Abstract
Background:
Diabetes mellitus is the fifth most common chronic condition and the sixth most frequent cause of death among the elderly. The objective of this research was to develop a new method for diabetes diagnosis by analysis of the glycogen content of the oral epithelial cells.
Materials and Methods:
Ten control subjects and ten diabetic patients (study group) were taken, four oral smears for both control and study group from the buccal mucosa were taken and stained with hematoxylin and eosin stain, Papanicolaou (PAP) stain, periodic acid Schiff (PAS) stain and PAS-Diastase (PAS-D) stain.
Results:
The results showed that in the diabetic group: (i) The epithelial cells stained with PAP stain exhibited figures of binucleation and occasional karyorrhexis, (ii) the epithelial cells treated with PAS-D showed that glycogen containing cells did not take up the stain as compared to the other cells.
Conclusion:
The results associated with clinical and histological observations suggest that diabetes mellitus can produce alterations of oral epithelial cells as well as in their glycogen content.
Keywords: Diabetes mellitus, exfoliative cytology, periodic acid Schiff, periodic acid Schiff-diastase
INTRODUCTION
Diabetes mellitus represents an extreme disturbance in glucose metabolism with severe hyperglycemia and insulin deficiency. Diabetes mellitus is the fifth most common chronic condition and the sixth most frequent cause of death among the elderly.[1] An estimated 100 million people are affected by diabetes mellitus worldwide.[2] Hyperglycemia can be associated with several oral complications. Tissue repair is affected and dysfunction of the oral mucosa occurs due to alterations in salivary flow[3,4,5] and constituents, changes in nutrition and reduced immune defenses leading to changes in microbial oral flora and a greater tendency to infections. As a result, xerostomia; candidiasis; increased incidence of dental caries, gingivitis and periodontitis; periapical abscess; parotid enlargement and burning mouth syndrome are prevalent in diabetics.[4,6] Therefore, early diagnosis of the diabetes mellitus is an important aspect of health care.
Glycogen fails to stain with conventional hematoxylin solutions and only stains weakly, if at all, with eosin. Diastase is the commonly employed form of glycogen digestion enzyme as it is easy to use, stable and comparatively cheap.[7] The aim of our study was to study the effects of diastase enzyme on the glycogen content of the epithelial cells of mucosa of normal and diabetic patients in exfoliative cytology and to compare the staining quality in mucosa of normal and diabetic patients using hematoxylin and eosin (H and E) stain; Papanicolaou (PAP) stain, periodic acid Schiff (PAS) and PAS-Diastase (PAS-D) stains, respectively.
MATERIALS AND METHODS
It is a case-control study involving a total of 20 subjects (10 cases and 10 controls). Diagnosed cases of diabetes were selected. The method used to estimate the values of blood sugar levels (BSL) (fasting and postprandial [PP]) was glucose oxidase method. An informed consent was taken from the patient.
Patients with habits (tobacco chewing and smoking), other systemic diseases/malignancies/taking medications other than the diabetic medications were excluded.
Subjects were instructed to gargle their mouth with water. Smears were taken from the mucosa of normal (control) and diabetic (study) patients using a wooden spatula and transferred onto clean glass slides. The glass slides (40) were then immediately fixed in 95% ethyl alcohol and stained using H and E (10 slides), PAP (10 slides), PAS (10 slides) and PAS-D (10 slides) stains for both the groups.
For PAS-D, the slides were first incubated in diastase solution at 37°C for 1-h, washed and then stained with PAS stain. The method given in Bancroft was followed for staining of PAS-D.[7] The staining quality in the cells and their glycogen content was studied in both the groups and then compared.
RESULTS
In our study, blood glucose level was estimated using glucose oxidase method. It is used to detect both hypoglycemia and hyperglycemia and helps in diagnosis of diabetes. Glucose oxidase is an enzyme highly specific for glucose and does not react with blood saccharides. So, it has been employed for the estimation of blood glucose. The principle used in PAS-D stain was that diastase (or α-amylase) acts on glycogen to depolymerize it into smaller sugar units, maltose and glucose, that are washed out of the section. Glycogen will be stained magenta on PAS [Figure 1] stained slide and will be absent on the PAS-D [Figure 2] stained slide because of glycogen digestion by the diastase enzyme. Such detection of glucose can help in the diagnosis of diabetes.
Figure 1.
