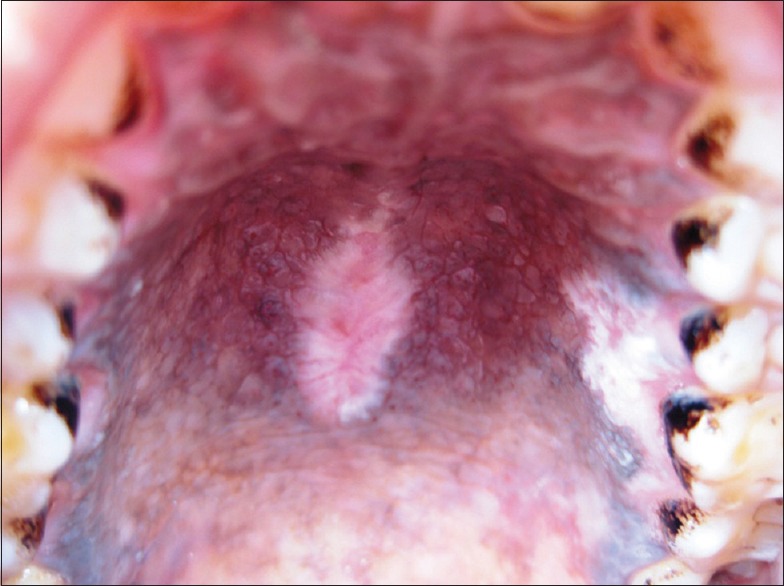
Hyperpigmentation refers to well-defined diffuse or focal greyish black pigmentation of the palatal mucosa due to increased melanin production by melanocyte [Figure 1]. The intensity was varying in all 87% of cases. This pigmentation was limited to the hard palate and had regular margins and was produced due to increased melanin deposition as a protective reaction to heat and its antioxidant properties against toxic products produced during combustion of tobacco within the oral cavity.[15,16] The possibility of drug-induced pigmentation was ruled out by recording history of medication such as quinacrine, amodiaquine, premarin etc.[17,18,19] Physiologic racial pigmentation is generalized, but our cases showed pigmentation only on the palatal mucosa. In the hard palate physiologic racial pigmentation presents as dark brown macules with less well-defined borders, is asymptomatic with no gender preference.[20,21] The results obtained in our study showed an increased association of palatal pigmentation with reverse smoking.
Figure 1.
Diffuse melanin pigmentation of palatal mucosa
Depigmentation
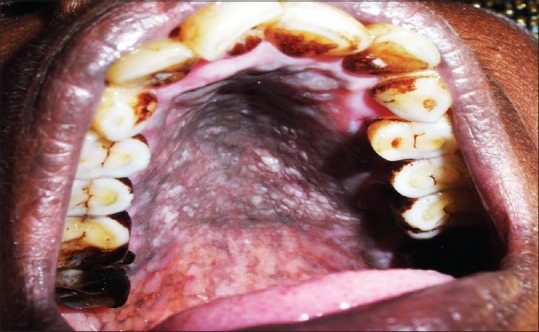
Depigmented areas indicate areas of the palatal mucosa that are clinically devoid of melanin pigmentation. It appeared as focal spots surrounded by hyperpigmentation [Figure 2]. Natali et al. in 1991 reported an observation that heavy drinking and smoking produced oral mucosal changes consisting of splotchy areas of depigmentation surrounded by hyperpigmentation. They concluded that these oral pigment changes identify patients who are heavy smokers and drinkers and may be a useful diagnostic sign.[22] Daftary in 1989 reported 17% of reverse smokers with clinically depigmented areas.[23] Hedin et al. in 1992 histologically studied 80 biopsies from the palate of Indian reverse smokers and reported well localized depigmented areas in 9 of the reverse smokers showing histologically sharp margins and distinct hyperchromasia of the basal epithelium within the depigmented area. Furthermore, the epithelium in these regions had intact rete pegs and only a slight inflammation was found in the connective tissue below these depigmented areas. They concluded that as this area has been deprived of its melanin content, it is probably more easily penetrated by the smoke toxins. It may be a weak area due to loss in the melanin defence barrier of the tissue and later develops into a “red area.”[24] Among 60 reverse smokers included in our study, 64% showed areas of focal depigmentation surrounded by hyperpigmentation. We hypothesize that melanin is a natural antioxidant secreted by melanocytes that plays a role in scavenging the toxic products produced during combustion of tobacco.[16] These depigmented areas are deprived of melanin defence barrier as intense toxic content over a period exceeded the levels of melanocyte to produce melanin or the toxic effects may cause melanocyte function loss.
