Abstract
Diverse environmental and physiological factors are known to induce the transcription of a set of genes encoding special protective molecules known as “molecular chaperones” within our cells. Literature abounds in evidence regarding the varied roles; these “guides” can effectively perform in our system. Highly conserved through evolution, from the prokaryotes to the eukaryotes, these make perfect study tools for verifying their role in both the pathogenesis as well as the therapeutics of varied neurodegenerative, autoimmune and potentially malignant disorders and varied cancer states. We present a concise review of this ever dynamic molecule, highlighting the probable role in a potentially malignant disorder, oral lichen planus.
Keywords: Cancer therapeutics, cytoprotection, molecular chaperones, heat shock response
INTRODUCTION
“The advent of science and new discoveries has unearthed the wonder lying within us. The very essence of life, the wondrous molecules defending us and laboring hard for every breath we take.”
Diverse environmental and physiological factors are known to induce a set of genes encoding special protective molecules known as “molecular chaperones” within our cells. This phenomenon is called as the heat shock response (HSR), which is an ordered genetic response.[1] The HSR was first discovered by Ritossa,[2] who observed a pattern of Drosophila salivary gland chromosome puffs which were induced as a response phenomenon to transient exposure to elevated temperatures. Since then, many investigators have proven it to be indeed ubiquitous and highly conserved – in all organisms from prokaryotes to eukaryotes, – an essential defense mechanism, protecting the cells from a wide range of harmful conditions.[3,4]
There are many “stressors” that can presumably activate the transcription of these heat shock genes. The list includes various acute and chronic conditions such as elevated temperatures, heavy metals, small molecule chemical toxicants, infection and oxidative stress. Mutations and environmental influences including inflammation, ischemia, tissue wounding and repair, cancer and neurodegenerative diseases are also associated with the aberrant expression of heat shock proteins (HSPs).[1,5] Once expressed, varied roles are modulated via these molecules [Figure 1].
Figure 1.
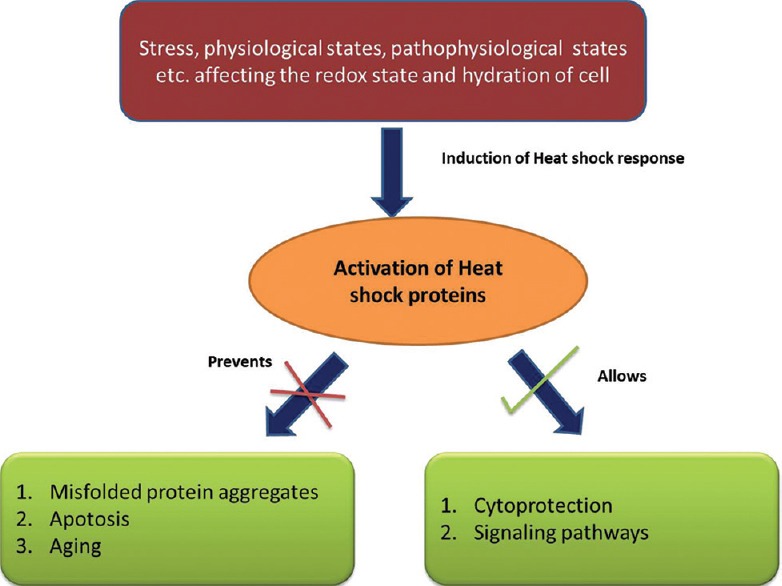
Activation of heat shock proteins and its varied effector functions
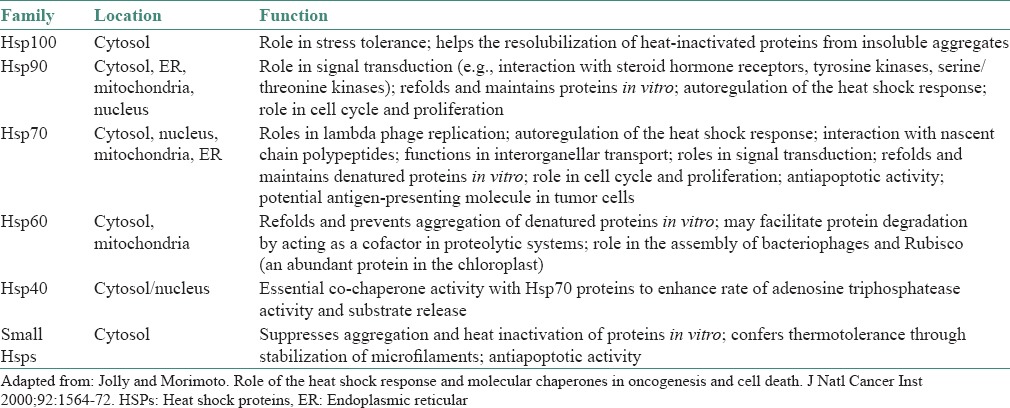
At the molecular level, the cellular response to stress is demonstrated by the induced synthesis of HSPs, of which molecular chaperones and proteases represent two well-characterized families of proteins. The molecular chaperones chiefly function in protein folding, translocation and refolding of intermediates, whereas the proteases such as the ubiquitin-dependent proteasome, ensure that damaged and short-lived proteins are degraded or destroyed in an effective manner. Under “stressed” conditions the molecular chaperones are directed toward the capture of folding intermediates to prevent misfolding and aggregation and to facilitate refolding or degradation.[6,7,8] HSPs have been classified into six major families according to their molecular sizes: Hsp100, Hsp90, Hsp70, Hsp60, Hsp40 and small HSPs (sHSPs) with sizes ranging from 15 kDa to 30 kDa. High molecular weight HSPs are also known as adenosine triphosphate (ATP)-dependent chaperones. They assist in the folding of newly synthesized or damaged proteins in an ATP-dependent active process. In contrast, sHSPs work in an ATP-independent fashion.[9] Members of each gene family are constitutively expressed, inducibly regulated and/or targeted to different compartments [Table 1].