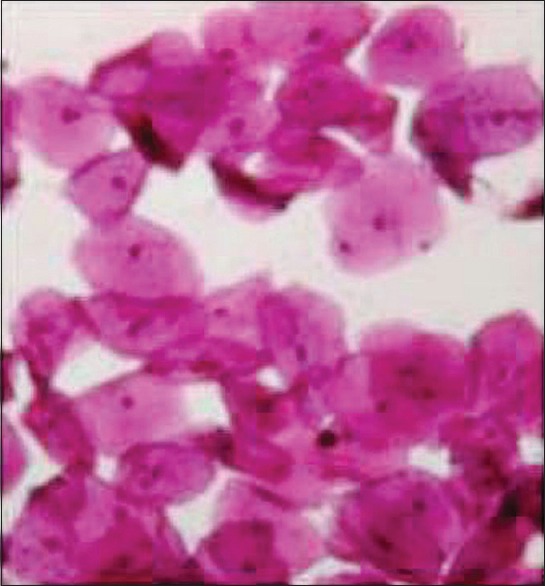
Photomicrograph of buccal exfoliated cells (PAS stain, ×400)
Figure 2.
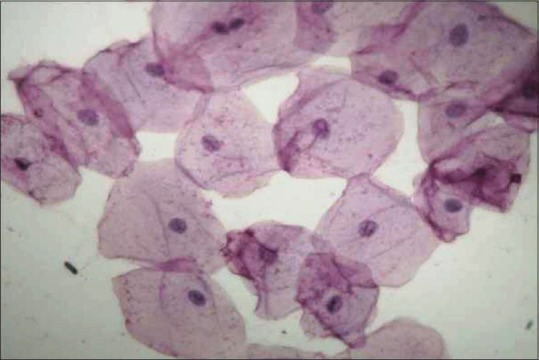
Photomicrograph of buccal exfoliated cells (PAS-D stain, ×400)
On comparison of the mean and standard deviation values during fasting and PP, we found that high BSLs were seen in the study group as compared to that of the control group [Table 1]. By applying Student's unpaired t-test, there was a highly significant difference between mean values of BSL when compared to control versus study group (i.e., P < 0.01). Also, it was seen that the mean values of BSL both fasting and PP was more in the study group as compared to the control group [Table 2]. By applying Student's unpaired t-test, there was a highly significant difference between mean values of BSL when compared to fasting versus PP in both control and study group (i.e., P < 0.01) [Table 3].
Table 1.
Mean and standard deviation values of blood sugar level in control and study group
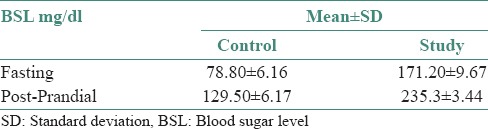
Table 2.
Comparison of mean values of blood sugar level in control and study group
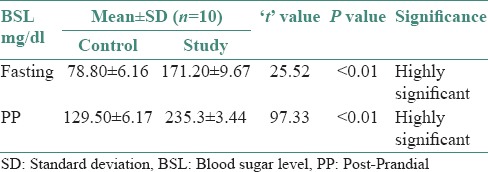
Table 3.
Comparison of mean values of both fasting and post-prandial blood sugar levels in control and study group
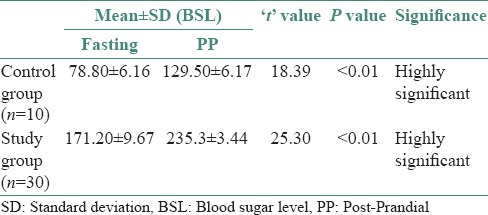
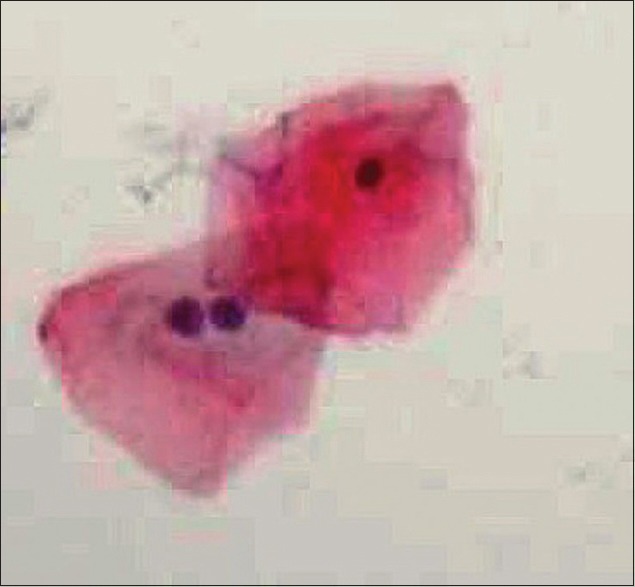
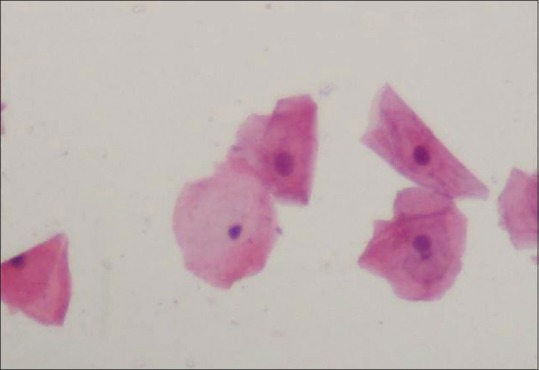
The study group exhibited nuclear changes such as binucleation [Figure 3], decreased cytoplasmic/nuclear ratio, nuclear enlargement [Figure 4] and enucleation [Figure 5] in PAP stained slides as compared to that of control group. Inflammatory cells were seen in both study and control group. No significant differences were found in H and E stained slides.