Figure 2.
Focal areas of depigmentation surrounded by hyperpigmentation
Excrescences
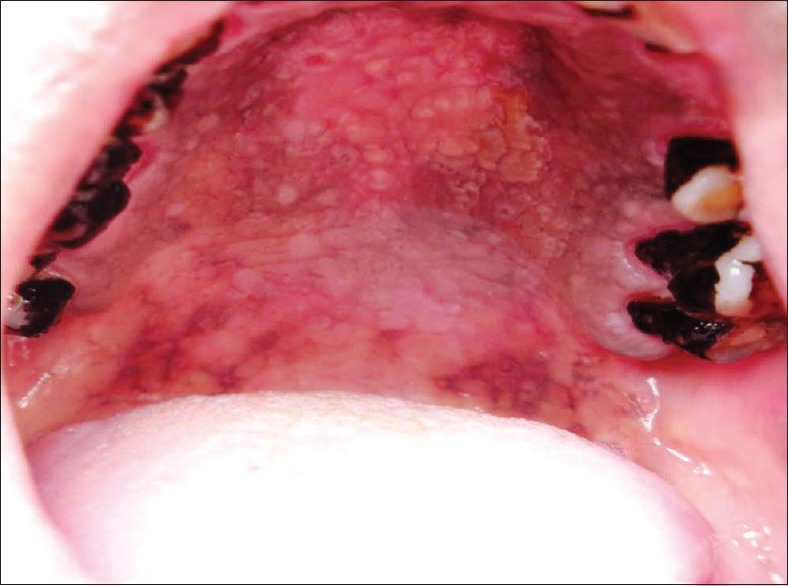
Excrescences clinically resembles nicotina palatine, comprising of 1–3 mm elevated area, often with central red dots marking the orifices of the palatal mucosal glands [Figure 3]. 51% of palatal changes among 60 reverse smokers consisted of excrescences. In few cases, excrescences represented the initial palatal reaction and milder forms of it resembled the smoker's palate in conventional smokers. Ramulu et al. in 1971 stated that when the glandular area of the hard palatal mucosa showed papular elevations upto 2–3 mm in height with central umbilications with or without pigmentation of the surrounding mucosa is taken as stomatitis nicotina.[25] Furthermore, they stated that there may be many of these painless papular lesions in the glandular part of the hard palatal mucosa and are not present in the soft palate or in the anterior half or third of the hard palate and will not extend up to the alveolar margin.[25] Thoma et al., described nicotine stomatitis as a lesion that occurs in pipe smokers in regions that are not covered by dentures.[26] Saunders described nicotine stomatitis as a papular leukoplakia in the palate that is caused by tobacco smoke striking the palate more directly than other regions. He described the red circular areas as the orifices of the mucous glands.[21] Schwartz also thought that this type of lesion was caused by tobacco.[27] Van Wyk considered these lesions to be associated with a long history of smoking, especially pipe smoking.[28] These lesions are reversible if smoking is stopped.[29] Gavarasana and Susarla in 1989 reported the frequency of stomatitis nicotina to be 7.13 times higher in reverse smokers than in conventional chutta smokers.[2] Excrescences in reverse smokers differ from the nicotina palatini seen in conventional smokers by its clinical, biological behavior and higher malignant potential.[30] On histopathological examination Reddy et al. reported that earliest atypical changes in the orifices of the ducts of the glands and the papules with umblication is due to hyperplasia of the mucous glands.[25] Ramulu et al., in 1973 noted a strong correlation between reverse smoking, nicotina palitini and carcinoma of the palate. They found that nicotina palitini showed atypical changes of the epithelium and 2.4% showed microinvasive carcinoma. Hence, they concluded that nicotina palitini among reverse smokers could be precancerous as both the surface epithelium and ductal epithelium undergoes hyperplasia and hypertrophy initially in response to heat and chemicals released during reverse smoking. The ductal lining undergoes dysplastic changes from which microinvasive carcinoma and then invasive carcinoma may arise.