Table 1.
The HSP family and its varied functions
The molecular analysis of HSP genes in eukaryotes have also identified the heat shock element (HSE). This is a promoter element essential for heat shock inducibility in response to the previously mentioned various conditions of stress and comprises multiple adjacent inverted arrays of the binding site (5_-nGAAn-3_). HSEs are positioned at various distances upstream of transcription initiation; in vertebrates, inducible transcription requires the de novo binding of heat shock transcription factors (HSFs) transiently to the HSEs.[10,11] A family of HSF regulate the HSR at the transcriptional level.[12] Of the three human HSF genes, HSF 1, -2 and -4; HSF1 is the best characterized and essential for the HSR. Under normal conditions, HSF1 largely exists as a repressed monomer in the cytoplasm and is thought to be bound, directly or indirectly, by the protein chaperones Hsp90, Hsp70 and Hsp40.
It is a multi-domain stress-activated transcription factor consisting of an amino-terminal helix, winged-loop helix DNA binding domain, three leucine zipper domains (LZ1-3) that form coiled-coil interactions to facilitate HSF1 multimerization, a central regulatory domain that is extensively modified by phosphorylation, acetylation and sumolyation, an additional LZ4 domain and a carboxyl-terminal transcription activation domain.[13] Under varied conditions of stress the HSF1 derepresses, trimerizes and accumulates in the nucleus. HSF1 trimers subsequently bind with high affinity to the previously mentioned HSEs. These consist of inverted repeats of consensus sequence nGAAn. The binding occurs in varied orientations.[14] This then leads to an up-regulation in the expression of HSPs in the cell-HSR. The elevated levels of protective and adaptive response, also known as induced thermotolerance, ensures that the cell responds rapidly to repeated sub-acute challenges by diverse conditions of cell stress.[15] This lead us to propose that the induction of the HSR may have broad therapeutic benefits in the treatment of various types of tissue trauma and disease.
Regulation of heat shock transcription factors
Apart from induction by misfolded protein aggregates, altered intracellular redox status caused by changes in temperature or other stresses have been suggested to be involved in the activation of mammalian HSF1.[16] A role for stress-specific pathways in HSF1 activation has also been suggested.[17,18] The balance of kinase and phosphatase activities acting on HSF1 is of fundamental importance to the regulation of the HSR, as suggested by mathematical modeling.[19] HSF1 is negatively regulated by feedback control through interaction with Hsp70 and Hsp90.[12] In cells expressing high levels of these chaperones, the inducible expression of heat shock genes is affected. Lately, HSF1 as well as other HSFs have also shown to be able to interact or cooperate with signal transducers and activators of transcriptions (STATs) (STAT1 and STAT3) or nuclear factor interleukin-6 family members. It has been concluded that STAT1 can interact with p53 and that both of these factors are able to modulate the effects of HSF1 on HSP expression.[20]
Heat shock proteins as the “coordinating mediators of immunology”
The coordinated response by the innate immunity and the adaptive immunity is essential for efficient immune response. Taking antitumor immunity as an example, the first line of defense is mediated by natural killer cells which are part of the innate immunity.[21,22] These cause lysis of the tumor cells and the cross-presentation of antigens by dendritic cells (DCs) to prime adaptive T-cells. Activated T-cells in turn release cytokines or express cluster of differentiation (CD40) ligand on their cell surface to reciprocally activate DCs. It is suggested that HSPs may play important roles in both innate and adaptive immunity. DCs are activated by a range of microbial molecules, one such being lipopolysaccharide (LPS) which in turn trigger adaptive T- and B-cell immunity.[23]
Studies thus far suggest that HSPs could be such endogenous molecules that activate DCs in manner similar to these microbial antigens.[24] The initial clues came from a study on the immune responses to purified endoplasmic reticular (ER) HSP, gp96,[25] which concluded that the interaction of purified gp96-peptide complexes with antigen presenting cells (APCs), such as macrophages or DCs, leads to binding of gp96-peptide complexes to common HSP receptor, CD91, on APCs,[26,27] followed by its internalization, processing of the gp96-chaperoned peptides and their re-presentation by major histocompatibility complex I (MHC I) and MHC II molecules. The MHC-peptide complexes act as “signal one” to stimulate the cognate CD4− and CD8− T-cells. Also, the interaction of gp96 with APCs causes activation and maturation of DCs, which secrets pro-inflammatory cytokines and provides costimulatory signals (“signal two”) for effective T-cell priming. These findings have been proven correct using cells expressing gp96 on the cell surface.[28] It was found that cell surface expression of gp96 by tumor cells leads to DC maturation and cross priming of tumor-specific T-cells [Figure 2].
Figure 2.