Figure 3.
Photomicrograph of exfoliated cells exhibiting binucleation (H&E stain, ×400)
Figure 4.
Photomicrograph of exfoliated cells exhibiting nuclear enlargement (PAS stain, ×400)
Figure 5.
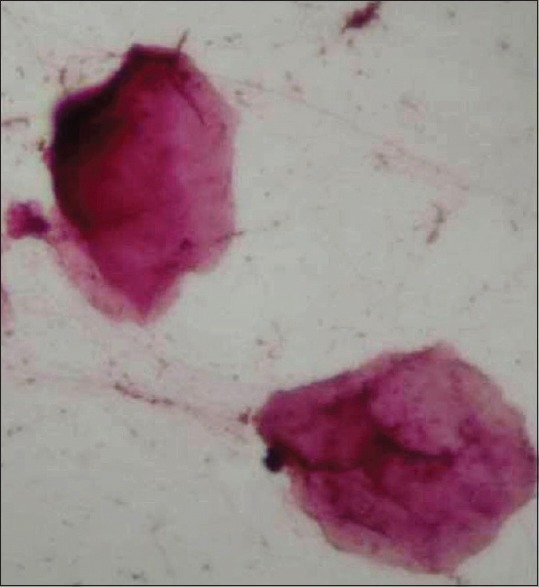
Photomicrograph of exfoliated cells exhibiting enucleation (H&E stain, ×400)
DISCUSSION
Diabetes mellitus, a complex metabolic disorder, is a syndrome characterized by abnormalities in carbohydrates, lipid and protein metabolism that results either from a profound or an absolute deficiency of insulin (Type I) or from target tissue resistance to its cellular metabolic effects (Type II).[8]
Although many of the pathological processes affecting the oral mucosa are clinically distinguishable, most lesions require a definitive diagnosis before the appropriate therapy may be commenced. The most accepted clinical technique for the diagnosis of lesions of the oral mucosa is an incisional or excisional biopsy. In specific clinical conditions, such as diabetes, a great many invasive techniques lose viability as a result of variations in blood glucose and the disease itself.[9] In these cases, oral exfoliative cytology may be more appropriate.
A cytologic smear is an advantageous diagnostic procedure because it is noninvasive, relatively painless, inexpensive and requires a minimum of technical skill. It is useful when a patient refuses to have a biopsy performed or when medically compromised patients would be exposed to unnecessary surgical risks and anxious patients can be reassured quickly about the nature of oral mucosal changes, especially when a fear of cancer or a family history of cancer accounts for their apprehension.[10] In a study developed in Sudan, the oral cytologic analysis is proposed as a useful early diagnostic method for epithelial atypia and therefore also for malignant oral lesions.[11,12]
In our study, we compared the slides of H and E, PAP, PAS, PAS-D in both control and study group and found that the cells in the study group exhibited binucleation, decreased cytoplasmic/nuclear ratio, nuclear enlargement, enucleation and inflammation as compared to that of control group, which is similar to the findings of Jajarm et al.[1] Shareef et al.[2] and Alberti et al.[9] who also found that diabetes mellitus can produce alterations in oral epithelial cells, detectable by microscopy and cytomorphometry, which can be used in evaluation of this disease.
Our study evaluated whether diabetes diagnosis can be made by analysis of the glycogen content of the oral epithelial cells in diabetics. We compared the mean values of BSL in control and study group and found that mean values of BSL fasting and PP is more in study group as compared control group which is highly significant with a P < 0.01. Because of the increased glucose in diabetics, their epithelial cells showed less staining when stained with PAS-D, since glycogen is digested by the diastase enzyme treatment prior to PAS staining.
In our study, the oral smears obtained from diabetic and control groups contained cellular representatives of all the epithelial strata, from nonkeratinized cells to the completely keratinized ones. These patients presented cellular alterations, both quantitative and qualitative, at all differentiation levels.