[31] Mehta et al. in 1977 stated that excrescences were transient in nature and tend to regress with the discontinuation of the habit. They concluded that this is the initial and most immediate response to reverse smoking habit. The hypertrophied ductal openings of minor salivary glands act as portal of entry for the toxins of tobacco smoke and the pseudostratified columnar epithelial linings of ducts is found to undergo squamous metaplasia in response to chronic irritation caused by heat and chemicals released during reverse smoking and this metaplastic squamous epithelium has got high malignant potential and may be responsible for malignancy in this component.[11] Lavanya et al. in 2006 stated that if carcinoma arises from an excrescence, it can be mainly from the metaplastic squamous epithelium of ductal lining of minor salivary glands but may not be from the palatal epithelium.[32] Ramulu et al. observed reversibility of stomatitis nicotina and papular lesions disappear and only pigmentation remains when the individuals stops practicing the habit.[25]
Figure 3.
Excrescences, elevated orifices of the palatal mucosal glands
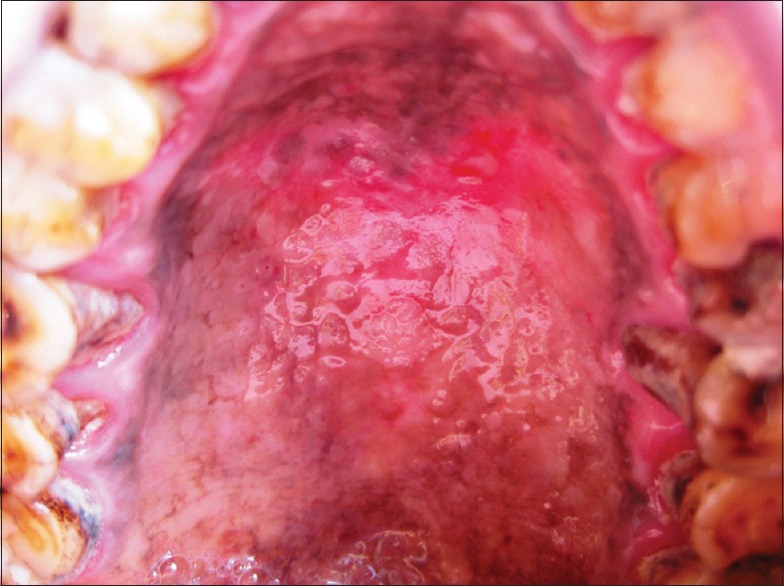
Potentially malignant lesions
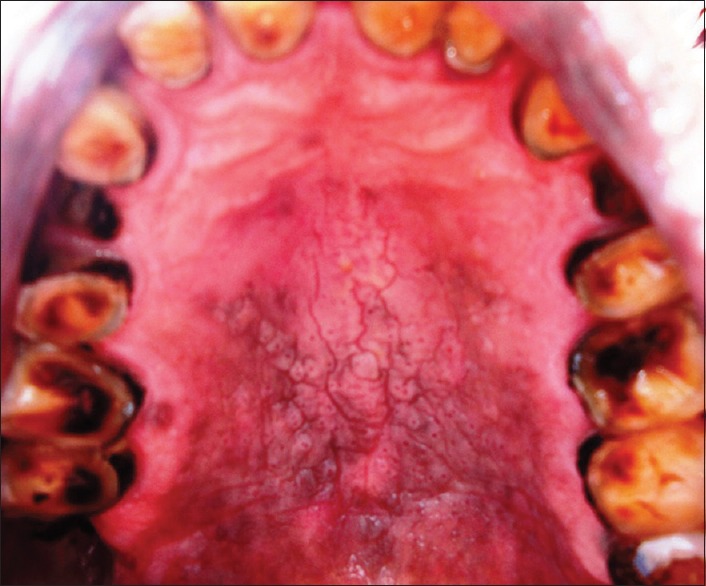
Non scrapable, well-defined, elevated plaques, white in color were observed and clinically termed as leukoplakia [Figure 4]. In few individuals, red lesions were also evident together with leukoplakia constituting 32% of our study sample. Gavarasana and Susarla in 1989 reported the frequency of preleukoplakia to be 2.26 times higher; leukoplakia was 13.84 times higher in reverse smokers compared to the conventional chutta smokers.[2] Van Der EB et al. in 1993 reported 9.8% prevalence rates of leukoplakia palati and confirmed a strong association of leukoplakia with reverse smoking than conventional chutta smoking.[12]
Figure 4.
Leukoplakia around areas of depigmentation
Ulcerations
We report 9.72% of 60 reverse smokers showed frank ulcerations which refers to red area with crater or excavation of varying degree [Figure 5]. These ulcerations were chronic, symptomatic and were observed in the depigmented areas surrounded by hyperpigmentation. On the basis of clinical history, we attribute that these chronic non- healing ulcers are of malignant nature. Carcinoma associated with reverse smoking and especially in women of Vishakapatnam district was reported first by Kini and Rao in 1937 and supported by Khanolkar and Suryabai in 1945.[25] Reddy et al. in 1974 stated that a reverse smoking female runs a relative risk of developing carcinoma of the hard palate 47 times more than that of a nonsmoking female.[33]
Figure 5.
Ulceration, red areas with crater or excavation of varying degree