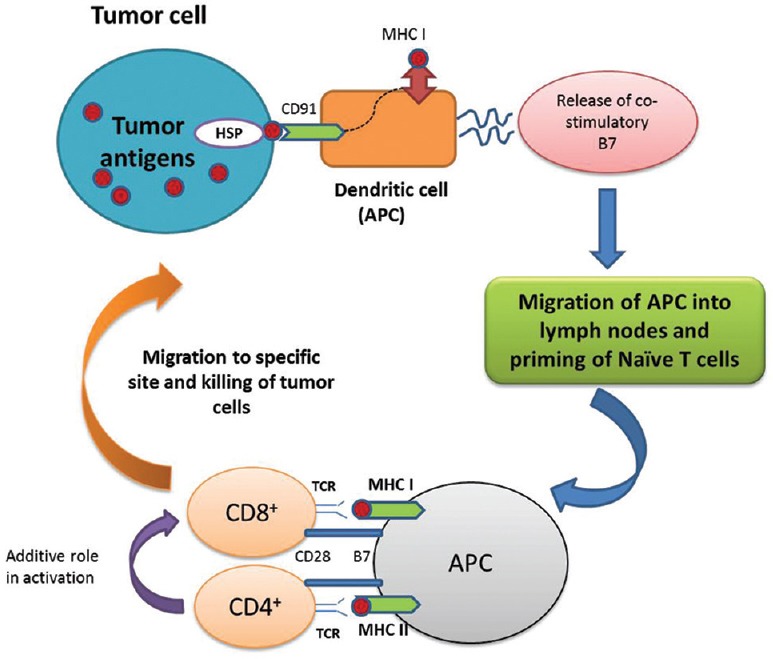
Role of heat shock proteins in cancer: Heat shock proteins (e.g., gp96) stimulating anticancer immunity. The interaction of gp96 with its specific receptors, such as CD91 on antigen presenting cells is followed by cross-presentation of antigens to major histocompatibility complex class I and increase in the release of co-stimulatory molecules such as B7. These dendritic cells subsequently migrate to the draining lymph nodes and prime antigen-specific naive T-cells. Those CD8+ T-cells (helped by CD4+ T-cells) exit from lymph into the tumor sites for lysis and clearance of tumors in an antigen specific manner
Liu has substantiated the role of gp96 (endoplasmin) as an important chaperone for inflammation and cancer. It has been well-established as a mediator in inducing toll-like receptor (TLR) signaling when challenged with pathogen-associated LPS molecules, thus leading to inflammatory responses. Its role in immunology can also be verified by the fact that gp96 is induced 10-folds on B-cell activation.[29]
The immunological features of HSPs have been summarized:
HSPs chaperone interact with immunologically important molecules such as MHC I,[30] immunoglobulin's,[31] T-cell receptors and TLRs[32]
HSPs chaperone bind cellular peptides
Extracellular HSPs serve as cytokines to activate the innate functions of APCs, such as DCs, because of their binding to specific receptors on APCs
HSPs can deliver their chaperoned peptides from non-APCs to MHC molecules of APCs and
Depending upon different modes of tissue damage, the release of HSPs may play immunoregulatory roles in in vivo.
Thus, they were hailed by some as the immune system's“swiss army knives”.[33]
Role of heat shock proteins in antigen processing and presentation
Formation of stable MHC complexes capable of presenting antigenic peptides to T-cells depends on their proper folding and assembly in the ER, as well as on the availability of peptide ligands. Folding and assembly of both MHC class I and class II molecules is initiated in the ER, whereas the site of peptide loading depends on the intracellular compartment in which degraded protein fragments are sampled.[34] MHC class I molecules are loaded in the ER with ligands derived from endogenous proteins present in the cytosol (viral, tumor or self-antigens). Peptides from the cytoplasm are transported into the ER by a specialized transport system, termed the transporter associated with antigen processing (TAP). In contrast, MHC class II molecules bind ligands of extracellular origin in the endosomal compartment. To prevent premature loading of the MHC class II molecule in the ER, its binding site is blocked by the invariant chain, which is released in the endosome, so that loading of MHC class II molecules with endosomal peptides becomes possible.[35] Several lines of evidence suggest that HSP plays a role in MHC-antigen processing.[36,37,38,39] Folding and assembly of MHC-peptide complexes are promoted by molecular chaperones, which holds true for many other proteins. Members of the Hsp70 family are critically involved in the processing and presentation of antigens.[37,39,40,41,42] Binding immunoglobulin protein (BiP) and another endoplasmic chaperone, calnexin, promote the assembly of both MHC class I and class II molecules in the ER.[43,44] Furthermore, for BiP and other chaperones such as gp96 and Hsp70 (ERp72), an interaction with misfolded MHC class II molecules has been demonstrated, resulting in their retention in the ER.[45]
Srivastava et al. have provided substantial evidence that peptide transport from the proteasome to the ER and subsequent peptide loading of MHC class I molecules in the ER depend on a battery of HSP including cytosolic and endoplasmic members of the Hsp70 and Hsp90 families.[46,47] Recent studies have revealed that gp96 in the ER acts as a peptide acceptor, receiving peptides of cytosolic origin after their transport through the ER membrane by TAP molecules.[48] Subsequently, gp96-peptide complexes bind to MHC and the peptides are then translocated from gp96 to MHC class I molecules in an ATP-dependent manner.[47] Due to its proteolytic activity, gp96 may also participate in further trimming of MHC class I peptides in the ER.[49,50]
The immunological roles of HSPs have come to light primarily because of their involvement in antitumor immunity and the ensuing implications for antigen presentation and re-presentation. According to Li et al., HSP polypeptides interact with macrophages, DCs, T-cells and platelets through known and yet to be discovered receptors. HSP/APC interaction leads to the secretion of cytokines and chemokines and to the maturation and migration of DCs, possibly as a result of the translocation of nuclear factor-kappa beta into the nucleus. They are effective in antigen presentation via the MHC I and MHC II pathway.[30]
Recently, Javid et al. have shown similarities in the peptide binding between HSP70 and MHC I molecules and detailed on the role of Hsp70 and Hsp90 in antigen processing and presentation in an ATP-dependent manner.[51]
Autoimmune diseases: Heat shock proteins role in breaking immune tolerance: A hypothesis
The beauty of the immune system lies in its ability to mount effective immune responses against pathogens, while remaining nonresponsive to more abundant and normal self-antigens.