We found that nuclear changes were significantly higher in the diabetic group than in the control group that was similar to the findings of Jajarm et al.[1] This could be related to increased cellular age in patients with diabetes. Decreased cellular turnover might be a secondary reaction to ischemia caused by atherosclerosis in diabetic patients.[13] Thus as a result of ischemia, cellular turnover would decrease and limited production of young cells would mean that the majority of cells are old or aged.
We found a significant increase in inflammation in the diabetic group in comparison with the control group. This might result from decreased salivary flow in diabetes, due to hypofunction of the salivary glands secondary to adverse hormonal, microvascular and neuronal changes.[3,14]
Conner et al.,[14] reported a decrease in the salivary flow of diabetic patients, probably related to systemic dehydration (polyuria), medicament interference (diuretics) and/or membranopathy of the ducts.
From our study, we can say that exfoliative cytology is useful as an additional tool to aid in the diagnosis of diabetes mellitus. Furthermore, studies with greater sample size and comparison to other conditions causing similar cytomorphometric changes are needed to determine the predictive value of this method.
CONCLUSION
Thus, from our study, it can be said that exfoliative cytology can be used as a tool for diagnosis as well as for screening the patients for diabetes using PAS-D stain. However, larger sample size studies should be performed to come to a definite conclusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jajarm HH, Mohtasham N, Moshaverinia M, Rangiani A. Evaluation of oral mucosa epithelium in type II diabetic patients by an exfoliative cytology method. J Oral Sci. 2008;50:335–40. doi: 10.2334/josnusd.50.335. [DOI] [PubMed] [Google Scholar]
- 2.Shareef BT, Ang KT, Naik VR. Qualitative and quantitative exfoliative cytology of normal oral mucosa in type 2 diabetic patients. Med Oral Patol Oral Cir Bucal. 2008;13:E693–6. [PubMed] [Google Scholar]
- 3.Chávez EM, Borrell LN, Taylor GW, Ship JA. A longitudinal analysis of salivary flow in control subjects and older adults with type 2 diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:166–73. doi: 10.1067/moe.2001.112054. [DOI] [PubMed] [Google Scholar]
- 4.Little JW, Falace DA, Miller CS, Rhodis NL. 6th ed. St. Louis: Mosby; 2002. Dental Management of the Medically Compromised Patient; pp. 248–68. [Google Scholar]
- 5.Newman MG, Takei HH, Klokkevold PR, Carranza FA. 10th ed. S.t Louis: Saunders; 2006. Carranza's Clinical Periodontology; pp. 320–2. [Google Scholar]
- 6.Greenburg MS, Glick M. 10th ed. Hamilton: BC Decker; 2003. Burket's Oral Medicine: Diagnosis and Treatment; pp. 563–72. [Google Scholar]
- 7.Bancroft J, Stevens A. 2nd ed. New York: Churchill-Livingstone; 1980. Theory and Practice of Histological Techniques; pp. 187–8. [Google Scholar]
- 8.Vernillo AT. Diabetes mellitus: Relevance to dental treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:263–70. doi: 10.1067/moe.2001.114002. [DOI] [PubMed] [Google Scholar]
- 9.Alberti S, Spadella CT, Francischone TR, Assis GF, Cestari TM, Taveira LA. Exfoliative cytology of the oral mucosa in type II diabetic patients: Morphology and cytomorphometry. J Oral Pathol Med. 2003;32:538–43. doi: 10.1034/j.1600-0714.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones AC, Pink FE, Sandow PL, Stewart CM, Migliorati CA, Baughman RA. The Cytobrush Plus cell collector in oral cytology. Oral Surg Oral Med Oral Pathol. 1994;77:95–9. [PubMed] [Google Scholar]
- 11.Acha A, Ruesga MT, Rodríguez MJ, Martínez de Pancorbo MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Med Oral Patol Oral Cir Bucal. 2005;10:95–102. [PubMed] [Google Scholar]
- 12.Ahmed HG, Idris AM, Ibrahim SO. Study of oral epithelial atypia among Sudanese tobacco users by exfoliative cytology. Anticancer Res. 2003;23:1943–9. [PubMed] [Google Scholar]
- 13.Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: Placement to 36 months. Ann Periodontol. 2000;5:157–65. doi: 10.1902/annals.2000.5.1.157. [DOI] [PubMed] [Google Scholar]
- 14.Conner S, Iranpour B, Mills J. Alteration in parotid salivary flow in diabetes mellitus. Oral Surg Oral Med Oral Pathol. 1970;30:55–9. doi: 10.1016/0030-4220(70)90011-3. [DOI] [PubMed] [Google Scholar]