According to Reddy et al. in 1976 carcinoma of palate in reverse smokers usually occurs in the middle of the posterior half of the hard palate and not in the soft palate or in the anterior half of the hard palate. They concluded that the localization of carcinoma in reverse smokers corresponds to the glandular area of the hard palate.[33] According to Qugley et al. in 1965 the reason for this malignant transformation may be due to excessive heat generated during reverse smoking rising the palatal temperature to upto 120° C and also because of the higher content of nicotine, total particulate matter and incomplete combustion of the chutta tobacco.[34] Reddy and Kumari in 1974 reported microinvasive carcinoma of the hard palate in reverse smoking females.[35] Mehta FS (1977) reported epithelial dysplasia as one of the histological change in various palatal changes as it was found in 23% of 101 biopsies with the highest prevalence being in the red areas.[11] Van Der EB et al. in 1993 stated that reverse chutta smoking was a major determinant of palatal cancer as all the cases diagnosed as palatal carcinoma were observed within the group of reverse smokers.[12]
CONCLUSION
Reverse smoking is one of the endemic tobacco habit still practised in the coastal rural Andhra Pradesh. It is a well-established habit among the adult females of this region. The clinically evident mucosal changes were very characteristic and unique. Precancerous palatal changes and carcinoma of the palate were increasingly associated with reverse smokers. In this study, an attempt was made to highlight the changes of the palatal mucosa of reverse chutta smokers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pindborg JJ, Mehta FS, Gupta PC, Daftary DK, Smith CJ. Reverse smoking in Andhra Pradesh, India: A study of palatal lesions among 10,169 villagers. Br J Cancer. 1971;25:10–20. doi: 10.1038/bjc.1971.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavarasana S, Susarla MD. Palatal mucosal changes among reverse smokers in an Indian Village. Jpn J Cancer Res. 1989;80:209–11. doi: 10.1111/j.1349-7006.1989.tb02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortiz GM, Pierce AM, Wilson DF. Palatal changes associated with reverse smoking in Filipino women. Acta Cytol. 1996;19:28–31. doi: 10.1111/j.1601-0825.1996.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 4.Stoykewych AA, DeBrouwere R, Curran JB. Reverse smoking and its effects on the hard palate: A case report. J Can Dent Assoc. 1992;58:215–6. [PubMed] [Google Scholar]
- 5.Hogewind WF, Greebe RB, van der Waal I. Reverse smoking in the Netherlands. Int J Oral Maxillofac Surg. 1987;16:500–4. doi: 10.1016/s0901-5027(87)80093-0. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez Gómez GJ, Alvarez Martínez E, Jiménez Gómez R, Mosquera Silva Y, Gaviria Núñez AM, Garcés Agudelo A, et al. Reverse smokers's and changes in oral mucosa. Department of Sucre, Colombia. Med Oral Patol Oral Cir Bucal. 2008;13:E1–8. [PubMed] [Google Scholar]