For T-lymphocytes, the vast majority of potentially self-reactive cells are eliminated during development in the thymus by what is called as negative selection.[52,53] When self-reactive T-cells do migrate into the periphery, multiple mechanisms play pivotal role to prevent these cells from inappropriate activation, including antigen sequestration, clonal exhaustion, anergy and antigen-specific suppression or regulation.[54] The prevalent hypothesis regarding antigen-driven peripheral tolerance is that antigen (signal 1) alone without the presence of costimulatory molecules (signal 2) leads to antigen-specific unresponsiveness or anergy.[55,56] The default pathway for immunological response to tumor thus might be tolerance attributable to lack of signal 2. This tolerance has been shown to be overcome by transfecting tumor cells with costimulatory molecules,[57,58] introducing proinflammatory cytokines/chemokines to the tumors,[59,60] administration of systemic cytokines or signal 2 agonist[61,62] and other means to activate/modulate the function of DCs.[63,64] Since HSPs are capable of activating DCs to up-regulate signal 2, in addition to delivering signal 1 through cross-presentation of HSP-chaperoned peptides, it is justified to hypothesize that HSPs can break peripheral tolerance against tumor associated antigens.
Under the condition of stress or “danger,” HSPs are not only increased in expression level (for the purpose of cytoprotection and antigen presentation) but could also undergo dynamic redistribution to gain access to the extracellular environment. Their cell surface expression or secretion might possibly lead to sending an “ON” signal to activate the immune system and thus break down peripheral tolerance. Liu et al.[65] and previously Zügel and Kaufmann[66] have beautifully illustrated the cytokine role of these molecules.
Heat shock proteins in disease
Neurodegenerative disorders like Alzheimers, Huntington disease, spinocerebellar ataxias, Parkinson's disease, etc., have been linked to the aberrant expression of HSPs.[67] Various cell imaging experiments have shown an increase in the level of Hsp70 in relation to the huntingtin aggregates.[68] This suggests that these chaperone interactions may reflect the efforts of Hsp70 to direct the unfolding and dissociation of substrates from the aggregate and dampen its damaging effects.[69]
Relation to aging
The HSR has recently been implicated in the regulation of longevity in Caenorhabditis elegans. RNA interference experiments show that a decrease in sHSPs and other HSPs leads to a decrease in longevity.[70,71] Therefore, in addition to the prevention of diseases of aging, increased levels of HSPs may lead to increase in life span.
Heat shock proteins and cancer
Tumor cells typically express higher levels of HSPs compared with nontransformed cells, leading to the suggestion that the aberrant expression of chaperones is associated with the tumorigenic state.[72] An intriguing proposition is that tumor cells are dependent on elevated levels of HSPs, perhaps is a generalized mechanism to suppress cumulative mutations that would otherwise result in the expression of deleterious proteins. The chronic upregulation of HSPs could also promote cancer by the anti-apoptotic functions demonstrated by all chaperones. Hsp70, Hsp40, Hsp27 and Hsp90 act at multiple points in apoptosis, including inhibition of c-Jun NH2-terminal kinase activation, prevention of cytochrome C release, regulation of the apoptosome, prevention of lysosomal membrane permeabilization and prevention of caspase activation.[73] Therefore, compounds that downregulate the HSR and chaperone levels, when given in combination with chemotherapy, may prove beneficial for cancer treatment.
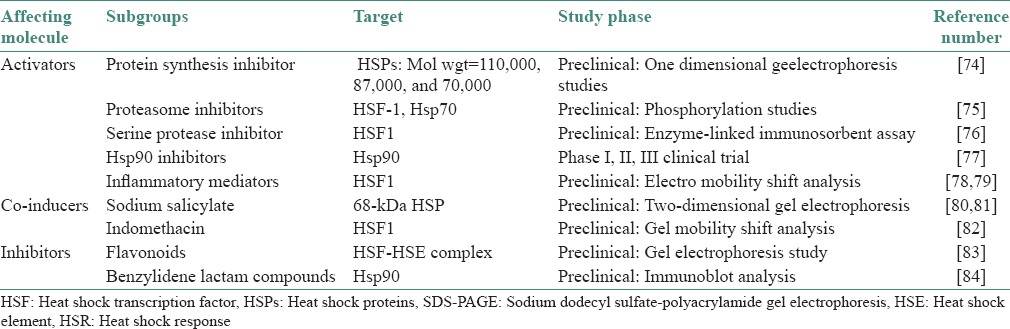
Therapeutically active small molecules that regulate HSF1 or modulate chaperone activities could benefit diseases that have in common alterations in protein conformation that cause an imbalance in protein homeostasis. The classes of small molecules that modulate the HSR are represented by a diverse set of chemically unrelated compounds consistent with the various environmental and physiological signaling pathways that induce the HSR [Table 2].[74,75,76,77,78,79,80,81,82,83,84]
Table 2.
Activators and inhibitors of the HSR
Heat shock proteins as effective cancer vaccine
Tumor-derived HSPs have been shown to be effective cancer vaccines not only for prophylaxis against cancers but also for the treatment of existing cancers in many preclinical tumor models.[25,85] These have prompted systemic clinical testing of tumor-derived HSPs for the treatment of human malignancy.[86,87] The current effort has been focused mainly on gp96 and Hsp70. Unlike traditional cancer drugs, HSP-peptide vaccine is individually based and tailored toward an individual tumor of an individual patient.[86] This is based on two reasons: (a) HSPs chaperone is antigenic fingerprint of cells from which they are isolated and (b) tumor-protective antigens are individually distinct. As early as in the 1940s, it was appreciated that tumors were antigenically distinct from one another, most likely because of the subsequent realization of the differences in peptide pools among different tumors, as a result of random mutations of DNAs in the transformed cell. Depending on the tumor types, grade, differentiation stage or genetic background, peptide pools that are associated with HSPs should also be individually unique. Therefore, to customize the tumor vaccine for the patient, tumor antigens (HSP-peptide complexes) should be effectively derived from autologous tumors of this patient and not from those of someone else. More than 300 patients have been treated with HSP vaccines thus far.[86] The diseases include lymphoma, renal cell carcinoma, melanoma, colorectal cancer, gastric cancer, pancreatic cancer, breast cancer and others.
On monitoring clinically and immunologically, no significant toxicities including the generation of autoantibodies have been reported.
The ability of human melanoma-derived Hsp70 to stimulate autologous melanoma-specific T-cells for producing interferon-gamma were demonstrated using peripheral monocytes pulsed with Hsp70 as targets.[87,88] This makes us ponder further on the prospect of using this modality for next phase clinical trials in advanced melanoma.
A detailed account of these findings have been reviewed by Jolly and Morimoto.[89]
Heat shock proteins 70 in oral lichen planus: Role in pathogenesis and proposal of a therapeutic model…
Oral lichen planus (OLP) has been the focus of study for its proposed potential malignant nature. Sugerman et al. drew comparisons in HSP staining among OLP, dysplastic OLP, normal oral mucosa and nonspecific oral ulceration.[90] The expression of HSP was prominently noticed in 94% of their cases. Hsp70 expression was found throughout the full thickness of the epithelium among 26 of the 30 samples of clinically and histologically confirmed cases of OLP. These were quantitatively and qualitatively analyzed by myself and colleagues in a previous study.[91] This finding was justified since these molecules are essential protein folding tools in the cellular machinery.[91,92,93] The normal, nondiseased mucosa of the oral cavity shows a faint expression of HSPs in the epithelium. This has been previously confirmed in literature by various studies conducted by Bramanti et al.[94] and Seoane et al.[95] The expression of HSPs was found to be de-regulated in OLP.
The basal cell zone in this particular premalignant condition is an apparent target for destruction by the sub epithelial T-lymphocytic population (CD8+).[96,97] The raised index of the HSPs here re-asserts the effect of “stress of dying”. HSPs probably represent the antigenic proteins that may potentially be involved in both the initiation and the persistence of the autoimmune lymphocytic response of lichen planus.[91,94,97]
In contrast, Chaiyarit et al. confirmed the expression of Hsp60 in the basal layer of OLP, but found no significant difference in the expression of Hsp70 between the OLP and oral fibroma groups.[98] Bramanti et al.[94] and Seoane et al.[95] reported that Hsp70 expression in OLP, when compared to the normal mucosa were slight and inconclusive.
There is literature pertaining to anticancer research that suggests the usage of inhibitors of the HSPs as a novel tool in cancer therapy. The benzoquinoid ansamycin antibiotics, first isolated from the actinomycete Streptomyces hygroscopicus var. geldanus var. nova, include geldanamycin and its semi-synthetic derivatives, 17-allylamino-17-demethoxygeldanamycin(17-AAG) and water-soluble 17- demethylam inoethylamino -17 -demethoxygeldanamycin. These inhibitors work by interacting specifically with a single molecule, Hsp90; cause destabilization and eventual degradation of multiple Hsp90 client proteins. The first-in-class Hsp90 inhibitor, 17-AAG is currently in phase II clinical trials. About 20 interventional studies are in clinical phase I and II trials, delivered either orally or via IV route. However, direct tumor monitoring either by biopsy or noninvasive methods is critical to optimal clinical efficacy.[99,100]
New alternatives and synthetic analogs based on 17-AAG (17-amino-17 demethoxygeldanamycin, in phase III clinical trial) backbone have been developed which have overcome in vivo inactivity, are safer and easier to produce.[101]
Lately, as there is evolving evidence that HSPs are present in the extracellular environment and identification of HSP and antibodies directed against it in normal individuals has shown that reactivity to these does not necessarily reflect adverse, pro-inflammatory responses and that the promotion of reactivity to self-HSPs can downregulate pathogenic processes, all suggesting a potential role for HSPs as therapeutic agents, rather than as therapeutic targets.[102,103] The potential therapeutic value of HSPs purified from appropriate tissues lies in their capacity to induce pro-inflammatory responses at low concentrations and induce regulatory immunity at high doses. The key lies in delivering the appropriate peptide.[104]
Recent studies
Over the past few years, the research in the field of HSR has intensified. Evaluation to establish the relationship between Hsp90 and Hsp70 was performed by Nakamoto et al.[105] A mutual supportive function, helping each other in protein refolding was found between the two chaperones.