- 7.Reddy CR, Prahlad D, Ramulu C. Incidence of oral cancer with particular reference to hard palate cancer in 1 million population in the District of Visakhapatnam. Indian J Cancer. 1975;12:72–6. [PubMed] [Google Scholar]
- 8.Rajkumar NG, Bharath TS, Manjunath K, Saraswathi TR, Ramachandran CR. Cytological changes and patterns of keratinization in palatal mucosa of reverse smokers: A pilot study. J Orofac Sci. 2010;2:7–11. [Google Scholar]
- 9.Malik SK, Behera D, Jindal SK. Reverse smoking and chronic obstructive lung disease. Br J Dis Chest. 1983;77:199–201. doi: 10.1016/0007-0971(83)90028-1. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz GM, Pierce AM, Wilson DF. Palatal changes associated with reverse smoking in Filipino women. Oral Dis. 1996;2:232–7. doi: 10.1111/j.1601-0825.1996.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 11.Mehta FS, Jalnawalla PN, Daftary DK, Gupta PC, Pindborg JJ. Reverse smoking in Andhra Pradesh, India: Variability of clinical and histologic appearances of palatal changes. Int J Oral Surg. 1977;6:75–83. doi: 10.1016/s0300-9785(77)80002-1. [DOI] [PubMed] [Google Scholar]
- 12.van der Eb MM, Leyten EM, Gavarasana S, Vandenbroucke JP, Kahn PM, Cleton FJ. Reverse smoking as a risk factor for palatal cancer: A cross-sectional study in rural Andhra Pradesh, India. Int J Cancer. 1993;54:754–8. doi: 10.1002/ijc.2910540508. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran R, Sivapathasundharam B. 6th ed. New Delhi (India): Elsevier Pvt. Limited; 2009. Shafer's Text Book of Oral Pathology. [Google Scholar]
- 14.Gupta PC, Mehta FS, Pindborg JJ. Mortality among reverse chutta smokers in South India. Br Med J (Clin Res Ed) 1984;289:865–6. doi: 10.1136/bmj.289.6449.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DS, Park SH, Kwon SB, Joo YH, Youn SW, Sohn UD, et al. Temperature regulates melanin synthesis in melanocytes. Arch Pharm Res. 2003;26:840–5. doi: 10.1007/BF02980030. [DOI] [PubMed] [Google Scholar]
- 16.Langfelder K, Streibel M, Jahn B, Haase G, Brakhage AA. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet Biol. 2003;38:143–58. doi: 10.1016/s1087-1845(02)00526-1. [DOI] [PubMed] [Google Scholar]
- 17.Lippard VW, Kauer GL., Jr Pigmentation of the palate and subungual tissues associated with suppressive quinacrine hydrochloride therapy. Am J Trop Med Hyg. 1945;25:469–71. doi: 10.4269/ajtmh.1945.s1-25.469. [DOI] [PubMed] [Google Scholar]
- 18.Watson IB, MacDonald DG. Amodioquine induced oral pigmentation – A light and electron microscopic study. J Oral Pathol. 1974;3:16–21. doi: 10.1111/j.1600-0714.1974.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 19.Pérusse R, Morency R. Oral pigmentation induced by Premarin. Cutis. 1991;48:61–4. [PubMed] [Google Scholar]