Messmer et al. reestablished the in vivo immunogenic role of HSPs.[106] Dysregulation in the intracellular expression of these molecules has been linked to functional and pathological aggregate formations.[107]
Strong pathological correlations[108] and association between autoimmunity and new related autoimmune diseases has been resurfacing in more recent literature.[109,110] Extensive database has accumulated to justify the role of these molecules in carcinogenesis and tumor advancement.[111,112]
CONCLUSION
Surprising immunological features have been linked with HSPs. Their functioning in the cells has both a role in normal as well as pathological states. In the current context, understanding the implications of these wonderful molecules is an ardent task, a challenge to be taken up in full swing. Using this technology for immunotherapy should involve designing well-planned customized therapies. More clinical studies should be conducted to be sure how to use these for battling against diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–66. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 2.Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–3. [Google Scholar]
- 3.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–91. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto RI. Cells in stress: Transcriptional activation of heat shock genes. Science. 1993;259:1409–10. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- 5.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 6.Ellis RJ. The general concept of molecular chaperones. Philos Trans R Soc Lond B Biol Sci. 1993;339:257–61. doi: 10.1098/rstb.1993.0023. [DOI] [PubMed] [Google Scholar]
- 7.Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–34. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 8.Welch WJ. Heat shock proteins functioning as molecular chaperones: Their roles in normal and stressed cells. Philos Trans R Soc Lond B Biol Sci. 1993;339:327–33. doi: 10.1098/rstb.1993.0031. [DOI] [PubMed] [Google Scholar]
- 9.Soo ET, Yip GW, Lwin ZM, Kumar SD, Bay BH. Heat shock proteins as novel therapeutic targets in cancer. In Vivo. 2008;22:311–5. [PubMed] [Google Scholar]
- 10.Wu C. Heat shock transcription factors: Structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–69. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto RI. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 12.Pirkkala L, Nykänen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–31. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 13.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–55. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaeger AM, Makley LN, Gestwicki JE, Thiele DJ. Genomic heat shock element sequences drive cooperative human heat shock factor 1 DNA binding and selectivity. J Biol Chem. 2014;289:30459–69. doi: 10.1074/jbc.M114.591578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landry J, Bernier D, Chrétien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982;42:2457–61. [PubMed] [Google Scholar]
- 16.Ahn SG, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–28. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol. 2004;24:5249–56. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson S, Hollis A, Hazzalin CA, Mahadevan LC. Distinct stimulus-specific histone modifications at hsp70 chromatin targeted by the transcription factor heat shock factor-1. Mol Cell. 2004;15:585–94. doi: 10.1016/j.molcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Rieger TR, Morimoto RI, Hatzimanikatis V. Mathematical modeling of the eukaryotic heat-shock response: Dynamics of the hsp70 promoter. Biophys J. 2005;88:1646–58. doi: 10.1529/biophysj.104.055301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephanou A, Latchman DS. Transcriptional modulation of heat-shock protein gene expression. Biochem Res Int 2011. 2011 doi: 10.1155/2011/238601. 238601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, et al. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 23.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–94. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Dai J, Zheng H, Liu B, Caudill M. An integrated view of the roles and mechanisms of heat shock protein gp96-peptide complex in eliciting immune response. Front Biosci. 2002;7:d731–51. doi: 10.2741/a808. [DOI] [PubMed] [Google Scholar]
- 26.Binder RJ, Han DK, Srivastava PK. CD91: A receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 27.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 28.Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731–5. doi: 10.4049/jimmunol.167.12.6731. [DOI] [PubMed] [Google Scholar]
- 29.Liu B. Heat shock protein gp96 as an immune chaperone of inflammation and cancer. Austin J Clin Immunol. 2014;1:1014–9. [Google Scholar]
- 30.Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. 2002;14:45–51. doi: 10.1016/s0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 31.Melnick J, Dul JL, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–5. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 32.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–6. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 33.Schild H, Rammensee HG. gp96 – The immune system's Swiss army knife. Nat Immunol. 2000;1:100–1. doi: 10.1038/77770. [DOI] [PubMed] [Google Scholar]
- 34.Neefjes JJ, Momburg F. Cell biology of antigen presentation. Curr Opin Immunol. 1993;5:27–34. doi: 10.1016/0952-7915(93)90077-6. [DOI] [PubMed] [Google Scholar]
- 35.Sant AJ, Miller J. MHC class II antigen processing: Biology of invariant chain. Curr Opin Immunol. 1994;6:57–63. doi: 10.1016/0952-7915(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 36.Cristau B, Schafer PH, Pierce SK. Heat shock enhances antigen processing and accelerates the formation of compact class II alpha beta dimers. J Immunol. 1994;152:1546–56. [PubMed] [Google Scholar]
- 37.DeNagel DC, Pierce SK. A case for chaperones in antigen processing. Immunol Today. 1992;13:86–9. doi: 10.1016/0167-5699(92)90147-Y. [DOI] [PubMed] [Google Scholar]
- 38.Melnick J, Argon Y. Molecular chaperones and the biosynthesis of antigen receptors. Immunol Today. 1995;16:243–50. doi: 10.1016/0167-5699(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 39.Williams DB, Watts TH. Molecular chaperones in antigen presentation. Curr Opin Immunol. 1995;7:77–84. doi: 10.1016/0952-7915(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 40.Jacquier-Sarlin MR, Fuller K, Dinh-Xuan AT, Richard MJ, Polla BS. Protective effects of hsp70 in inflammation. Experientia. 1994;50:1031–8. doi: 10.1007/BF01923458. [DOI] [PubMed] [Google Scholar]
- 41.Schirmbeck R, Reimann J. Peptide transporter-independent, stress protein-mediated endosomal processing of endogenous protein antigens for major histocompatibility complex class I presentation. Eur J Immunol. 1994;24:1478–86. doi: 10.1002/eji.1830240704. [DOI] [PubMed] [Google Scholar]
- 42.Vanbuskirk A, Crump BL, Margoliash E, Pierce SK. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989;170:1799–809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson MR, Cohen-Doyle MF, Peterson PA, Williams DB. Regulation of MHC class I transport by the molecular chaperone, calnexin (p88, IP90) Science. 1994;263:384–7. doi: 10.1126/science.8278813. [DOI] [PubMed] [Google Scholar]
- 44.Anderson KS, Cresswell P. A role for calnexin (IP90) in the assembly of class II MHC molecules. EMBO J. 1994;13:675–82. doi: 10.1002/j.1460-2075.1994.tb06306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaiff WT, Hruska KA, Jr, McCourt DW, Green M, Schwartz BD. HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells. J Exp Med. 1992;176:657–66. doi: 10.1084/jem.176.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: Role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153–77. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–8. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 48.Lammert E, Arnold D, Nijenhuis M, Momburg F, Hämmerling GJ, Brunner J, et al. The endoplasmic reticulum-resident stress protein gp96 binds peptides translocated by TAP. Eur J Immunol. 1997;27:923–7. doi: 10.1002/eji.1830270418. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, Srivastava PK. Tumor rejection antigen gp96/grp94 is an ATPase: Implications for protein folding and antigen presentation. EMBO J. 1993;12:3143–51. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–9. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Javid B, MacAry PA, Lehner PJ. Structure and function: Heat shock proteins and adaptive immunity. J Immunol. 2007;179:2035–40. doi: 10.4049/jimmunol.179.4.2035. [DOI] [PubMed] [Google Scholar]
- 52.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–80. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 53.von Boehmer H, Kisielow P. Self-nonself discrimination by T cells. Science. 1990;248:1369–73. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]
- 54.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 55.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–9. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 56.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 58.Chen L, Ashe S, Brady WA, Hellström I, Hellström KE, Ledbetter JA, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 59.Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995;13:399–415. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- 60.Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, et al. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: Augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–70. [PubMed] [Google Scholar]
- 61.Vallera DA, Taylor PA, Aukerman SL, Blazar BR. Antitumor protection from the murine T-cell leukemia/lymphoma EL4 by the continuous subcutaneous coadministration of recombinant macrophage-colony stimulating factor and interleukin-2. Cancer Res. 1993;53:4273–80. [PubMed] [Google Scholar]
- 62.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94:8099–103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baggers J, Ratzinger G, Young JW. Dendritic cells as immunologic adjuvants for the treatment of cancer. J Clin Oncol. 2000;18:3879–82. doi: 10.1200/JCO.2000.18.23.3879. [DOI] [PubMed] [Google Scholar]
- 64.Morse MA, Mosca PJ, Clay TM, Lyerly HK. Dendritic cell maturation in active immunotherapy strategies. Expert Opin Biol Ther. 2002;2:35–43. doi: 10.1517/14712598.2.1.35. [DOI] [PubMed] [Google Scholar]
- 65.Liu B, DeFilippo AM, Li Z. Overcoming immune tolerance to cancer by heat shock protein vaccines. Mol Cancer Ther. 2002;1:1147–51. [PubMed] [Google Scholar]
- 66.Zügel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kakizuka A. Protein precipitation: A common etiology in neurodegenerative disorders? Trends Genet. 1998;14:396–402. doi: 10.1016/s0168-9525(98)01559-5. [DOI] [PubMed] [Google Scholar]
- 68.Kim S, Nollen EA, Kitagawa K, Bindokas VP, Morimoto RI. Polyglutamine protein aggregates are dynamic. Nat Cell Biol. 2002;4:826–31. doi: 10.1038/ncb863. [DOI] [PubMed] [Google Scholar]
- 69.Warrick JM, Chan HY, Gray-Board GL, Chai Y, Paulson HL, Bonini NM. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet. 1999;23:425–8. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- 70.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–5. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 71.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–64. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jäättelä M. Escaping cell death: Survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- 73.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–18. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 74.Lee YJ, Dewey WC. Induction of heat shock proteins in Chinese hamster ovary cells and development of thermotolerance by intermediate concentrations of puromycin. J Cell Physiol. 1987;132:1–11. doi: 10.1002/jcp.1041320102. [DOI] [PubMed] [Google Scholar]
- 75.Holmberg CI, Illman SA, Kallio M, Mikhailov A, Sistonen L. Formation of nuclear HSF1 granules varies depending on stress stimuli. Cell Stress Chaperones. 2000;5:219–28. doi: 10.1379/1466-1268(2000)005<0219:fonhgv>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossi A, Elia G, Santoro MG. Activation of the heat shock factor 1 by serine protease inhibitors. An effect associated with nuclear factor-kappaB inhibition. J Biol Chem. 1998;273:16446–52. doi: 10.1074/jbc.273.26.16446. [DOI] [PubMed] [Google Scholar]
- 77.Bagatell R, Paine-Murrieta GD, Taylor CW, Pulcini EJ, Akinaga S, Benjamin IJ, et al. Progressive decrease in chaperone protein levels in a mouse model Huntingtin's diseaseand induction of stress proteins as a therapeutic approach. Clin Cancer Res. 2000;6:3312–8. [PubMed] [Google Scholar]
- 78.Jurivich DA, Sistonen L, Sarge KD, Morimoto RI. HSP70 accumulation in chondrocyticcells exposed to high continuous hydrostatic pressure coincides with mRNA stabilizationrather than transcrioptional activation. Proc Natl Acad Sci U S A. 1994;91:2280–4. [Google Scholar]