- 20.Mirowski GW, Waibel JS. Pigmented lesions of the oral cavity. Dermatol Ther. 2002;15:218–28. [Google Scholar]
- 21.Kauzman A, Pavone M, Blanas N, Bradley G. Pigmented lesions of the oral cavity: Review, differential diagnosis, and case presentations. J Can Dent Assoc. 2004;70:682–3. [PubMed] [Google Scholar]
- 22.Natali C, Curtis JL, Suarez L, Millman EJ. Oral mucosa pigment changes in heavy drinkers and smokers. J Natl Med Assoc. 1991;83:434–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Daftary DK. Personal Communication. 1989 [Google Scholar]
- 24.Hedin CA, Pindborg JJ, Daftary DK, Mehta FS. Melanin depigmentation of the palatal mucosa in reverse smokers: A preliminary study. J Oral Pathol Med. 1992;21:440–4. doi: 10.1111/j.1600-0714.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 25.Reddy CR, Kameswari VR, Ramulu C, Reddy PG. Histopathological study of stomatitis nicotina. Br J Cancer. 1971;25:403–10. doi: 10.1038/bjc.1971.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thoma KN. Stomatitis nicotina and its effect on the palate. Am J Orthod. 1941;27:38–47. [Google Scholar]
- 27.Saunders WH. Nicotine stomatitis of the palate. Ann Otol Rhinol Laryngol. 1958;67:618–27. doi: 10.1177/000348945806700303. [DOI] [PubMed] [Google Scholar]
- 28.van Wyk CW. Nicotinic stomatitis of the palate: A clinico-histological study. J Dent Assoc S Afr. 1967;22:106–11. [PubMed] [Google Scholar]
- 29.Sutherland KG. The pathology and treatment of diseases of the palate. Aust Dent J. 1968;13:111–24. doi: 10.1111/j.1834-7819.1968.tb02248.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramulu C, Reddy CR. Carcinoma of the hard palate and its relation to reverse smoking. Int Surg. 1972;57:636–41. [PubMed] [Google Scholar]
- 31.Ramulu C, Ratnam GV, Kameswari VR, Raju MV, Reddy CR. Regression of stomatitis nicotina in reverse smokers. Indian J Med Res. 1973;61:1328–34. [PubMed] [Google Scholar]
- 32.Lavanya S, Sriram G, Sivapathasundharam B. Cytomorphometric analysis of palatal mucosal cells in reverse smokers. J Oral Maxillofac Pathol. 2006;10:69–75. [Google Scholar]
- 33.Reddy CR, Venkatarathnam G, Kameswari VR. Distribution of glands in the mucosa of the hard palate and its relation to carcinoma. J Oral Surg. 1976;34:232–6. [PubMed] [Google Scholar]
- 34.Quigley LF, Jr, Cobb CM, Hunt EE., Jr Measurement of oral and burning zone temperatures during conventional and reverse cigarette smoking. Arch Oral Biol. 1965;10:35–44. doi: 10.1016/0003-9969(65)90055-5. [DOI] [PubMed] [Google Scholar]
- 35.Reddy CR, Kumari KR. Microinvasive carcinoma of hard palate in reverse smoking females. Indian J Cancer. 1974;11:386–93. [PubMed] [Google Scholar]