- 79.Jurivich DA, Pangas S, Qiu L, Welk JF. Phospholipase A2 triggers the first phase of the thermal stress response and exhibits cell-type specificity. J Immunol. 1996;157:1669–77. [PubMed] [Google Scholar]
- 80.Ohno K, Fukushima M, Fujiwara M, Narumiya S. Induction of 68,000-dalton heat shock proteins by cyclopentenone prostaglandins. Its association with prostaglandin-induced G1 block in cell cycle progression. J Biol Chem. 1988;263:19764–70. [PubMed] [Google Scholar]
- 81.Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–5. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 82.Lee BS, Chen J, Angelidis C, Jurivich DA, Morimoto RI. Pharmacological modulation of heat shock factor 1 by antiinflammatory drugs results in protection against stress-induced cellular damage. Proc Natl Acad Sci U S A. 1995;92:7207–11. doi: 10.1073/pnas.92.16.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagai N, Nakai A, Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun. 1995;208:1099–105. doi: 10.1006/bbrc.1995.1447. [DOI] [PubMed] [Google Scholar]
- 84.Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000;60:2942–8. [PubMed] [Google Scholar]
- 85.Li Z. Priming of T cells by heat shock protein-peptide complexes as the basis of tumor vaccines. Semin Immunol. 1997;9:315–22. doi: 10.1006/smim.1997.0087. [DOI] [PubMed] [Google Scholar]
- 86.Caudill MM, Li Z. HSPPC-96: A personalised cancer vaccine. Expert Opin Biol Ther. 2001;1:539–47. doi: 10.1517/14712598.1.3.539. [DOI] [PubMed] [Google Scholar]
- 87.Srivastava PK. Immunotherapy of human cancer: Lessons from mice. Nat Immunol. 2000;1:363–6. doi: 10.1038/808795. [DOI] [PubMed] [Google Scholar]
- 88.Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, et al. Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res. 2001;61:222–7. [PubMed] [Google Scholar]
- 89.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–72. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 90.Sugerman PB, Savage NW, Xu LJ, Walsh LJ, Seymour GJ. Heat shock protein expression in oral lichen planus. J Oral Pathol Med. 1995;24:1–8. doi: 10.1111/j.1600-0714.1995.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 91.Tyagi N, Shetty DC, Urs AB. Altered expression of HSP70 in oral lichen planus. J Oral Maxillofac Pathol. 2012;16:189–94. doi: 10.4103/0973-029X.98497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deuerling E, Bukau B. Chaperone-assisted folding of newly synthesized proteins in the cytosol. Crit Rev Biochem Mol Biol. 2004;39:261–77. doi: 10.1080/10409230490892496. [DOI] [PubMed] [Google Scholar]
- 93.Beere HM. “The stress of dying”: The role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117(Pt 13):2641–51. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 94.Bramanti TE, Dekker NP, Lozada-Nur F, Sauk JJ, Regezi JA. Heat shock (stress) proteins and gamma delta T lymphocytes in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:698–704. doi: 10.1016/s1079-2104(05)80254-9. [DOI] [PubMed] [Google Scholar]
- 95.Seoane J, Ramírez JR, Romero MA, Varela-Centelles P, Garcia-Pola MJ. Expression of heat shock protein (HSP70) in oral lichen planus and non-dysplastic oral leucoplakia. Clin Otolaryngol Allied Sci. 2004;29:191–6. doi: 10.1111/j.0307-7772.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 96.Khan A, Farah CS, Savage NW, Walsh LJ, Harbrow DJ, Sugerman PB. Th1 cytokines in oral lichen planus. J Oral Pathol Med. 2003;32:77–83. doi: 10.1034/j.1600-0714.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 97.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 98.Chaiyarit P, Kafrawy AH, Miles DA, Zunt SL, Van Dis ML, Gregory RL. Oral lichen planus: An immunohistochemical study of heat shock proteins (HSPs) and cytokeratins (CKs) and a unifying hypothesis of pathogenesis. J Oral Pathol Med. 1999;28:210–5. doi: 10.1111/j.1600-0714.1999.tb02026.x. [DOI] [PubMed] [Google Scholar]
- 99.Koga F, Kihara K, Neckers L. Inhibition of cancer invasion and metastasis by targeting the molecular chaperone heat-shock protein 90. Anticancer Res. 2009;29:797–807. [PubMed] [Google Scholar]
- 100.Den RB, Lu B. Heat shock protein 90 inhibition: Rationale and clinical potential. Ther Adv Med Oncol. 2012;4:211–8. doi: 10.1177/1758834012445574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823:742–55. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci U S A. 1997;94:13146–51. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–77. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 104.Pockley AG. Heat shock proteins in health and disease: Therapeutic targets or therapeutic agents? Expert Rev Mol Med. 2001;3:1–21. doi: 10.1017/S1462399401003556. [DOI] [PubMed] [Google Scholar]
- 105.Nakamoto H, Fujita K, Ohtaki A, Watanabe S, Narumi S, Maruyama T, et al. Physical interaction between bacterial heat shock protein (Hsp) 90 and Hsp70 chaperones mediates their cooperative action to refold denatured proteins. J Biol Chem. 2014;289:6110–9. doi: 10.1074/jbc.M113.524801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Messmer MN, Pasmowitz J, Kropp LE, Watkins SC, Binder RJ. Identification of the cellular sentinels for native immunogenic heat shock proteins in vivo . J Immunol. 2013;191:4456–65. doi: 10.4049/jimmunol.1300827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang HY, Hou SC, Way TD, Wong CH, Wang IF. Heat-shock protein dysregulation is associated with functional and pathological TDP-43 aggregation. Nat Commun. 2013;4:2757. doi: 10.1038/ncomms3757. [DOI] [PubMed] [Google Scholar]
- 108.Kakkar V, Meister-Broekema M, Minoia M, Carra S, Kampinga HH. Barcoding heat shock proteins to human diseases: Looking beyond the heat shock response. Dis Model Mech. 2014;7:421–34. doi: 10.1242/dmm.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moudgil KD, Thompson SJ, Geraci F, De Paepe B, Shoenfeld Y. Heat-shock proteins in autoimmunity. Autoimmune Dis 2013. 2013 doi: 10.1155/2013/621417. 621417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mosenson JA, Eby JM, Hernandez C, Le Poole IC. A central role for inducible heat-shock protein 70 in autoimmune vitiligo. Exp Dermatol. 2013;22:566–9. doi: 10.1111/exd.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch Toxicol. 2013;87:19–48. doi: 10.1007/s00204-012-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou YJ, Messmer MN, Binder RJ. Establishment of tumor-associated immunity requires interaction of heat shock proteins with CD91. Cancer Immunol Res. 2014;2:217–28. doi: 10.1158/2326-6066.CIR-13-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]


